Abstract
Hydrogen gas pressure is an important test parameter when considering materials for high-pressure hydrogen applications. A large set of data on the effect of hydrogen gas pressure on mechanical properties in gaseous hydrogen experiments was reviewed. The data were analyzed by converting pressures into fugacities (f) and by fitting the data using an power law. For 95% of the data sets, was smaller than 0.37, which was discussed in the context of (i) rate-limiting steps in the hydrogen reaction chain and (ii) statistical aspects. This analysis might contribute to defining the appropriate test fugacities (pressures) to qualify materials for gaseous hydrogen applications.
1. Introduction
Mobility based on hydrogen-powered vehicles such as fuel cell vehicles is a promising way to reduce greenhouse emissions. For high market penetration, the prognosis is that the costs of a hydrogen infrastructure are significantly lower compared to a battery-charging infrastructure [1]. However, there are special issues associated with the realization of a hydrogen infrastructure. One issue is the susceptibility of most structural materials, especially steels, to hydrogen embrittlement. Despite this issue, the overall goal is to realize safe and affordable infrastructure and mobility.
For automotives as well as for many infrastructure applications, the design must be safe, affordable and lightweight. The qualification of materials for use in hydrogen applications is specified in relevant standards. It is generally accepted that a material’s qualification for gaseous hydrogen applications should be performed under conditions reflecting the final use of the material, i.e., in gaseous hydrogen atmospheres. Material testing in gaseous hydrogen atmospheres requires very specialized equipment [2,3] as well as strong safety protocols. Altogether, this results in high technological efforts, reflected by the costs being 15 to 150 times more expensive compared to tests in ambient air, depending on the test method (tensile test, crack growth test, etc.) [4]. Generating and maintaining a high hydrogen gas pressure throughout the test duration remains a technical challenge, especially at sub-ambient temperatures. The primary motivation of this study was to review the experimental data investigating the influence of external gas pressure on the degree of hydrogen effects in materials. The data were analyzed to quantify the effect of high hydrogen gas pressures of up to 100 MPa on the mechanical properties of steels and to propose a rationale for the definition of test pressures for high-pressure hydrogen applications.
2. Experimental and Analytical Details
This study reviews the experimental results of the influence of external high-pressure hydrogen on the various mechanical properties of steels. This study only reviews the results obtained in gaseous hydrogen environments at room temperature, with a focus on hydrogen gas pressures of up to at least 70 MPa (Table 1). Such experiments were typically performed with apparatuses similar to those described in [2,3]. Studies investigating the influence of hydrogen gas pressure at temperatures other than room temperature were not found in the existing literature. Only results obtained by the hydrogen pre-charging of steels (gaseous or electrochemical) simulating external high hydrogen gas pressures were used to interpret the results.

Table 1.
Steel designations, similar grades for other regions and mechanical properties of the steels reviewed in this study. Normal letters refer to data explicitly given in the respective reference. (*) refers to data which were estimated from graphs or heat treatment conditions given in the respective references. F = ferrite, P = pearlite, TM = tempered martensite, B = bainite, AF = acicular ferrite. Nf = number of cycles to failure in Wöhler-type fatigue life tests, K = fracture toughness, da/dN = crack growth rate, na = not annotated.
Since the effect of hydrogen on the mechanical properties of steels typically increases with increasing hydrogen gas pressure (p), resulting in increased hydrogen concentration (c) [5,6,7], the experimental data must be fitted using an equation reflecting the rate-limiting steps in environmental hydrogen experiments. Such rate-limiting steps are [8]:
- Transport of hydrogen to the crack tip, proportional to p;
- Physical adsorption, proportional to p;
- Dissociative chemical adsorption, proportional to p0.5;
- Hydrogen absorption, Sievert’s law, proportional to p0.5;
- Transport of hydrogen to regions of tensile stress, not dependent on p;
- Hydrogen–material interactions, not dependent on p.
At high pressures (and low temperatures), the behavior of a gas deviates from that of an ideal gas. The appropriate thermodynamic parameter is fugacity (f), which is correlated to the pressure according to
with b = constant, R = universal gas constant, and T = temperature [9]. The correlation between pressure and fugacity is shown in Figure 1a. At room temperature, f ≈ p for pressures lower than 1 MPa, which is known as Sievert’s law. For higher pressures, the non-ideal gas behavior must be considered especially in the power laws of rate limiting steps 3 and 4. Using Sievert’s law as an example, p0.5 translates to f0.5 [9].
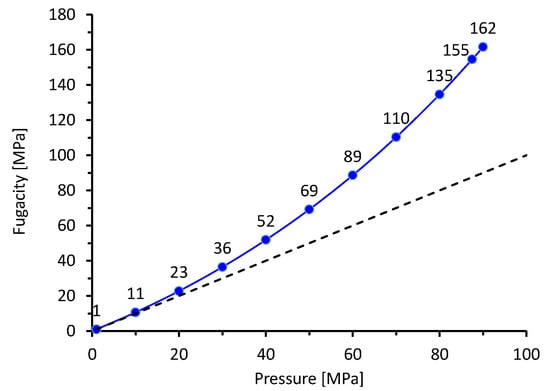
Figure 1.
Correlation between pressure and fugacity at room temperature calculated by Equation (1).
In the following, the data were analyzed in terms of fugacity instead of pressure. Based on this analysis, the experimental data were fitted using the following equation:
where HEI = any hydrogen embrittlement index, m = the factor and n = the exponent. Typical hydrogen embrittlement indices use the ratio of the mechanical property measured in H2 and in air, in percent. In this review, the relative fracture toughness (KH2/Kair), relative crack growth rate (da/dNH2/da/dNair), relative reduction of area (RRA = RAH2/RAair) of the tensile specimens and the relative number of cycles to the failure (Nf_H2/Nf_air) of fatigue life tests were calculated based on the published experimental data. In most references, the mechanical property in the control atmosphere (in most cases, air) was not measured as a function of pressure. In other words, the value measured in ambient air was used to calculate the embrittlement index for all hydrogen fugacities. Using such indices, the degree of embrittlement increases as the index decreases, except for the crack growth tests, because crack growth is accelerated in hydrogen compared to air. The experimental data were fitted using the method of least squares. The most important experimental details of the results reviewed here are summarized in Table 1, including the fit exponent (n) from Equation (2), as well as the coefficient of determination (R2). Further details can be found in the respective references.
3. Results
Figure 2 shows the quasi-static properties, i.e., the relative reduction of area (RRA) of the tensile specimens for heat-treatable steels (Figure 2a,b), carbon steels (Figure 2c) and austenitic stainless steels (Figure 2d,e), as well as the relative fracture toughness (relative K) of heat-treatable and low alloyed steels (Figure 2f,g) as a function of hydrogen fugacity. Microstructures, tensile properties in air, fit parameters according to Equation (2), as well as the coefficient of determination (R2), are summarized in Table 1. All experimental data were fitted using Equation (2) with a reasonably high R2 value greater than approximately 0.6. For most of the data, the R2 value was greater than 0.8. The analysis of the absolute value of the fit exponent reveals a range of between approximately 0.05 and 0.3.
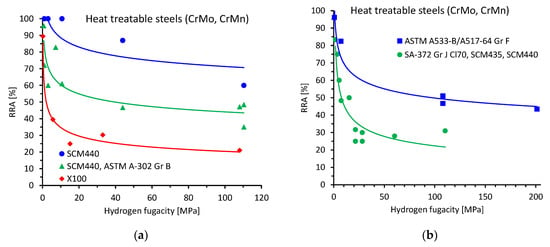
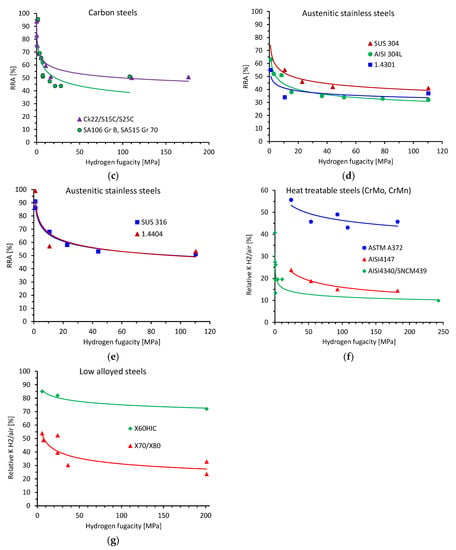
Figure 2.
Results of the quasi-static tensile and fracture toughness tests as a function of hydrogen fugacity. Relative reduction of area (RRA) in the tensile tests for (a,b) heat-treatable steels, (c) carbon steels, (d,e) austenitic stainless steels. Relative fracture toughness of (f) heat-treatable steels, (g) low alloyed pipeline steels. References, test details and fit parameters are given in Table 1.
Figure 3 summarizes the cyclic properties as a function of hydrogen fugacity, i.e., the relative crack growth rates (relative da/dN) for carbon steels (Figure 3a), heat-treatable steels (Figure 3b) and pure iron (Figure 3c), as well as the relative number of cycles to the failure of heat-treatable steels (Figure 3d). It should be noted here that the trend for the X80 pipeline steel was extrapolated (Figure 3d). Extrapolation of trends is generally associated with a high risk. However, the relative Nf at a fugacity of 8 MPa was already at a very low value of 0.1 and a significant further reduction in Nf with increasing fugacity was not expected. This allowed a meaningful extrapolation of the trend for this specific data set. Cyclic data systematically studying the influence of pressure for austenitic stainless steels were not found in the existing literature, but results in [10] suggest a negligible influence between 10 and 103 MPa. All data in Figure 3 were fitted using Equation (2) with a reasonably high R2 value greater than 0.55. For most of the data, the R2 value was greater than 0.9. The two different results for carbon steel SM490B were remarkable and even surprising (Figure 3a). Although the reported mechanical properties of the steels were very similar and the test conditions were identical (Table 1), significantly different crack growth rates were reported. A reason for this discrepancy could not be identified based on the available information in the respective references. Most relative da/dN values were calculated at ΔK ≈ 25 MPa m0.5. For steel HY-100, the data were acquired at ΔK = 55 MPa m0.5 (Figure 3b). Quasi-static fracture toughness values between 30 and 80 MPa m0.5 measured in high-pressure hydrogen were reported in [11] for steels with similar tensile properties. In other words, the cyclic stress intensity range of ΔK = 55 MPa m0.5 might be close to the fracture toughness of HY-100 and the increasing relative da/dN values for fugacities higher than 100 MPa might be explained by approaching the transition region to unstable crack growth at such high fugacities. For the cyclic data sets, the analysis of the absolute value of the fit exponent revealed a range of between approximately 0.08 and 0.4. (Table 1). Based on this analysis, the values for cyclic tests and quasi-static tests are in the same range (Table 1).
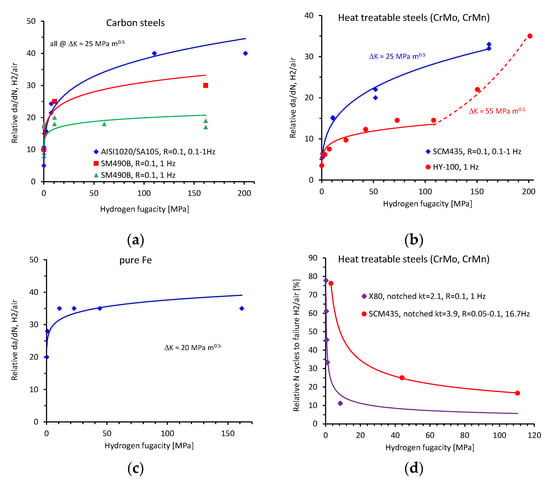
Figure 3.
Results of the cyclic crack growth tests (da/dN) and fatigue life tests measuring the number of cycles to failure (Nf) as a function of hydrogen test pressure. Relative da/dN for (a) carbon steels, (b) heat-treatable steels and (c) pure iron. (d) Relative number of cycles to the failure of heat-treatable steels. References, test details and fit parameters are given in Table 1.
4. Discussion
As mentioned previously, the experimental data were fitted using Equation (2). For = 0.5, all rate limiting steps (Figure 4) would be undistorted. However, the analysis performed in this study shows that the influence of hydrogen fugacity upon the mechanical properties of steels does not follow a law for a wide variety of microstructures, strength levels, and test methods (Table 1).
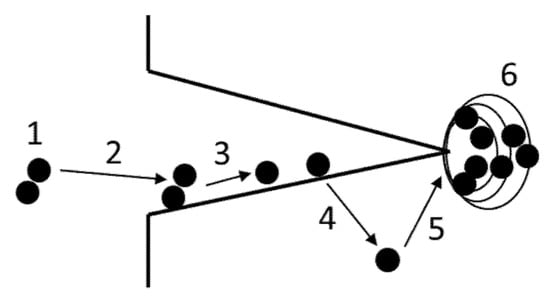
Figure 4.
Rate-limiting steps for the transport of hydrogen to the fracture propagation zone. 1 = transport of hydrogen to the crack tip, 2 = physical adsorption, 3 = dissociative chemical adsorption, 4 = hydrogen absorption, 5 = transport of hydrogen to regions of tensile stress, 6 = hydrogen–material interactions.
The first part of the discussion focuses on the possible rate-limiting steps in the hydrogen reaction chain (Figure 4). It is known that for metals loaded in a hydrogen gas environment, hydrogen-assisted crack initiation typically starts at the gas–metal interface. Upon further loading, this crack propagates into the material until a final cross-section fails by overload fracture. In other words, special attention must be paid to the possible rate-limiting steps at the gas–metal interface.
As outlined in [8], the collision rate of gas molecules with a metal surface is linearly proportional to the gas pressure, and a sticking coefficient of approximately 1 can be assumed for pure metal surfaces. Under such conditions, around one monolayer of H2 adsorbs within one second at a pressure of 10−10 MPa hydrogen partial pressure. With this estimation, it appears very unlikely that Steps 1 and 2 (the transport of hydrogen to the crack tip and physical adsorption) are rate-controlling under the test conditions discussed in this study with fugacities up to approximately 250 MPa.
The role of dissociative chemical adsorption was discussed in detail in [49]. It was shown that on Fe and Ni single-crystal surfaces, the coverage of dissociative sites is 100% at hydrogen gas pressures in the order of 10−1 MPa. Whether this is also true for highly distorted poly-crystals along a crack surface has not been investigated so far [8]. However, when considering fugacities up to approximately 250 MPa, it appears unlikely that dissociative chemical adsorption is rate-limiting under the test conditions discussed in this study.
The role of hydrogen absorption can be assessed by comparing test results obtained in gaseous hydrogen atmospheres with those of hydrogen pre-charged specimens. Hydrogen pre-charging of materials is typically performed under charging conditions to ensure a homogeneously saturated microstructure, and it appears that the fugacity-modified Sievert’s law accurately predicts the hydrogen concentration in austenitic stainless steels pre-charged at high fugacities (Figure 5a). This method eliminates Steps 1 to 4 from the reaction chain (Figure 4). However, the analysis of data obtained with hydrogen pre-charged materials (Figure 5b) reveals values in the same range as obtained with tests in gaseous hydrogen atmosphere being significantly lower than 0.5. Since this trend does not change after eliminating Step 4 from the reaction chain, it is unlikely that hydrogen absorption is a primary rate-limiting step when testing in gaseous hydrogen atmospheres.

Figure 5.
(a) Fugacity-modified Sievert’s law with data from [50,51], (b) RRA of 304 L austenitic stainless steel [5] and relative fracture stress (RFS) of AISI 4135 martensitic high strength steel [7] pre-charged with hydrogen as a function of hydrogen concentration.
Although not fugacity-dependent rather than microstructure-dependent, the transport of hydrogen to regions of tensile stress, i.e., the crack tip, can also be rate-limiting. Hydrogen transport over distances larger than a few grain diameters is controlled by bulk Fickian diffusion, characterized by the diffusivity D. The diffusivities at room temperature are in the order of 10−15 to 10−16 m2/s for face cubic centered (fcc) austenitic stainless steels [9] and 10−8 m2/s to 10−11 m2/s for body cubic centered (bcc) steels depending on the microstructure [52,53]. Although the diffusivities of bcc steels and austenitic steels differ by several orders of magnitude, a significant difference in their mechanical response as a function of fugacity could not be identified (Figure 2). In other words, if D were a primary rate-controlling parameter (at temperatures around room temperature), the fucacity dependence of the HEI data of bcc and fcc austenitic steels should be clearly different, which is not the case. Therefore, it appears unlikely that hydrogen bulk diffusivity is a primary rate-limiting parameter when testing in a gaseous hydrogen atmosphere.
If Steps 1 to 5 of the hydrogen reaction chain are not rate-limiting, then the mechanisms involved in the hydrogen–material interactions (Step 6) must control the hydrogen embrittlement effects. The length scale is in the order of the plastic zone in front of a flaw or crack tip, typically around a few 10 to a few 100 µm. Although the size of this zone comprises several grain diameters, the mechanisms are dominated by the so-called strain rate factor of the hydrogen transport equation, which is not a function of D [54,55,56]. There is experimental evidence that hydrogen influences dislocation motion and it can fairly be assumed that this effect increases with increasing local hydrogen concentration around the dislocation core. In this case, the interaction of hydrogen with dislocations plays a predominant role [8,57,58]. Unfortunately, currently, such mechanisms are not understood at the level of detail to quantify such effects. However, modern simulation tools may provide a framework to study such effects, since a model based on hydrogen accumulation and transport around a micro-crack using embedded atom methods and density functional theory calculations showed promising results [59].
The second part of the discussion focuses on the selection of material test fugacities (pressures) for high-pressure hydrogen applications. Figure 6a shows a histogram of the fit exponents () from Table 1, which suggests an asymmetric distribution. A statistical analysis revealed that these experimental data were well represented by a Weibull distribution. This appears reasonable because Weibull distributions are often found in statistics where values are bound to natural limits—here, . The corresponding Weibull curves are shown in Figure 6b with an expected value of approximately 0.12 and 95% of the values are lower than 0.37.
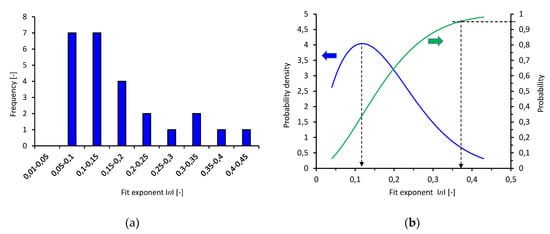
Figure 6.
(a) Histogram of the fit exponents () from Table 1, (b) corresponding Weibull curves, scale parameter 1.727, shape parameter 0.195, coefficient of determination R2 = 0.895.
The following discussion focuses on the two characteristic values, i.e., = 0.12 and = 0.37. To discuss the effect of these results on the definition of hydrogen test fugacities (pressures), two engineering applications were selected as use cases. The typical test pressure for automotive hydrogen tanks is 87.5 MPa (f1 = 155 MPa) and the typical operating pressure of gas pipelines is 10 MPa (f2 = 11 MPa). These nominal fugacities were used to calculate severity factors as
using = 0.12 and = 0.37 (Figure 7). SF indicates how much less severe a test at f is compared to the reference fugacities f1 and f2, respectively. For example, for f1 = 155 MPa and = 0.12, the severity factor of SF = 0.94 indicates that a fugacity of approximately 93 MPa is less severe by a factor of 0.94 compared to a fugacity of 155 MPa (Figure 7).
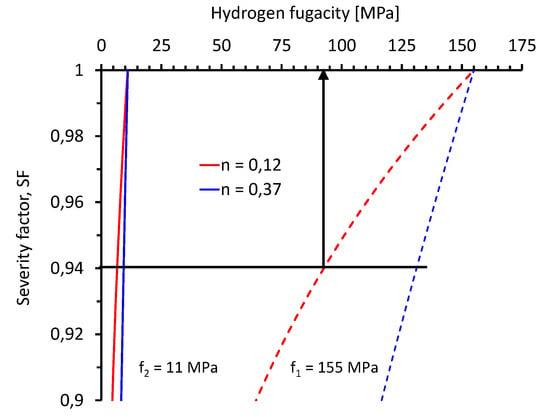
Figure 7.
Severity factor (SF) quantifying the effect of hydrogen fugacity on hydrogen embrittlement indices assuming an fn law with f1 = 155 MPa and f2 = 11 MPa as use cases. For example, for f1 = 155 MPa and = 0.12, the severity factor of SF = 0.94 indicates that a fugacity of approximately 93 MPa is less severe by a factor of 0.94 compared to a fugacity of 155 MPa.
The severity factor of the fugacity can then be compared with the inherent variation in the measured mechanical property. Repeatability and reproducibility studies on high-pressure gaseous hydrogen’s effects on the tensile properties of various structural materials [60,61] revealed that when only considering the scatter of quasi-static tensile test results in a control atmosphere, the RRA value must be lower than 0.89 to 0.99 (89% to 99%), depending on the material, in order to account for this reduction in hydrogen effects with a 95% confidence level (Table 2). The average value of 0.94 is represented by the solid horizontal line in Figure 7. Using again f1 = 155 MPa and = 0.12 as an example, the severity factor of SF = 0.94 indicates that a fugacity of approximately 93 MPa is less severe by a factor of 0.94 compared to a fugacity of 155 MPa. However, the measured hydrogen effects lie within the scatter of the results in the control atmosphere and, thus, are not statistically relevant with a 95% confidence level. In other words, for materials following an f0.12 law, any hydrogen effects measured between 93 and 155 MPa are not statistically relevant with a 95% confidence level. The lower bound fugacities for f2 = 11 MPa and = 0.37 are given in Table 3. This analysis may provide a data-based rationale for defining appropriate test fugacities (pressures) for high-pressure hydrogen applications.

Table 2.
Results of repeatability and reproducibility studies of tensile tests in ambient control atmospheres (helium, nitrogen or air). RR = round robin.

Table 3.
Calculated lower bound fugacities (pressures) for SF = 0.94.
5. Summary
The findings of this review can be summarized as follows:
- The relative reduction in mechanical properties in gaseous hydrogen experiments follows an power law (f = fugacity).
- The exponent was found to be smaller than 0.4. Since theoretical assumptions predict n = 0.5 for a fully undistorted hydrogen reaction chain, the reviewed data are interpreted in a way that hydrogen–dislocation interactions are the rate-limiting step controlling hydrogen effects in steels.
- A statistical analysis of the reviewed data revealed that 95% of the values are lower than 0.37.
- This analysis might be used to define appropriate test fugacities (pressures) to qualify materials for high-pressure gaseous hydrogen applications.
Author Contributions
Conceptualization, T.M.; data curation, T.M.; writing—original draft preparation, T.M.; writing—review and editing, F.S. and K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful for their discussion with Christopher San Marchi from Sandia National Laboratories, Livermore, USA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robinius, M.; Linßen, J.; Grube, T.; Reuß, M.; Stenzel, P.; Syranidis, K.; Kuckertz, P.; Stolten, D. Comparative Analysis of Infrastructures: Hydrogen Fueling and Electric Charging of Vehicles. In Schriften des Forschungszentrums Jülich Reihe Energie & Umwelt/Energy & Environment Band/Volume 408; Forschungszentrum Jülich: Jülich, Germany, 2018; p. 127. ISBN 978-3-95806-295-5. [Google Scholar]
- Deimel, P.; Hanisch, C. Tests on the steels 15 MnNi6 3 and X56TM in high pressure hydrogen gas of high purity. Int. J. Hydrogen Energy 1989, 14, 147–151. [Google Scholar] [CrossRef]
- Fukuyama, S.; Imade, M.; Yokogawa, K. Hydrogen and Evaluation of Hydrogen Gas Embrittlement of Metals. In Proceedings of the ASME 2008 Pressure Vessel and Piping Conference (PVP 2008), Chicago, IL, USA, 27–31 July 2008; ASME Press: San Antonio, TX, USA, 2008. [Google Scholar]
- Vesely, E.J.; Bhat, B.N.; McPherson, W.B.; Grethlein, C.E. Reproducibility and repeatability of tensile and low cycle fatigue properties in propulsion grade hydrogen. In Proceedings of the 5th International Conference on the Effect of Hydrogen on the Behavior of Materials, Moran, WY, USA, 11–14 September 1994; Moody, N.R., Thompson, A.W., Ricker, R.E., Was, G.W., Jones, R.H., Eds.; The Minerals, Metals & Materials Society: Moran, WY, USA, 1994; pp. 363–374. [Google Scholar]
- Buckley, J.R.; Hardie, D. The effect of pre-straining and d-ferrite on the embrittlement of 304L stainless steel by hydrogen. Corros. Sci. 1993, 34, 93–107. [Google Scholar] [CrossRef]
- Omura, T.; Kobayashi, K.; Miyahara, M.; Kudo, T. Effect of surface hydrogen contents on hydrogen embrittlement properties of stainless steels. Corros. Eng. 2006, 55, 747–759. [Google Scholar]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen on the fracture behavior of high strength steel during slow strain rate test. Corros. Sci. 2007, 49, 4081–4097. [Google Scholar] [CrossRef]
- Borchers, C.; Michler, T.; Pundt, A. Effect of hydrogen on the mechanical properties of stainless steels. Adv. Eng. Mater. 2008, 10, 11–23. [Google Scholar] [CrossRef]
- Marchi, C.S.; Somerday, B.P.; Robinson, S.L. Permeability, solubility and diffusivity of hydrogen isotopes in stainless steels at high gas pressures. Int. J. Hydrogen Energy 2007, 32, 100–116. [Google Scholar] [CrossRef]
- San Marchi, C.; Gibbs, P.; Foulk, J.; Nibur, K. Fatigue life of austenitic stainless steels in hydrogen environments. In Proceedings of the 43rd MPA Seminar, Stuttgart, Germany, 11–12 October 2017. [Google Scholar]
- Loginow, A.W.; Phelbs, E.H. Steels for seamless hydrogen pressure vessels. Corros. NACE 1975, 31, 404–412. [Google Scholar] [CrossRef]
- Hofmann, W.; Rauls, W. Ductility of steel under the influence of external high pressure hydrogen. Weld. Res. Suppl. 1965, 5, 225–230. [Google Scholar]
- Zhang, L.; Wen, M.; Fukuyama, S.; Yokogawa, K. Effect of Temperature on Hydrogen Environment Embrittlement of Carbon Steels at Low Temperatures. J. Jpn. Inst. Met. 2003, 67, 460–463. [Google Scholar] [CrossRef]
- Fukuyama, S.; Imade, M.; Lin, Z.; Yokogawa, K. Hydrogen embrittlement of metals in 70 MPA hydrogen at room temperature. Am. Soc. Mech. Eng. Press. Vessel. Pip. Div. PVP 2005, 6, 493–497. [Google Scholar]
- New Energy and Industrial Technology Development Organization (NEDO). 2011 Oil Refining Industry Security Measures Project. R&D on Material Usage Standards Associated with Hydrogen Energy Utilization; National Institute of Advanced Industrial Science and Technology (AIST): Tsukuba, Japan, 2012. [Google Scholar]
- Ogawa, Y.; Hino, M.; Nakamura, M.; Matsunaga, H. Pearlite-driven surface-cracking and associated loss of tensile ductility in plain-carbon steels under exposure to high-pressure gaseous hydrogen. Int. J. Hydrogen Energy 2020, 46, 6945–6959. [Google Scholar] [CrossRef]
- Xu, K.; Rana, M. Tensile and Fracture Properties of Carbon and Low Alloy Steels in High Pressure Hydrogen. In Proceedings of the 2008 International Hydrogen Conference—Effects of Hydrogen on Materials, Jackson Lake Lodge, WY, USA, 7–10 September 2008; pp. 349–356. [Google Scholar]
- Walter, R.J.; Chandler, W.T. Effects of high-pressure hydrogen on metals at ambient temperature. In NASA-R-7780-1; National Aeronautics and Space Administration: Huntsville, AL, USA, 1969; p. 328. [Google Scholar]
- New Energy and Industrial Technology Development Organization (NEDO). Development for Safe Utilization and Infrastructure of Hydrogen. Common Base Technology Development Relating Hydrogen. The Study of Basic Properties of Materials for Hydrogen; The Japan Research and Development Center for Metals: Tokyo, Japan, 2005. [Google Scholar]
- Imade, M.; Fukuyama, S.; Zhang, L.; Wen, M.; Yokogawa, K. Hydrogen environment embrittlement of SCM440 steel in high pressure hydrogen at room temperature. J. Jpn. Inst. Met. 2005, 69, 190–193. [Google Scholar] [CrossRef]
- Walter, R.J.; Chandler, W.T. Influence of hydrogen pressure and notch severity on hydrogen-environment embrittlement at ambient temperatures. Mater. Sci. Eng. 1971, 8, 90–97. [Google Scholar] [CrossRef]
- Jewett, R.P.; Walter, R.J.; Chandler, W.T.; Frohmberg, R.P. Hydrogen Environment Embrittlement of Metals. In NASA-CR-2163; National Aeronautics and Space Administration: Washington, DC, USA, 1973; p. 238. [Google Scholar]
- Walter, R.J.; Chandler, W.T. Influence of Gaseous Hydrogen on Metals—Final Report. In NASA-CR-124410; National Aeronautics and Space Administration: Huntsville, AL, USA, 1973; p. 170. [Google Scholar]
- Walter, R.J.; Chandler, W.T. Effects of high-pressure hydrogen on storage vessel materials. In NASA-R-6851; National Aeronautics and Space Administration: Huntsville, AL, USA, 1967; p. 180. [Google Scholar]
- New Energy and Industrial Technology Development Organization (NEDO). 2010 Oil Refining Industry Security Measures Project. R&D on Material Usage Standards Associated with Hydrogen Energy Utilization; National Institute of Advanced Industrial Science and Technology (AIST): Tsukuba, Japan, 2011. [Google Scholar]
- Han, G.; He, S.; Fukuyama, S.; Yokogawa, K. Effect of Nickel Equivalent on Hydrogen Environment Embrittlement of Austenitc Stainless Steels at Low Temperatures. Acta Mater. 1998, 46, 4570–4599. [Google Scholar] [CrossRef]
- Fukuyama, S.; Zhang, L.; Yokogawa, K. Development of materials testing equipment in high pressure hydrogen and hydrogen environment embrittlement of austenitic stainless steels. J. Jpn. Inst. Met. 2004, 68, 62–65. [Google Scholar] [CrossRef]
- Caskey, G.R. Hydrogen Compatibility Handbook for Stainless Steels. In Report DP-1643, E.I. du Pont de Nemours & Co; Savannah River National Laboratory: Aiken, SC, USA, 1983; p. 160. [Google Scholar]
- Michler, T.; Yukhimchuk, A.A.; Naumann, J. Hydrogen environment embrittlement testing at low temperatures and high pressures. Corros. Sci. 2008, 50, 3519–3526. [Google Scholar] [CrossRef]
- Clark, W.G. Effect of temperature and pressure on hydrogen cracking in high strength type 4340 steel. J. Mater. Energy Syst. 1979, 1, 33–40. [Google Scholar] [CrossRef]
- Fukuyama, S.; Yokogawa, K.; Araki, M. Fatigue Crack Growth of SNCM439 Steel in High Pressure Hydrogen at Room Temperature. J. Soc. Mater. Sci. Jpn. 1985, 34, 709–714. [Google Scholar] [CrossRef]
- Matsuoka, S.; Matsunaga, H.; Yamabe, J.; Hamada, S.; Iijima, T. Various strength properties of SCM435 and SNCM439 low-alloy steels in 115 MPa hydrogen gas and proposal of design guideline. Trans. JSME 2017, 83, 1–20. [Google Scholar]
- An, T.; Peng, H.; Bai, P.; Zheng, S.; Wen, X.; Zhang, L. Influence of hydrogen pressure on fatigue properties of X80 pipeline steel. Int. J. Hydrogen Energy 2017, 42, 15669–15678. [Google Scholar] [CrossRef]
- Miyamoto, T.; Matsuo, T.; Kobayashi, N.; Mukaie, Y.; Matsuoka, S. Characteristics of fatigue life and fatigue crack growth of SCM435 steel in high-pressure hydrogen gas. Trans. Jpn. Soc. Mech. Eng. A 2012, 78, 531–546. [Google Scholar] [CrossRef]
- Nelson, H.G. Hydrogen-induced slow crack growth of a plain carbon pipeline steel under conditions of cyclic loading. In Proceedings of the 2nd International Conference on Effect of Hydrogen on the Behaviour of Materials, Moran, WY, USA, 7 September 1975; Thompson, A.W., Bernstein, I.M., Eds.; The Metallurcical Society of AIME: New York, NY, USA, 1976; pp. 602–611. [Google Scholar]
- Walter, R.J.; Chandler, W.T. Cyclic-load growth in ASME SA-105 grade 2 steel in high-pressure hydrogen at ambient temperature. In Proceedings of the Effect of Hydrogen on Behavior of Materials, Moran, WY, USA, 7 September 1975; Thompson, A.W., Bernstein, I.M., Eds.; AIME: Moran, WY, USA, 1975; pp. 273–287. [Google Scholar]
- Matsuoka, S.; Takakuwa, O.; Okazaki, S.; Yoshikawa, M.; Yamabe, J.; Matsunaga, H. Peculiar temperature dependence of hydrogen-enhanced fatigue crack growth of low-carbon steel in gaseous hydrogen. Scr. Mater. 2018, 154, 101–105. [Google Scholar] [CrossRef]
- Yamabe, J.; Yoshikawa, M.; Matsunaga, H.; Matsuoka, S. Effects of hydrogen pressure, test frequency and test temperature on fatigue crack growth properties of low-carbon steel in gaseous hydrogen. Procedia Struct. Integr. 2016, 2, 525–532. [Google Scholar] [CrossRef]
- Yamabe, J.; Matsunaga, H.; Furuya, Y.; Hamada, S.; Itoga, H.; Yoshikawa, M.; Takeuchi, E.; Matsuoka, S. Qualification of chromium-molybdenum steel based on the safety factor multiplier method in CHMC1-2014. Int. J. Hydrogen Energy 2015, 40, 719–728. [Google Scholar] [CrossRef]
- Ogawa, Y.; Birenis, D.; Matsunaga, H.; Thøgersen, A.; Prytz, Ø.; Takakuwa, O.; Yamabe, J. Multi-scale observation of hydrogen-induced, localized plastic deformation in fatigue-crack propagation in a pure iron. Scr. Mater. 2017, 140, 13–17. [Google Scholar] [CrossRef]
- Ogawa, Y.; Birenis, D.; Matsunaga, H.; Takakuwa, O.; Yamabe, J.; Prytz, Ø.; Thøgersen, A. The role of intergranular fracture on hydrogen-assisted fatigue crack propagation in pure iron at a low stress intensity range. Mater. Sci. Eng. A 2018, 733, 316–328. [Google Scholar] [CrossRef]
- Birenis, D.; Ogawa, Y.; Matsunaga, H.; Takakuwa, O.; Yamabe, J.; Prytz, Ø.; Thøgersen, A. Interpretation of hydrogen-assisted fatigue crack propagation in BCC iron based on dislocation structure evolution around the crack wake. Acta Mater. 2018, 156, 245–253. [Google Scholar] [CrossRef]
- Ogawa, Y.; Umakoshi, K.; Nakamura, M.; Takakuwa, O. Hydrogen-assisted, intergranular, fatigue crack-growth in ferritic iron: Influences of hydrogen-gas pressure and temperature variation. Int. J. Fatigue 2020, 140, 105806. [Google Scholar] [CrossRef]
- Nanninga, N.E.; Levy, Y.S.; Drexler, E.S.; Condon, R.T.; Stevenson, A.E.; Slifka, A.J. Comparison of hydrogen embrittlement in three pipeline steels in high pressure gaseous hydrogen environments. Corros. Sci. 2012, 59, 1–9. [Google Scholar] [CrossRef]
- San Marchi, C.; Somerday, B.P.; Nibur, K.A.; Stalheim, D.G.; Boggess, T.; Jansto, S. Fracture and fatigue of commercial grade api pipeline steels in gaseous hydrogen. In Proceedings of the ASME 2010 Pressure Vessel and Piping Conference (PVP 2010), Bellevue, WA, USA, 18–22 July 2010; pp. 2010–25825. [Google Scholar]
- San Marchi, C.; Somerday, B.P.; Nibur, K.A.; Stahlheim, D.G.; Boggess, T.; Jansto, S. Fracture Resistance and Fatigue Crack Growth of X80 Pipeline. In Proceedings of the ASME 2011 Pressure Vessel and Piping Conference (PVP 2011), Baltimore, MA, USA, 17–21 July 2011; ASME Press: Baltimore, MA, USA, 2011. [Google Scholar]
- Cialone, H.J.; Holbrook, J.H. Sensitivity of steels to degradation in gaseous hydrogen. In ASTM STP 962; Raymond, E.L., Ed.; ASTM International: Philadelphia, PA, USA, 1988; pp. 134–152. [Google Scholar]
- Briottet, L.; Moro, I.; Lemoine, P. Quantifying the hydrogen embrittlement of pipeline steels for safety considerations. Int. J. Hydrogen Energy 2012, 37, 17616–17623. [Google Scholar] [CrossRef]
- Vehoff, H. Hydrogen related material problems. In Hydrogen in Metals III. Topics in Applied Physics, Vol 73; Wipf, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 215–278. [Google Scholar]
- San Marchi, C.; Michler, T.; Nibur, K.A.; Somerday, B.P. On the physical differences between tensile testing of type 304 and 316 austenitic stainless steels with internal hydrogen and in external hydrogen. Int. J. Hydrogen Energy 2010, 35, 9736–9745. [Google Scholar] [CrossRef]
- San Marchi, C.; Somerday, B.P.; Tang, X.; Schiroky, G.H. Effects of alloy composition and strain hardening on tensile fracture of hydrogen-precharged type 316 stainless steels. Int. J. Hydrogen Energy 2008, 33, 889–904. [Google Scholar] [CrossRef]
- San Marchi, C.; Somerday, B.P. Effects of High-Pressure Gaseous Hydrogen on Structural Metals. SAE Technical Paper 2007-01-0433; SAE: Warrendale, PA, USA, 2007. [Google Scholar]
- Hurtado Noreña, C.; Bruzzoni, P. Effect of microstructure on hydrogen diffusion and trapping in a modified 9%Cr-1%Mo steel. Mater. Sci. Eng. A 2010, 527, 410–416. [Google Scholar] [CrossRef]
- Díaz, A.; Cuesta, I.I.; Rodríguez, C.; Alegre, J.M. Influence of non-homogeneous microstructure on hydrogen diffusion and trapping simulations near a crack tip in a welded joint. Theor. Appl. Fract. Mech. 2021, 112, 102879. [Google Scholar] [CrossRef]
- Krom, A.H.M.; Koers, R.W.J.; Bakker, A.D. Hydrogen transport near a blunting crack tip. J. Mech. Phys. Solids 1999, 47, 971–992. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Dou, D.; Shen, L.; Gong, J. FE analysis of hydrogen diffusion around a crack tip in an austenitic stainless steel. Int. J. Hydrogen Energy 2016, 41, 6053–6063. [Google Scholar] [CrossRef]
- Wu, X.Q.; Kim, I.S. Effects of strain rate and temperature on tensile behavior of hydrogen-charged SA508 Cl.3 pressure vessel steel. Mater. Sci. Eng. A 2003, 348, 309–318. [Google Scholar] [CrossRef]
- Dadfarnia, M.; Martin, M.L.; Nagao, A.; Sofronis, P.; Robertson, I.M. Modeling hydrogen transport by dislocations. J. Mech. Phys. Solids 2014, 78, 511–525. [Google Scholar] [CrossRef]
- Xing, X.; Cheng, R.; Cui, G.; Liu, J.; Gou, J.; Yang, C.; Li, Z.; Yang, F. Quantification of the temperature threshold of hydrogen embrittlement in X90 pipeline steel. Mater. Sci. Eng. A 2021, 800, 140118. [Google Scholar] [CrossRef]
- Vesely, E.J.; Jacobs, R.K.; Watwood, M.C.; McPherson, W.B. Influence of strain rate on tensile properties in high-pressure hydrogen. In Hydrogen Effects in Materials; Thompson, A.W., Moody, N.R., Eds.; The Minerals, Metals & Materials Society: Moran, WY, USA, 1996; pp. 363–374. [Google Scholar]
- Current status of round-robin tests for hydrogen material compatibility. In Proceedings of the 6th Meeting of the Informal Working Group on GTR No.13 (Phase 2), Tianjin, China, 18–20 June 2019.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).