Analysis of Valorization Process of Aluminum Breakage Scraps to Obtain Green Hydrogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Aluminum
2.1.1. Aluminum Plate (A1)
2.1.2. Aluminum Chips (A2 and A3)
2.1.3. Pure Grainy Aluminum (A4)
2.2. Sodium Hydroxide
2.3. Alcohol
3. Components and Methodology Used in Tests
Determining the Theoretical Model
4. Tests
4.1. Test 1: Effect of Chips Size
4.2. Test 2: Temperature Effect
4.3. Test 3: Effect of Adding Alcohol
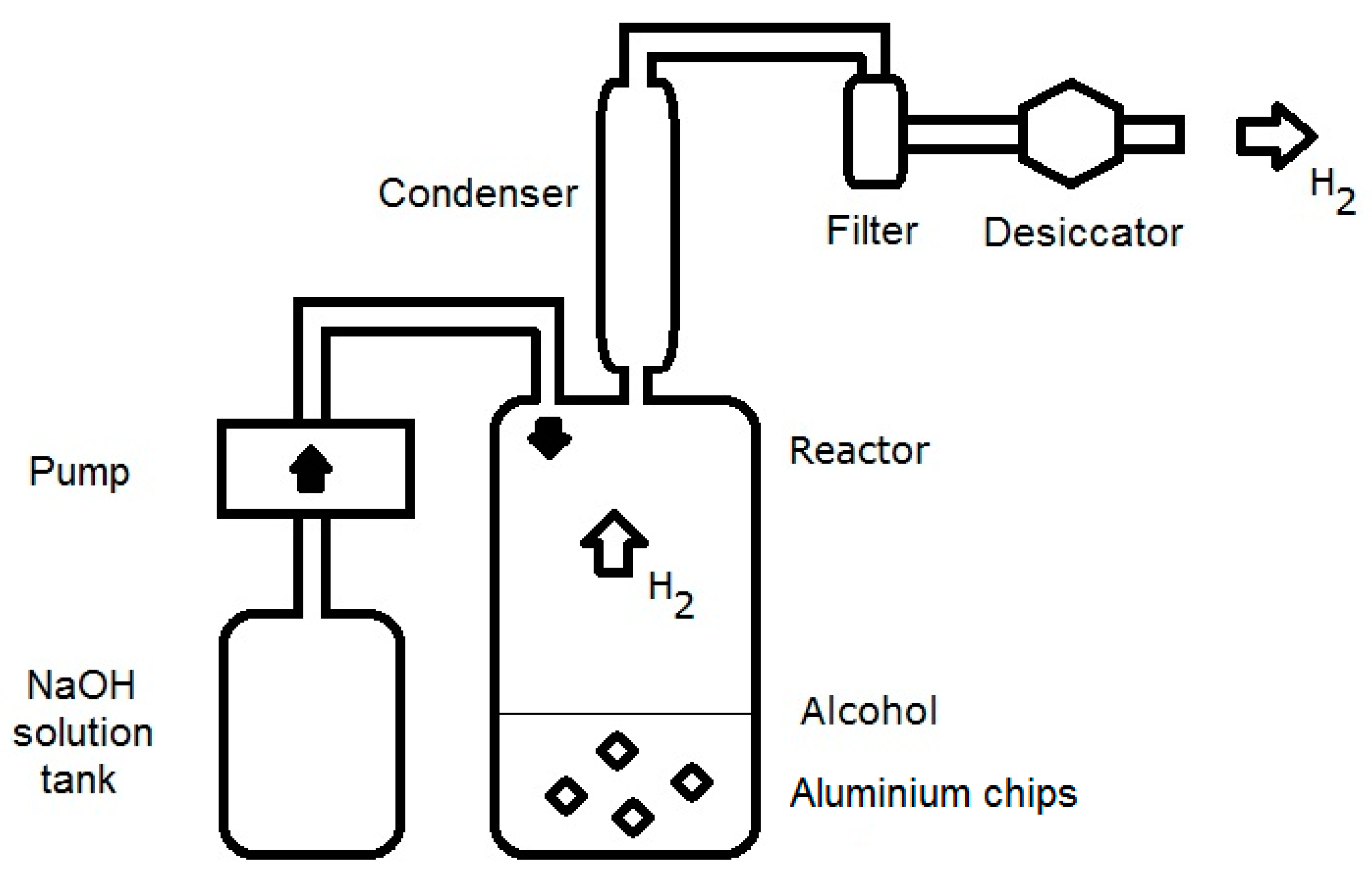
5. Hydrogen Generation Reactor from Chips
5.1. Hydrogen Generation Procedure
5.2. Analysis of Hydrogen Purity
5.3. Analysis of Aluminum Hydroxide Purity
6. Theoretical Kinetic Model
6.1. Chemical Reactions
ΔH = −278 kJ/mol H2
6.2. Theoretical Hydrogen Flow
6.2.1. The Hypothesis of Enough Active Aluminum Surface
6.2.2. The Hypothesis of Not Enough Active Aluminum Surface
6.3. Active Aluminum Surface
6.3.1. Aluminum Waste in the Grainy Form
6.3.2. Aluminum Waste in Chip Shape
7. Results and Discussion
7.1. Result of Test 1: Effect of Chips Size
7.2. Result of Test 2: Temperature Effect
7.3. Result of Test 3: Effect of Adding Alcohol
7.4. Results in the Hydrogen Generation Reactor from Different Types of Industrial Aluminum Chips
7.5. Purity of Hydrogen
7.6. Purity of Aluminum Hydroxide
7.7. Economic Study
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abánades, A. The challenge of Hydrogen production for the transition to a CO2-free economy. Agron. Res. Biosyst. Eng. Spec. 2012, 1, 11–16. [Google Scholar]
- Widera, B. Renewable Hydrogen implementations for combined energy storage, transportation, and stationary application. Therm. Sci. Eng. Prog. 2020, 16, 100460. [Google Scholar] [CrossRef]
- Ball, M.; Weeda, M. The hydrogen economy, vision or reality? Compend. Hydrogen Energy 2016, 40, 237–266. [Google Scholar]
- Wang, H.Z.; Leung, D.Y.C. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Woodall, J.M.; Ziebarth, J.T.; Allen, C.R.; Jeon, J.; Choi, G.; Kramer, R. Generating hydrogen on demand by splitting water with Al-rich alloys. Clean Technol. 2008, 5, 313–315. [Google Scholar]
- Lin, Y.; Genzer, J.; Dickey, M.D. Attributes, Fabrication and Applications of Gallium-Based Liquid Metal Particles. Adv. Sci. 2020, 7, 2000192. [Google Scholar] [CrossRef]
- Pyun, S.I.; Moon, S. Corrosion mechanism of pure aluminum in aqueous alkaline solution. J. Solid State Electrochem. 2000, 4, 267–272. [Google Scholar] [CrossRef]
- Huang, X.N.; Liu, S.; Wang, C.; Chen, D.; Huang, Y.X. On-Demand Hydrogen Generator Based on the Reaction between Aluminum Slurry and Alkaline Solution. Adv. Mater. Res. 2012, 347–353, 3242–3245. [Google Scholar] [CrossRef]
- Rami, A.; Lasia, A. Kinetics of hydrogen evolution on Ni-Al alloy electrodes. J. Appl. Electrochem. 1992, 22, 376–382. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Effect of structural evolution of aluminum powder during ball milling on hydrogen generation in aluminum water reaction. Int. J. Hydrogen Energy 2012, 38, 795–806. [Google Scholar] [CrossRef]
- Hakenjos, A.; Muenter, H.; Wittstadt, U.; Hebling, C. A PEM fuel cell for the combined measurement of current and temperature distribution, and flow field flooding. J. Power Sources 2004, 131, 213–216. [Google Scholar] [CrossRef]
- Petrovic, J.; Thomas, G. Reaction of Aluminum with Water to Produce Hydrogen. A Study of Issues Related to the Use of Aluminum for On-Board Vehicular Hydrogen Storage; U.S. Department of Energy: Washington, DC, USA, 2008.
- Parmuzina, A.V.; Kravchenko, O.V.; Bulychev, B.M.; Shkolnikov, E.I.; Burlakova, A.G. Oxidation of activated aluminum with water as a method for hydrogen generation. Russ. Chem. Bull. 2009, 58, 493–498. [Google Scholar] [CrossRef]
- Eom, K.S.; Cho, E.A.; Kwon, H.S. Feasibility of on-board hydrogen production from hydrolysis of AleFe alloy for PEMFCs. Int. J. Hydrogen Energy 2011, 36, 12338–12342. [Google Scholar] [CrossRef]
- Wang, E.D.; Shi, P.F.; Du, C.Y.; Wang, X.R. A mini-type hydrogen generator from aluminum for proton exchange membrane fuel cells. J. Power Sources 2008, 181, 144–148. [Google Scholar] [CrossRef]
- Akansu, S.O.; Dulger, Z.; Kahraman, N.; Veziroǧlu, T.N. Internal combustion engines fueled by natural gas—Hydrogen mixtures. Int. J. Hydrogen Energy 2004, 29, 1527–1539. [Google Scholar] [CrossRef]
- Teng, H.T.; Lee, T.Y.; Chen, Y.K.; Wang, H.W.; Cao, G. Effect of Al(OH)3 on the hydrogen generation of aluminum-water system. J. Power Sources 2012, 219, 16–21. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–357. [Google Scholar] [CrossRef]
- Salueña-Berna, X.; Mujal-Rosas, R.; Dagà-Monmany, J.M.; Colom, X. Método para generar hidrógeno a partir de residuos de aluminio, para alimentar la pila de combustible de un coche de radio control. DYNA 2019, 94, 12–15. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Mechanism of corrosion of activated aluminum particles by hot water. Electrochim. Acta 2014, 127, 95–105. [Google Scholar] [CrossRef]
- Shaytura, N.S.; Laritchev, M.N.; Laritcheva, O.O.; Shkolnikov, E.I. Study of texture of hydroxides formed by aluminum oxidation with liquid water at various activation techniques. Curr. Appl. Phys. J. 2010, 10, 1567–1739. [Google Scholar] [CrossRef]
- Hu, H.; Qiao, M.; Pei, Y.; Fan, K.; Li, H.; Zong, B.; Zhang, X. Kinetics of hydrogen evolution in alkali leaching of rapidly quenched Ni-Al alloy. Appl. Catal. A. Gen. 2003, 252, 173–183. [Google Scholar] [CrossRef]
- Mahmoodi, K.; Alineiad, B. Enhancement of hydrogen generation rate in reaction of aluminum with water. Int. J. Hydrogen Energy 2010, 35, 5227–5232. [Google Scholar] [CrossRef]
- Park, Y.K.; Tadd, E.H.; Zubris, M.; Tannenbaum, R. Size-controlled synthesis of alumina nanoparticles from aluminum alkoxides. Mater. Res. Bull. 2005, 40, 1506–1512. [Google Scholar] [CrossRef]
- Macanás, J.; Soler, L.; Candela, A.M.; Muñoz, M.; Casado, J. Hydrogen generation by aluminum corrosion in aqueous alkaline solutions of inorganic promoters. The Alhydrox process. Energy 2011, 36, 2493–2501. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Wang, C.-C.; Lin, C.-L.; Tsai, Y.-L.; Chao, C.-G.; Liu, T.-F. Mechanism of aluminum-induced lateral crystallization of amorphous silicon. Jpn. J. Appl. Phys. 2010, 49, 2493–2501. [Google Scholar] [CrossRef]
- Khodadad, E.; Lei, M.K. Mathematical modeling for hard trivalent chromium coatings thickness with thin zincates interlayer on pure aluminum. Int. J. Electrochem. Sci. 2014, 9, 1250–1263. [Google Scholar]
- Keane, E.; Browne, D.J.; Carr, A.J. Experimental and economic study of aluminium-gallium alloys as a fuel/catalyst for hydrogen propulsion. In Proceedings of the SEEP 2009: International Conference on Sustainable Energy & Environmental Protection, Dublin, Ireland, 12–15 August 2009; pp. 12–15. [Google Scholar]
- Takehito, H.; Takeuchi, M.; Hisa, M.; Akiyama, T. Hydrogen Production from Waste Aluminum at Different Temperatures, with LCA. Mater. Trans. 2005, 46, 1052–1057. [Google Scholar]







| Aluminum | Si(%) | Cu(%) | Zn(%) | Fe(%) | Mn(%) | Others (%) | Al(%) |
|---|---|---|---|---|---|---|---|
| A1 | 0.25 | 0.05 | 0.1 | 0.4 | 0.05 | <0.05 | 99.5 |
| Aluminum | SI (%) | Cu (%) | Zn (%) | Fe (%) | Mn (%) | Others (%) | Al (%) |
|---|---|---|---|---|---|---|---|
| A2 | 5 | 1.5 | 0.7 | 0.5 | 0.15 | <0.05 | 92 |
| A3 | 0.5 | 1 | 5 | 0.2 | 0.1 | <0.05 | 94 |
| Aluminum | Si (%) | Cu (%) | Zn (%) | Fe (%) | Mn (%) | Others (%) | Al (%) |
|---|---|---|---|---|---|---|---|
| A4 | - | - | - | - | - | <0.05 | 99.7 |
| Alcohol (99%) | Formula | Tboiling (°C) | Density (g/cm3) at 20 °C | Solubility in H2O |
|---|---|---|---|---|
| Isopropyl alcohol | C3H8O | 83 | 0.79 | miscible |
| Typology | Quantity | Dimensions (mm3) | Weight (g) | Initial Contact Surface (mm2) |
|---|---|---|---|---|
| Plates (sheets) | 1 | 20 × 30 × 0.5 | 0.788 | 1240 |
| Chips (bits) | 104 | 2 × 2 × 0.5 | 0.534 | 1240 |
| Temperature (°C) | Thickness Reduction (mm/min) |
|---|---|
| 25 | 0.00117 ± 0.00003 |
| 60 | 0.01473 ± 0.00003 |
| Aluminum A1 Sheet (mm3) | Temperature (°C) | Thickness Depletion e (mm/min) | Time (min) | Average Flow Rate (mL/min) |
|---|---|---|---|---|
| 20 × 30 × 0.5 | 25 | 0.00117 ± 0.00003 | 208 | 4.7 |
| 20 × 30 × 0.5 | 60 | 0.01473 ± 0.00003 | 16 | 64.3 |
| Aluminum Type | Hydrogen 100 mL Solution (L) | Hydrogen End-of-Process (L) | Hydrogen Total (L) | ηprocess (%) | ηtotal (%) |
|---|---|---|---|---|---|
| Pure aluminum A4 | 30.5 | 38.9 | 38.9 | 99.7 | 99.7 |
| Automotive aluminum A2 | 21.8 | 32.2 | 34.6 | 82.6 | 88.7 |
| Aeronautical aluminum A3 | 20.8 | 29.9 | 33.3 | 76.7 | 85.4 |
| Aluminum Type | Initial Impurities (%) Cu-Zn-Fe-Si | Final Impurities (%) Cu-Zn-Fe-Si | Content Al (%) |
|---|---|---|---|
| A4 (pure) | <0.05 | <0,01 | 24.4 |
| A2 (automotive) | 1.5-0.7-0.5-5 | <0.01-0.11-0.02-0.19 | 24.3 |
| A3 (aeronautical) | 1-5-0.2-0.5 | <0.01-0.75-0-0.04 | 23.2 |
| Price Aluminum Breakage Scrap | Price Al2O3 | Cost/kg Hydrogen (9 kg Al) | Sale Al2O3 (3.7 kgAl2O3/kg Al) | Cost/kg Hydrogen Total |
|---|---|---|---|---|
| USD 0.6/kg Al | 0.3 $/kg Al2O3 | 5.4 $/kg H2 | 1.1 $/kg H2 | 4.3 $/kg H2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salueña Berna, X.; Marín-Genescà, M.; Dagà-Monmany, J.M. Analysis of Valorization Process of Aluminum Breakage Scraps to Obtain Green Hydrogen. Metals 2021, 11, 598. https://doi.org/10.3390/met11040598
Salueña Berna X, Marín-Genescà M, Dagà-Monmany JM. Analysis of Valorization Process of Aluminum Breakage Scraps to Obtain Green Hydrogen. Metals. 2021; 11(4):598. https://doi.org/10.3390/met11040598
Chicago/Turabian StyleSalueña Berna, Xavier, Marc Marín-Genescà, and José María Dagà-Monmany. 2021. "Analysis of Valorization Process of Aluminum Breakage Scraps to Obtain Green Hydrogen" Metals 11, no. 4: 598. https://doi.org/10.3390/met11040598
APA StyleSalueña Berna, X., Marín-Genescà, M., & Dagà-Monmany, J. M. (2021). Analysis of Valorization Process of Aluminum Breakage Scraps to Obtain Green Hydrogen. Metals, 11(4), 598. https://doi.org/10.3390/met11040598







