Microstructure and Mechanical Properties of High Relative Density γ-TiAl Alloy Using Irregular Pre-Alloyed Powder
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Sample Preparation
2.2. Testing and Characterization
3. Results and Discussion
3.1. PA Powder Analysis
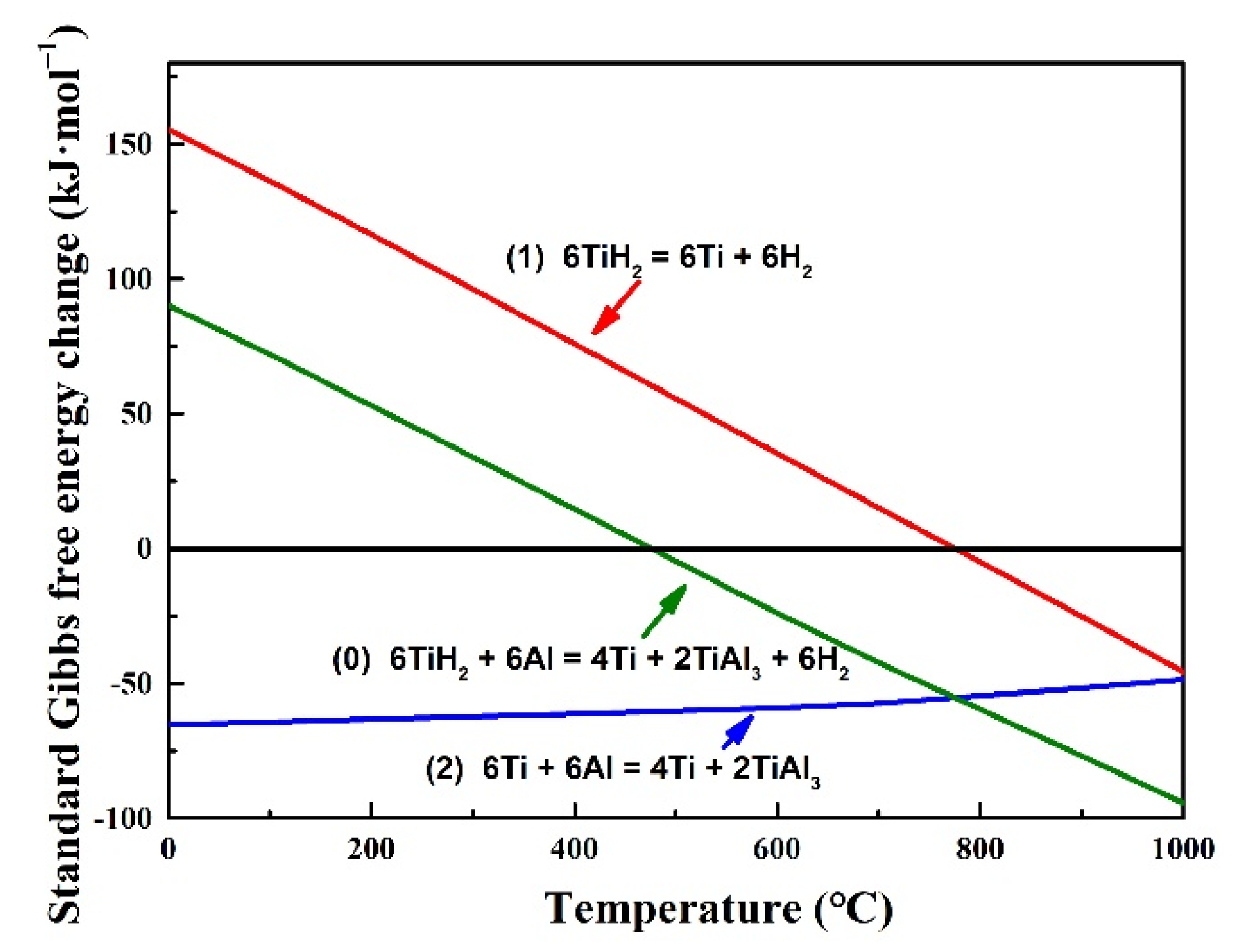
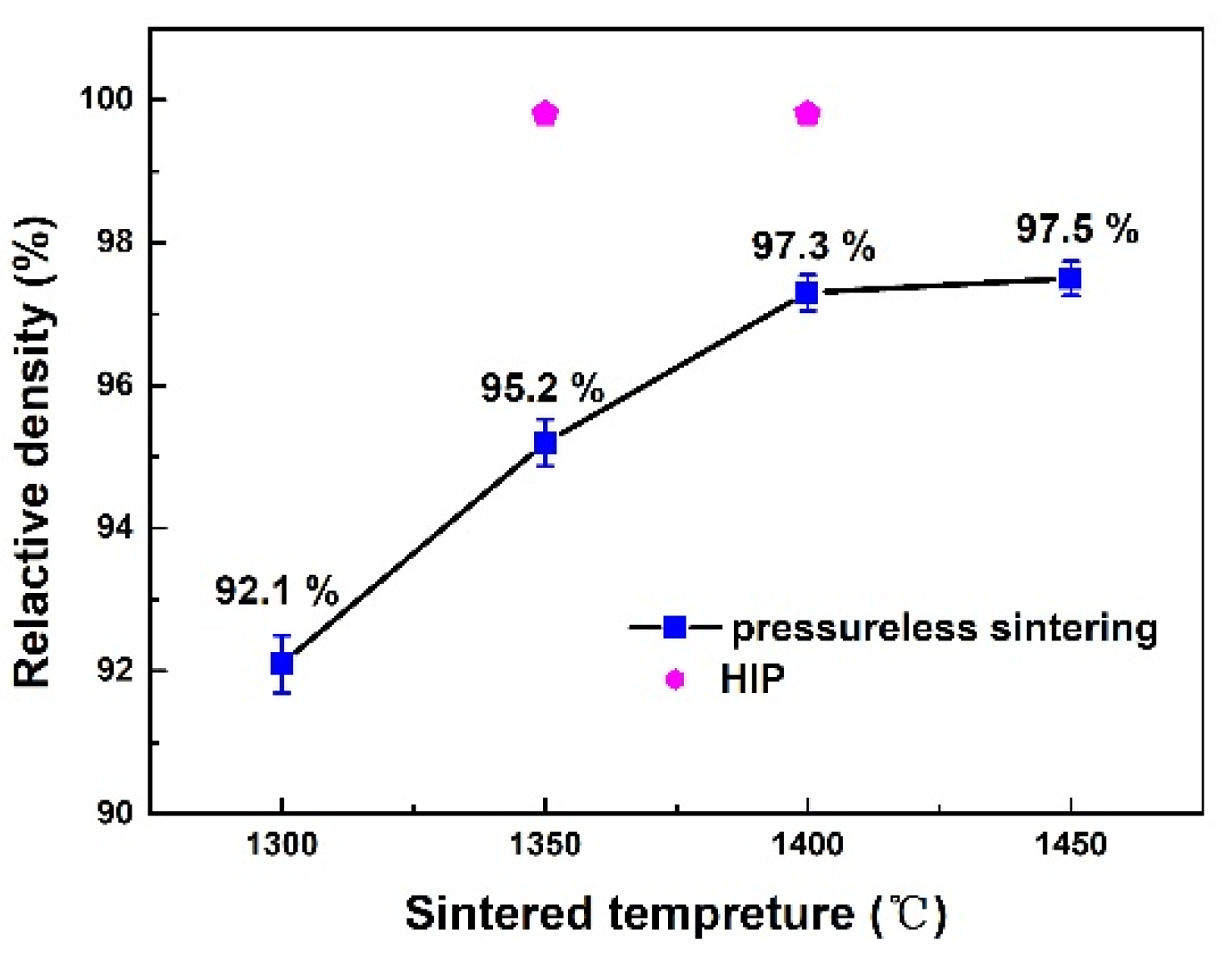
3.2. γ-TiAl Alloy Preparation
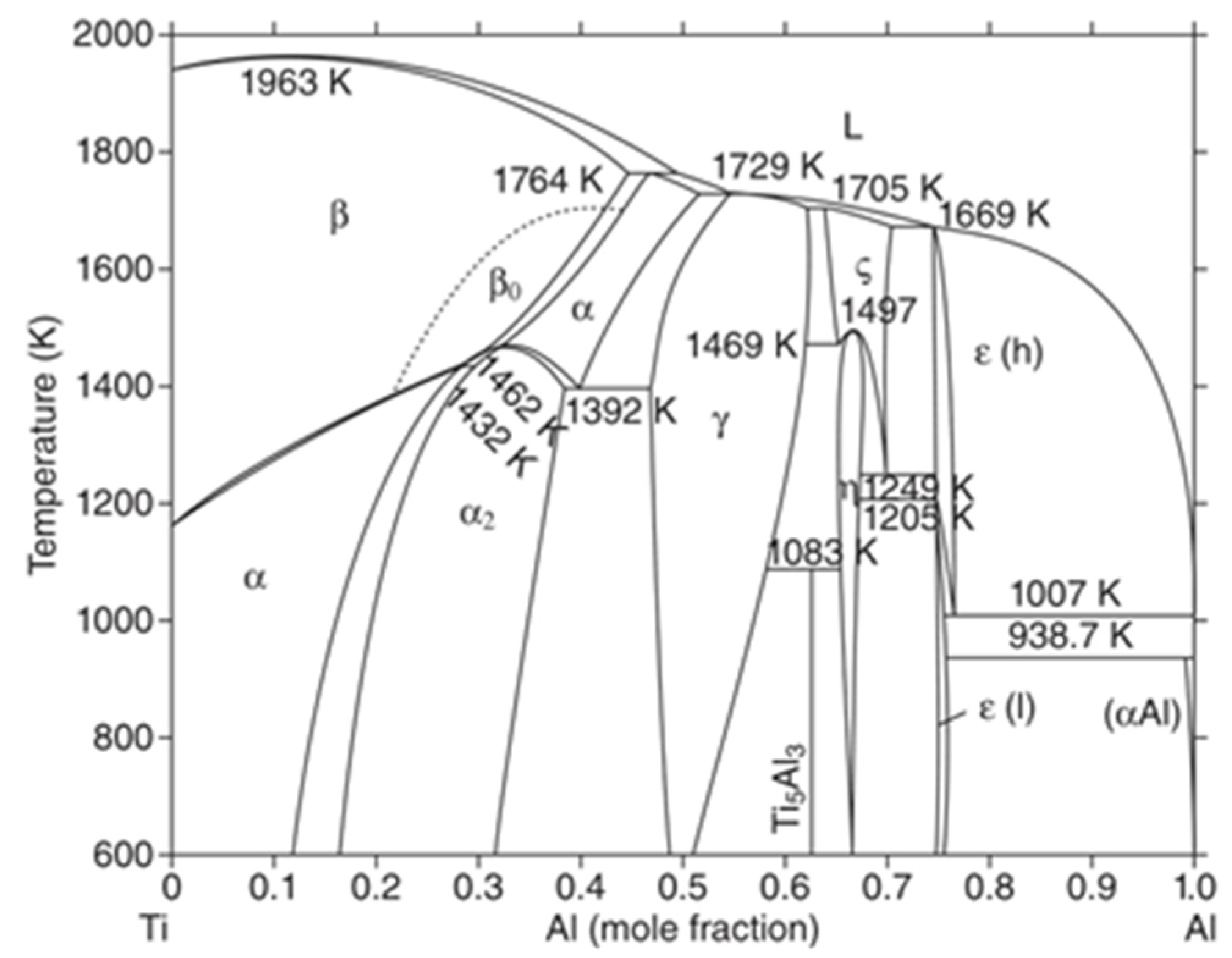
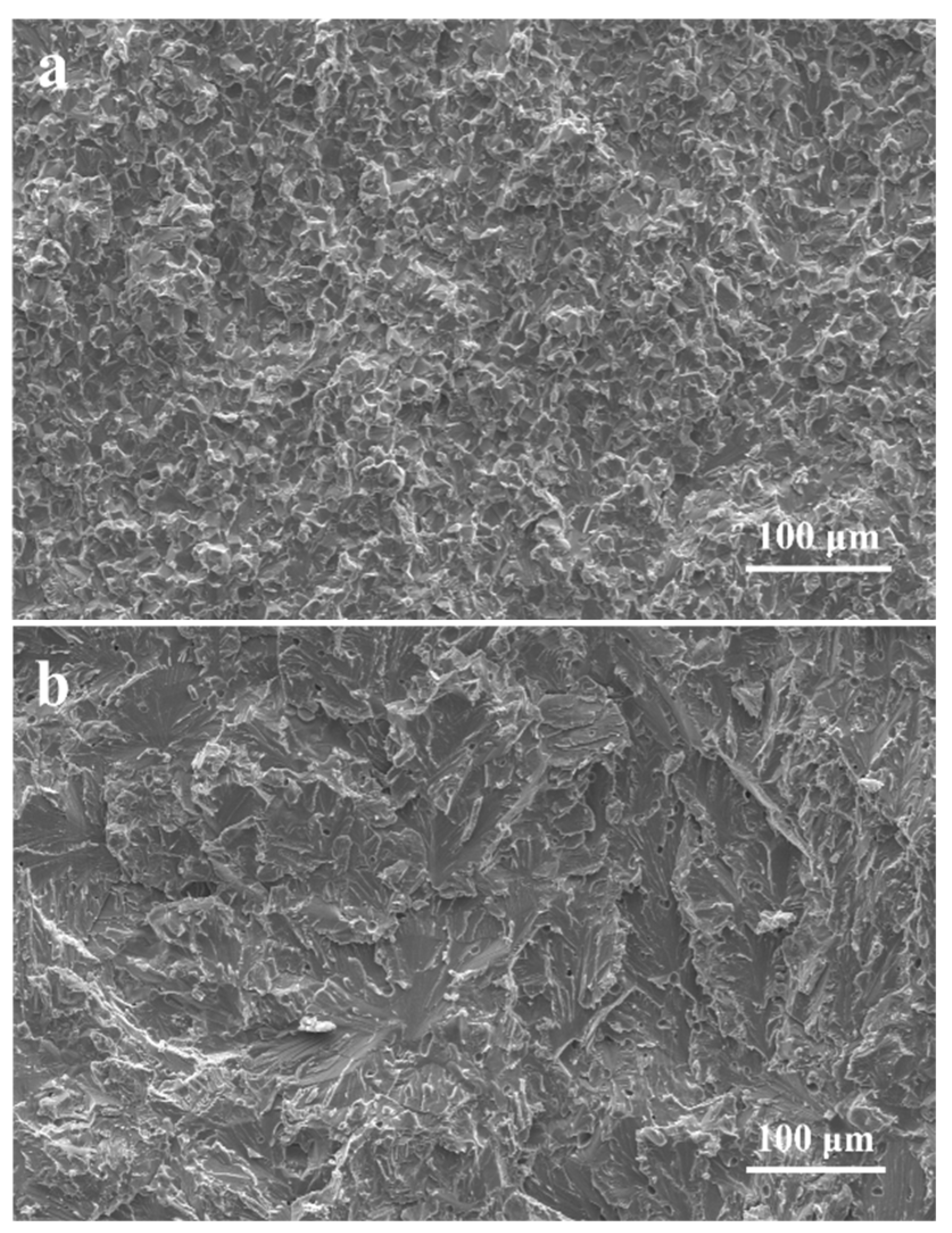
3.3. Phase and Microstructure
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemens, H.; Kestler, H. Processing and applications of intermetallic γ-TiAl-based alloys. Adv. Eng. Mater. 2020, 18, 551–570. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, processing, microstructure, properties, and applications of advanced intermetallic TiAl alloys. Adv. Eng. Mater. 2020, 15, 191–215. [Google Scholar] [CrossRef]
- Noda, T. Application of cast gamma TiAl for automobiles. Intermetallics 1998, 6, 709–713. [Google Scholar] [CrossRef]
- Kim, Y.W.; Dimiduk, D.M. Progress in the understanding of gamma titanium aluminides. Jom 1991, 43, 40–47. [Google Scholar] [CrossRef]
- Beranoagirre, A.; Olvera, D.; De Lacalle, L.N.L. Milling of gamma titanium–aluminum alloys. Int. J. Adv. Manuf. Technol. 2012, 62, 83–88. [Google Scholar] [CrossRef]
- Beranoagirre, A.; De Lacalle, L.N.L. Grinding of gamma TiAl intermetallic alloys. Procedia Eng. 2013, 63, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Bartolotta, P.; Barrett, J.; Kelly, T.; Smashey, R. The use of cast Ti-48Al-2Cr-2Nb in jet engines. Jom 1997, 49, 48–50. [Google Scholar] [CrossRef]
- Hyman, M.E.; McCullough, C.; Levi, C.G.; Mehrabian, R. Evolution of boride morphologies in TiAl-B alloys. Metall. Trans. A 1991, 22, 1647–1662. [Google Scholar] [CrossRef]
- He, S.Z.; He, Y.H.; Huang, B.Y.; Zhang, J.H.; Lu, S.Q.; Tang, J.C. Effect of quenching temperature and tempering time on grain refinement in TiAl base alloy. J. Aeronaut. Mater. 2003, 23, 5–10. [Google Scholar]
- Hu, D. Effect of composition on grain refinement in TiAl-based alloys. Intermetallics 2001, 9, 1037–1043. [Google Scholar] [CrossRef]
- Gerling, R.; Clemens, H.; Schimansky, F.P. Powder metallurgical processing of intermetallic gamma titanium aluminides. Adv. Eng. Mater. 2004, 6, 23–38. [Google Scholar] [CrossRef]
- Daloz, D.; Hecht, U.; Zollinger, J.; Combeau, H.; Hazotte, A.; Založnik, M. Microsegregation, macrosegregation and related phase transformations in TiAl alloys. Intermetallics 2011, 19, 749–756. [Google Scholar] [CrossRef]
- Yang, Y.F.; Luo, S.D.; Schaffer, G.B.; Qian, M. Impurity scavenging, microstructural refinement and mechanical properties of powder metallurgy titanium and titanium alloys by a small addition of cerium silicide. Mater. Sci. Eng., A 2013, 573, 166–174. [Google Scholar] [CrossRef]
- Xia, Y.; Yu, P.; Schaffera, G.B.; Qian, M. Cobalt-doped Ti-48Al-2Cr-2Nb alloy fabricated by cold compaction and pressureless sintering. Mater. Sci. Eng. A 2013, 574, 176–185. [Google Scholar] [CrossRef]
- Alman, D.E. Reactive sintering of TiAl-Ti5Si3 in situ composites. Intermetallics 2005, 13, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Zhang, C.; Yang, F.; Cao, P.; Guo, Z.M.; Lu, B.X.; Chen, C.G.; Liu, P.; Volinsky, A.A. High-density and low-interstitial Ti-23Al-17Nb prepared by vacuum pressureless sintering from blended elemental powders. Vacuum 2019, 164, 62–65. [Google Scholar] [CrossRef]
- Kim, D.J.; Seo, D.Y.; Saari, H.; Sawatzky, T.; Kim, Y.W. Isothermal oxidation behavior of powder metallurgy beta gamma TiAl–2Nb–2Mo alloy. Intermetallics 2011, 19, 1509–1516. [Google Scholar] [CrossRef]
- Yan, M.; Mei, B.C.; Zhu, J.Q.; Tian, C.G.; Wang, P. Synthesis of high-purity bulk Ti2AlN by spark plasma sintering (SPS). Ceram. Int. 2008, 34, 1439–1442. [Google Scholar] [CrossRef]
- Lee, T.K.; Kim, J.H.; Hwang, S.K. Direct consolidation of γ-TiAl-Mn-Mo from elemental powder mixtures and control of porosity through a basic study of powder reactions. Metall. Mater. Trans. A 1997, 28, 2723–2729. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, B.Y.; He, Y.H.; Zhou, K.C. Processing TiAl-based alloy by elemental powder metallurgy. J. Mater. Sci. Technol. 2000, 16, 605–610. [Google Scholar] [CrossRef]
- Yumoto, A.; Yamamoto, T.; Hiroki, F.; Shiota, I.; Niwa, N. Ti-Al, graded Al/AlTi, and Ti-Al-N coatings prepared by supersonic free-jet PVD. Mater. Trans. 2004, 45, 1620–1623. [Google Scholar] [CrossRef] [Green Version]
- Trzaska, Z.; Bonnefont, G.; Fantozzi, G.; Monchoux, J.P. Comparison of densification kinetics of a TiAl powder by spark plasma sintering and hot pressing. Acta Mater. 2017, 135, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Shagiev, M.R.; Senkov, O.N.; Salishchev, G.A.; Froes, F.H. High temperature mechanical properties of a submicrocrystalline Ti-47Al-3Cr alloy produced by mechanical alloying and hot isostatic pressing. J. Alloys Compd. 2000, 313, 201–208. [Google Scholar] [CrossRef]
- Kulkarni, K.N.; Sun, Y.; Sachdev, A.K.; Lavernia, E. Field-activated sintering of blended elemental γ-TiAl powder compacts: Porosity analysis and growth kinetics of Al3Ti. Scr. Mater. 2013, 68, 841–844. [Google Scholar] [CrossRef]
- Liu, H.W.; Bishop, D.P.; Plucknett, K.P. Densification behaviour and microstructural evolution of Ti-48Al consolidated by spark plasma sintering. J. Mater. Sci. 2017, 52, 613–627. [Google Scholar] [CrossRef]
- Smith, P.R.; Rosenberger, A.H.; Shepard, M.J.; Wheeler, R. Review AP/M approach for the fabrication of an orthorhombic titanium aluminide for MMC applications. J. Mater. Sci. 2000, 35, 3169–3179. [Google Scholar] [CrossRef]
- Zollinger, J.; Lapin, J.; Daloz, D.; Combeau, H. Influence of oxygen on solidification behaviour of cast TiAl-based alloys. Intermetallics 2007, 15, 1343–1350. [Google Scholar] [CrossRef]
- Seifert, H.J.; Kussmaul, A.; Aldinger, F. Phase equilibria and diffusion paths in the Ti-Al-O-N system. J. Alloys Compd. 2001, 317, 19–25. [Google Scholar] [CrossRef]
- Epifano, E.; Hug, G. First-principle study of the solubility and diffusion of oxygen and boron in γ-TiAl. Comput. Mater. Sci. 2020, 174, 109475. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, B.X.; Wang, H.Y.; Guo, Z.M.; Paley, V.; Volinsky, A.A. Vacuum Pressureless Sintering of Ti-6Al-4V Alloy with Full Densification and Forged-Like Mechanical Properties. J. Mater. Eng. Perform. 2018, 27, 282–292. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Z.M.; Yang, F.; Wang, H.Y.; Shao, Y.R.; Lu, B.X. In situ formation of low interstitials Ti-TiC composites by gas-solid reaction. J. Alloys Compd. 2018, 769, 37–44. [Google Scholar] [CrossRef]
- Liang, Y.F.; Yang, F.; Zhang, L.Q.; Lin, L.P.; Shang, S.L.; Liu, Z.K. Reaction behavior and pore formation mechanism of TiAl–Nb porous alloys prepared by elemental powder metallurgy. Intermetallics 2014, 44, 1–7. [Google Scholar] [CrossRef]
- Rawers, J.C.; Wrzesinski, W.R. Reaction-sintered hot-pressed TiAl. J. Mater. Sci. 1992, 27, 2877–2886. [Google Scholar] [CrossRef]
- Van Loo, F.J.J.; Rieck, G.D. Diffusion in the titanium-aluminium system—I. Interdiffusion between solid Al and Ti or Ti-Al alloys. Acta Metall. 1973, 21, 61–71. [Google Scholar] [CrossRef]
- Dlouhý, A.; Kuchařová, K. Creep and microstructure of near-gamma TiAl alloys. Intermetallics 2004, 12, 705–711. [Google Scholar] [CrossRef]
- Imayev, V.M.; Imayev, R.M.; Salishchev, G.A.; Shagiev, M.R.; Kuznetsov, A.V.; Povarova, K.B. Effect of strain rate on twinning and room temperature ductility of TiAl with fine equiaxed microstructure. Scr. Mater. 1997, 36, 891–897. [Google Scholar] [CrossRef]
- Camagu, S.T.; Mathabathe, N.M.; Motaung, D.E.; Muller, T.F.G.; Arendse, C.J.; Bolokang, A.S. Investigation into the thermal behaviour of the B2–NiAl intermetallic alloy produced by compaction and sintering of the elemental Ni and Al powders. Vacuum 2019, 169, 108919. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, M.H.; Liang, Y.F.; Ren, Y.; Dong, C.L.; Lin, J.P. Enhanced high-temperature tensile property by gradient twin structure of duplex high-Nb-containing TiAl alloy. Acta Mater. 2018, 161, 1–11. [Google Scholar] [CrossRef]
- Shu, S.L.; Qiu, F.; Jin, S.B.; Lu, J.B.; Jiang, Q.C. Compression properties and work-hardening behavior of Ti2AlC/TiAl composites fabricated by combustion synthesis and hot press consolidation in the Ti–Al–Nb–C system. Mater. Des. 2011, 32, 5061–5065. [Google Scholar] [CrossRef]
- Maloy, S.A.; Gray, G.T., III. High strain rate deformation of Ti48Al2Nb2Cr. Acta Mater. 1996, 44, 1741–1756. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, S.D.; Wu, X.; Schaffer, G.B.; Qian, M. The sintering densification, microstructure and mechanical properties of gamma Ti-48Al-2Cr-2Nb alloy with a small addition of copper. Mater. Sci. Eng., A 2013, 559, 293–300. [Google Scholar] [CrossRef]






| Powder | TiH2 | Al | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| D50 (μm) | 12 | 5.6 | 23.7 | 120.4 |
| Particle Size of Al Powder (μm) | Ti (wt%) | Al (wt%) | O (wt%) | N (wt%) | H (wt%) |
|---|---|---|---|---|---|
| D50 = 5.6 | Bal. | 33.9 | 0.505 | 0.028 | 0.005 |
| D50 = 23 | Bal. | 34.5 | 0.324 | 0.022 | 0.006 |
| D50 = 120.4 | Bal. | 34.1 | 0.171 | 0.019 | 0.005 |
| Sample | Point | Al (at.%) | Ti (at.%) |
|---|---|---|---|
| T1300 | 1 | 37.85 | 62.15 |
| 2 | 48.57 | 51.43 | |
| T1350 | 3 | 37.09 | 62.91 |
| 4 | 48.54 | 51.46 | |
| T1400 | 5 | 37.61 | 62.39 |
| 6 | 36.87 | 63.13 | |
| 7 | 48.60 | 51.40 | |
| T1450 | 8 | 39.74 | 60.26 |
| 9 | 39.50 | 60.50 | |
| 10 | 48.79 | 51.21 |
| Alloy (at%) | Processing | Compressive strength (MPa) | Reference |
|---|---|---|---|
| Ti-48Al | Sintering + HIP(H1350) | 2241 ± 52 | This work |
| Ti-48Al | Sintering + HIP(H1400) | 1931 ± 17 | This work |
| Ti-50Al | Hot pressing | 1300 | [34] |
| Ti-50Al-10wt%(Nb-C) | Hot pressing | 1649 | [34] |
| Ti-48Al-2Nb-2Cr | Cast | 950 | [35] |
| Ti-48Al-2Nb-2Cr-2Cu | sintering | 1700 | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.; Yang, F.; Lu, B.; Chen, C.; Sui, Y.; Guo, Z. Microstructure and Mechanical Properties of High Relative Density γ-TiAl Alloy Using Irregular Pre-Alloyed Powder. Metals 2021, 11, 635. https://doi.org/10.3390/met11040635
Yan M, Yang F, Lu B, Chen C, Sui Y, Guo Z. Microstructure and Mechanical Properties of High Relative Density γ-TiAl Alloy Using Irregular Pre-Alloyed Powder. Metals. 2021; 11(4):635. https://doi.org/10.3390/met11040635
Chicago/Turabian StyleYan, Mengjie, Fang Yang, Boxin Lu, Cunguang Chen, Yanli Sui, and Zhimeng Guo. 2021. "Microstructure and Mechanical Properties of High Relative Density γ-TiAl Alloy Using Irregular Pre-Alloyed Powder" Metals 11, no. 4: 635. https://doi.org/10.3390/met11040635







