Mechanical Alloying of Elemental Powders into Nanocrystalline (NC) Fe-Cr Alloys: Remarkable Oxidation Resistance of NC Alloys
Abstract
1. Introduction
2. Mechanical Alloying for Nanocrystallinity
3. Mechanical Alloying of Fe-Cr Alloy Powders and Their Consolidation
4. Oxidation Resistance of Mechanically Alloyed NC Fe-Cr Alloys
5. Mechanical Alloying of Fe-Cr-Ni-Zr Alloy Powders and Their Consolidation
6. Oxidation Resistance of Mechanically Alloyed NC Fe-Cr-Ni-Zr Alloys
7. Opportunities
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, K.; Van Swygenhoven, H.; Suresh, S. Mechanical behavior of nanocrystalline metals and alloys. Acta Mater. 2003, 51, 5743–5774. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Froes, F. The structure and mechanical properties of metallic nanocrystals. Metall. Trans. A 1992, 23, 1071–1081. [Google Scholar] [CrossRef]
- Mahesh, B.V.; Raman, R.K.S. Role of Nanostructure in Electrochemical Corrosion and High Temperature Oxidation: A Review. Metall. Mater. Trans. A 2014, 45, 5799–5822. [Google Scholar] [CrossRef]
- Koch, C.C.; Youssef, K.M.; Scattergood, R.O.; Murty, K.L. Breakthroughs in Optimization of Mechanical Properties of Nanostructured Metals and Alloys. Adv. Eng. Mater. 2005, 7, 787–794. [Google Scholar] [CrossRef]
- Rofagha, R.; Langer, R.; El-Sherik, A.M.; Erb, U.; Palumbo, G.; Aust, K.T. The corrosion behaviour of nanocrystalline nickel. Scripta Metall. Mater. 1991, 25, 2867. [Google Scholar] [CrossRef]
- Rofagha, R.; Erb, U.; Ostrander, D.; Palumbo, G.; Aust, K.T. The effects of grain size and phosphorus on the corrosion of nanocrystalline Ni-P alloys. Nanostruct. Mater. 1993, 2, 1–10. [Google Scholar]
- Karimpoor, A.A.; Erb, U.; Aust, K.T.; Palumbo, G. High strength nanocrystalline cobalt with high tensile ductility. Scripta Mater. 2003, 49, 651–656. [Google Scholar] [CrossRef]
- Cheng, S.; Ma, E.; Wang, Y.M.; Kecskes, L.J.; Youssef, K.M.; Koch, C.C.; Trociewitz, U.P.; Han, K. Tensile properties of in situ consolidated nanocrystalline Cu. Acta Mater. 2005, 53, 1521. [Google Scholar] [CrossRef]
- Gleiter, H. Nanocrystalline materials. Prog. Mater. Sci. 1989, 33, 223. [Google Scholar]
- Trapp, S.; Limbach, C.T.; Gonser, U.; Campbell, S.J.; Gleiter, H. Enhanced Compressibility and Pressure-Induced Structural Changes of Nanocrystalline Iron: In Situ Mössbauer Spectroscopy. Phys. Rev. Lett. 1995, 75, 3760. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.A.; Roy, R. Diphasic xerogels: I. Ceramic-metal composites. Mater. Res. Bull. 1984, 19, 169–177. [Google Scholar] [CrossRef]
- Seo, J.H.; Kim, J.K.; Yim, T.H.; Park, Y.B. Textures and Grain Growth in Nanocrystalline Fe-Ni Alloys. Mater. Sci. Forum 2005, 475–479, 3483–3488. [Google Scholar] [CrossRef]
- Hibbard, G.D.; Aust, K.T.; Erb, U. Thermal stability of electrodeposited nanocrystalline Ni–Co alloys. Mater. Sci. Eng. A 2006, 433, 195–202. [Google Scholar] [CrossRef]
- Groza, J.R. Nanocrystalline Powder Consolidation Methods. In Nanostructured Materials: Processing, Properties, and Applications; Koch, C.C., Ed.; William Andrew Pub.: Norwich, NY, USA, 2007. [Google Scholar]
- Rawers, F.; Biancaniello, R.; Jiggets, R.; Fields, R.; Williams, M. Warm-hip compaction of attrition-milled iron alloy powders. Scr. Mater. 1999, 40, 277. [Google Scholar] [CrossRef]
- Youssef, K.M.; Scattergood, R.O.; Murty, K.L.; Koch, C.C. Ultratough nanocrystalline copper with a narrow grain size distribution. Appl. Phys. Lett. 2004, 85, 929. [Google Scholar] [CrossRef]
- Elkedim, O.; Cao, H.S.; Guay, D. Preparation and corrosion behavior of nanocrystalline iron gradient materials produced by powder processing. J. Mater. Process. Technol. 2002, 121, 383. [Google Scholar] [CrossRef]
- Guruswamy, S.; Loveless, M.R.; Srisukhumbowornchai, N.; McCarter, M.K.; Teter, J.P. Processing of Terfenol-D alloy based magnetostrictive composites by dynamic compaction. IEEE Trans. 2000, 36, 3219–3222. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Gupta, R.K. Oxidation Resistance of Nanocrystalline vis-à-vis Microcrystalline Fe-Cr Alloys. Corros. Sci. 2009, 51, 316–321. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 1974. [Google Scholar]
- Gupta, R.K. Synthesis and Corrosion Behavior of Nanocrystalline Fe-Cr Alloys. Ph.D. Thesis, Monash University, Melbourne, Australia, 6 May 2010. [Google Scholar]
- Siegel, R.W. Mechanical properties of nanophase materials. Mater. Sci. Forum 1997, 235–238, 851. [Google Scholar]
- Malow, T.A.; Koch, C.C. Grain growth in nanocrystalline iron prepared by mechanical attrition. Acta Mater. 1997, 45, 2177. [Google Scholar] [CrossRef]
- Gupta, R.K.; Raman, R.K.S.; Koch, C.C. Grain Growth Behaviour and Consolidation of Ball Milled Nanocrystalline Fe-10Cr Alloy. Mater. Sci. Eng. A 2008, 494, 253–256. [Google Scholar] [CrossRef]
- Moelle, C.H.; Fecht, H.J. Thermal stability of nanocrystalline iron prepared by mechanical attrition. NanoStructured Mater. 1995, 6, 421. [Google Scholar] [CrossRef]
- Bonetti, E.; del Bianco, L.; Pasquini, L.; Sampaolesi, E. Thermal evolution of ball milled nanocrystalline iron. NanoStructured Mater. 1999, 12, 685. [Google Scholar] [CrossRef]
- Natter, H.; Schmelzer, M.; Loeffler, M.S.; Hempelmann, R. In-situ X-ray crystallite growth study on nanocrystalline Fe. J. Metastable Nanocryst. Mater. 2000, 8, 683–688. [Google Scholar] [CrossRef]
- Perez, R.J.; Jiang, H.G.; Lavernia, E.J. Grain size stability of nanocrystalline cryomilled Fe-3wt.% Al alloy. NanoStructured Mater. 1997, 9, 71. [Google Scholar] [CrossRef]
- Kofstad, P. High Temperature Corrosion; Elsevier Applied Science and Publishers Ltd.: New York, NY, USA, 1988. [Google Scholar]
- Wagner, C. Oxidation of alloys involving noble metals. J. Electrochem. Soc. 1952, 99, 103. [Google Scholar] [CrossRef]
- Wang, Z.B.; Tao, N.R.; Tong, W.P.; Lu, J.; Lu, K. Diffusion of chromium in nanocrystalline iron produced by means of surface mechanical attrition treatment. Acta Mater. 2003, 51, 4319. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Gupta, R.K.; Koch, C.C. Synthesis Challenges and Extraordinary Resistance to Environmental Degradation of Nanocrystalline vis-à-vis Microcrystalline Fe-Cr Alloys. Philos. Mag. 2010, 90, 3233–3260. [Google Scholar] [CrossRef]
- Mahesh, B.V.; Raman, R.K.S.; Koch, C.C. Bimodal grain size distribution: An effective approach for improving the mechanical and corrosion properties of Fe-Cr-Ni alloys. J. Mater. Sci. 2012, 47, 7735–7743. [Google Scholar] [CrossRef]
- Kumar, R.; Bakshi, S.R.; Joardar, J.; Parida, S.; Raja, V.S.; Raman, R.K.S. Structural Evolution During Milling, Annealing and Rapid Consolidation of Nanocrystalline Fe-10Cr-3Al Powder. Materials 2017, 10, 272. [Google Scholar] [CrossRef] [PubMed]
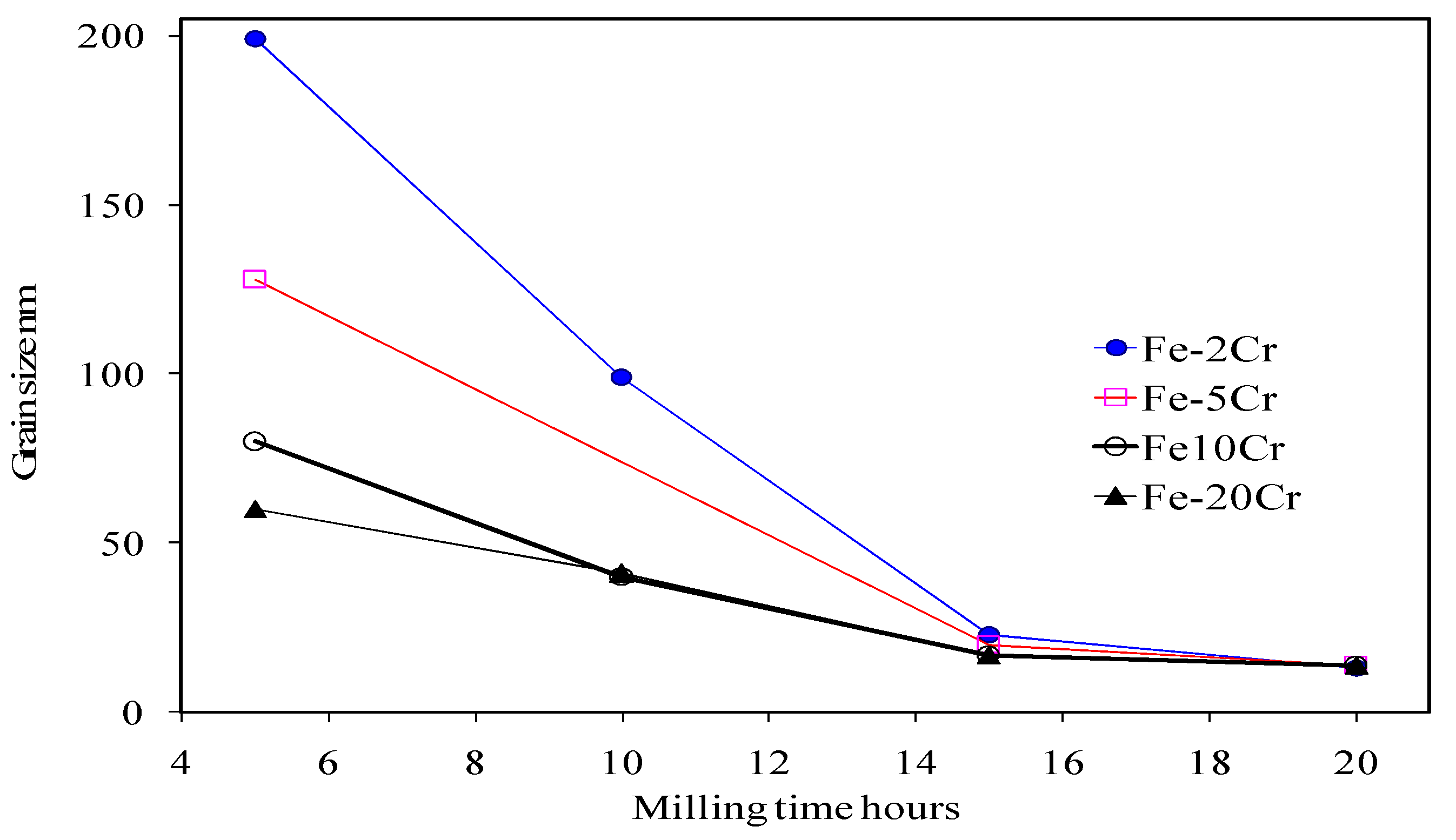
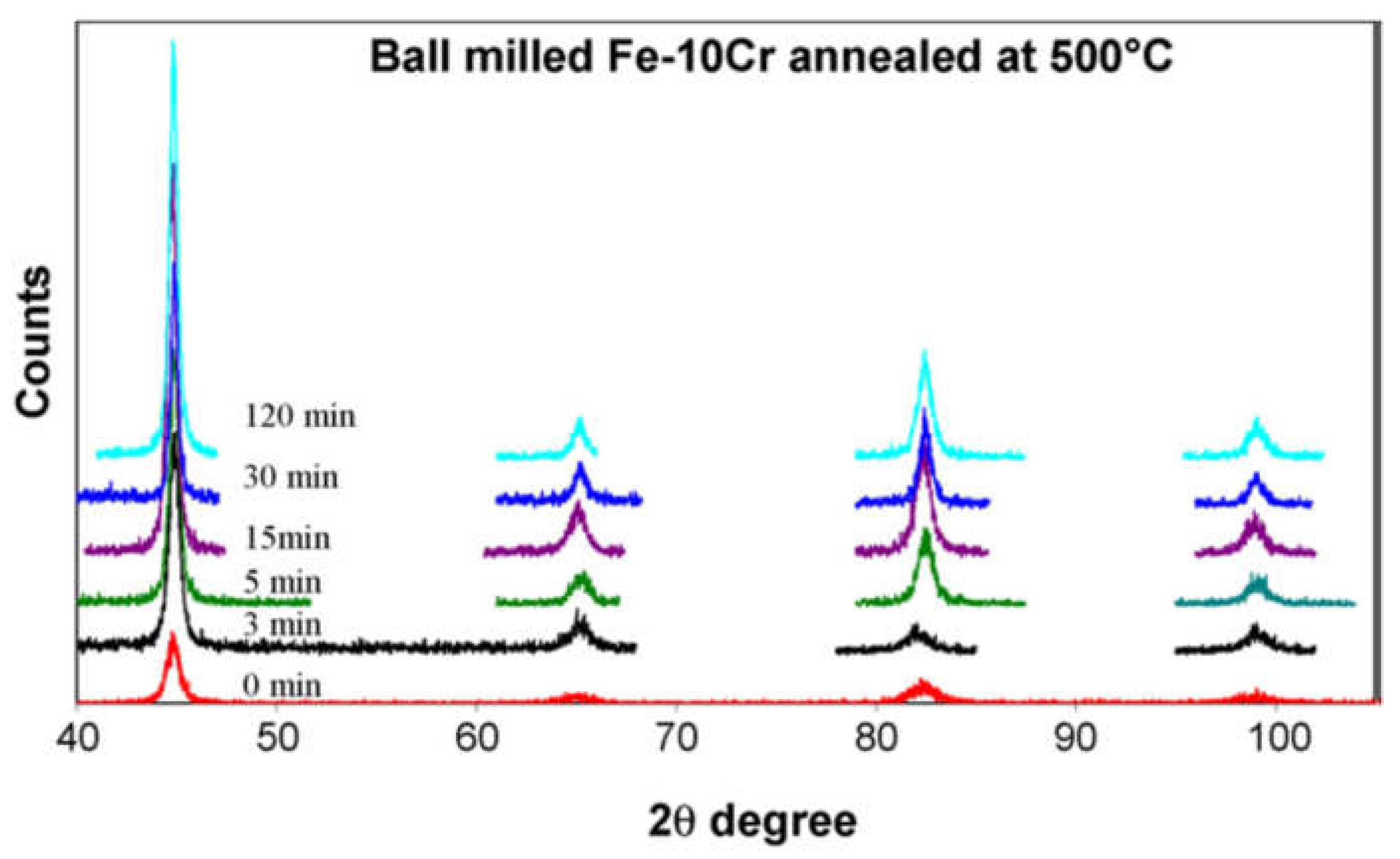

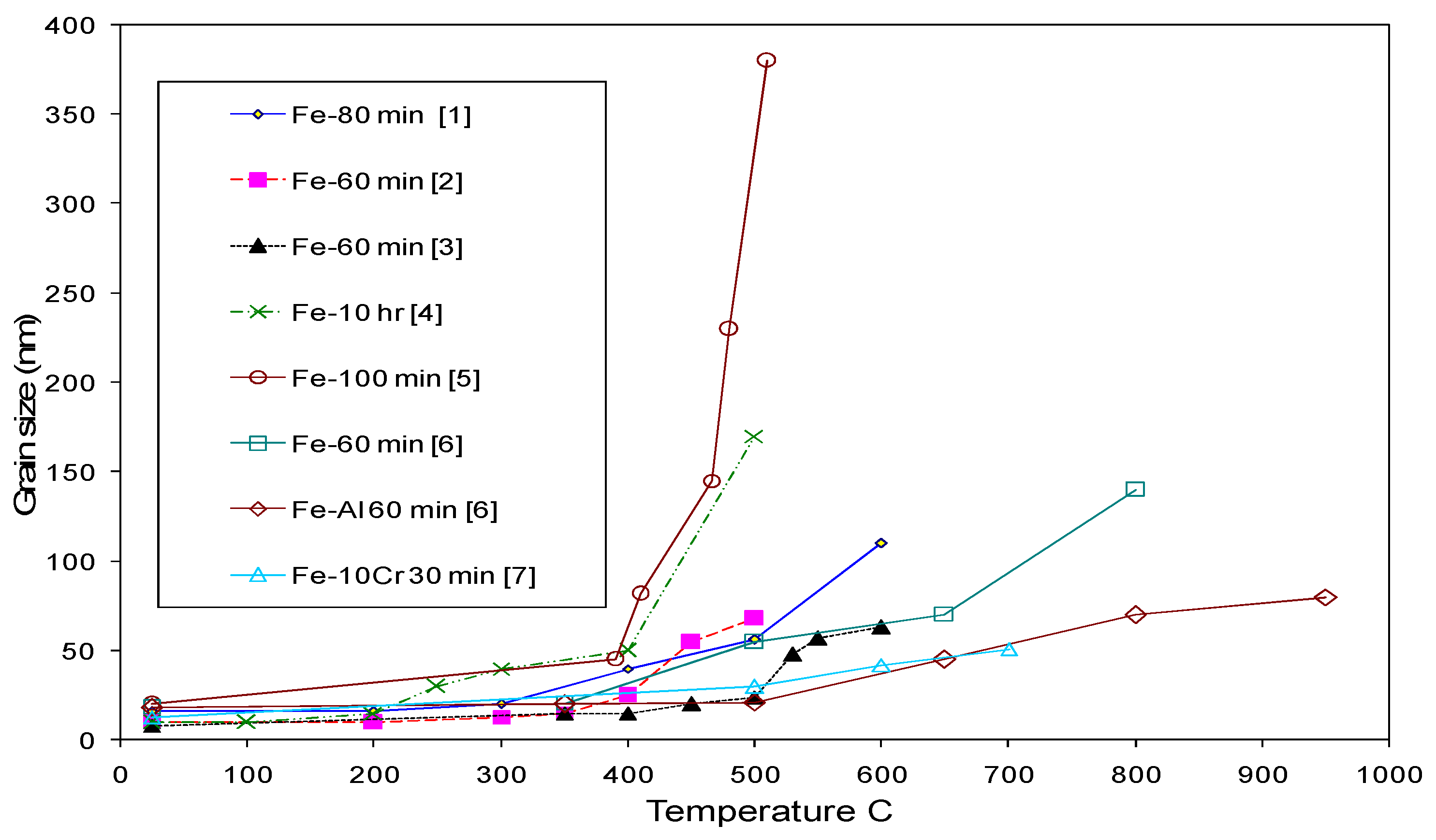
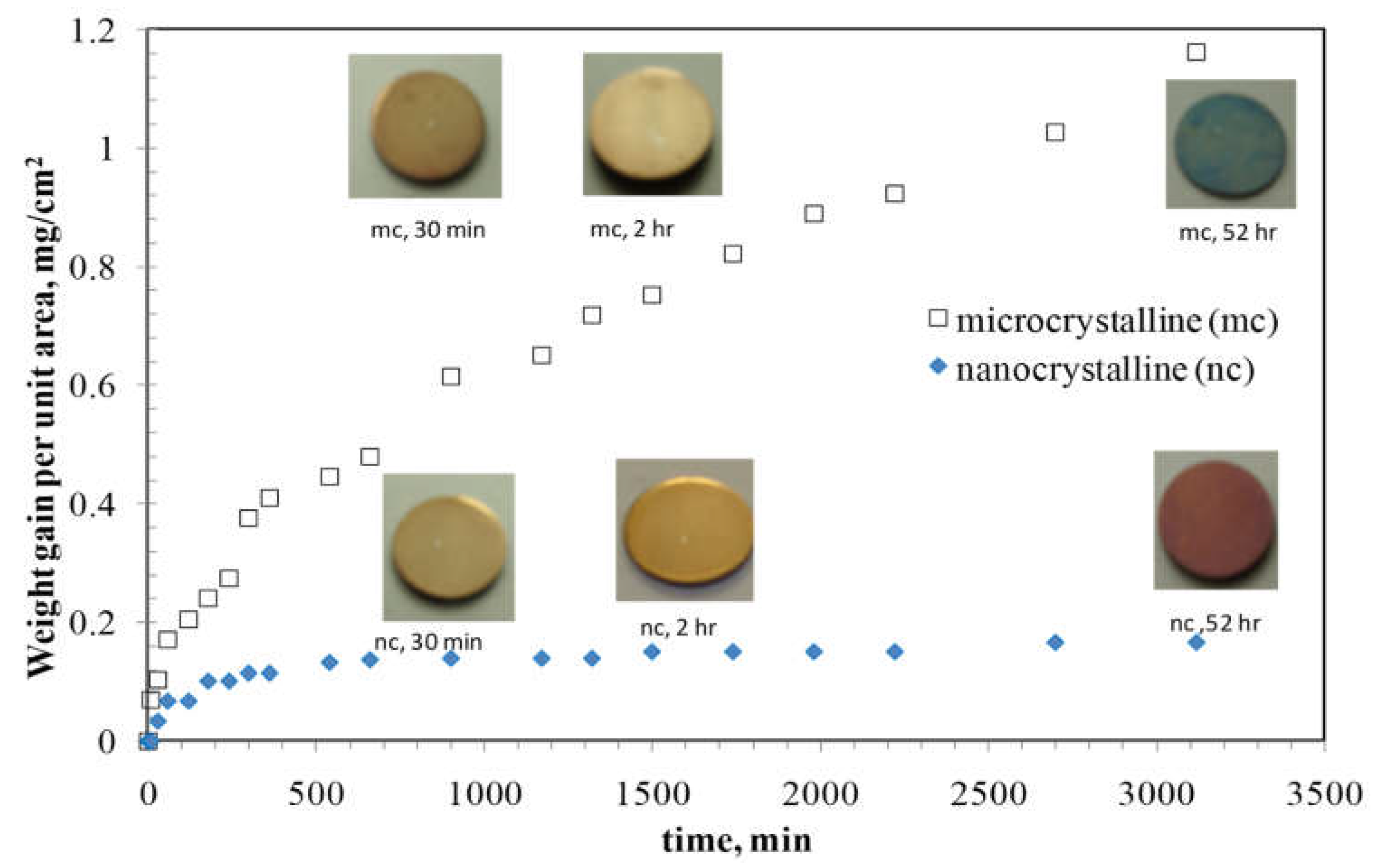
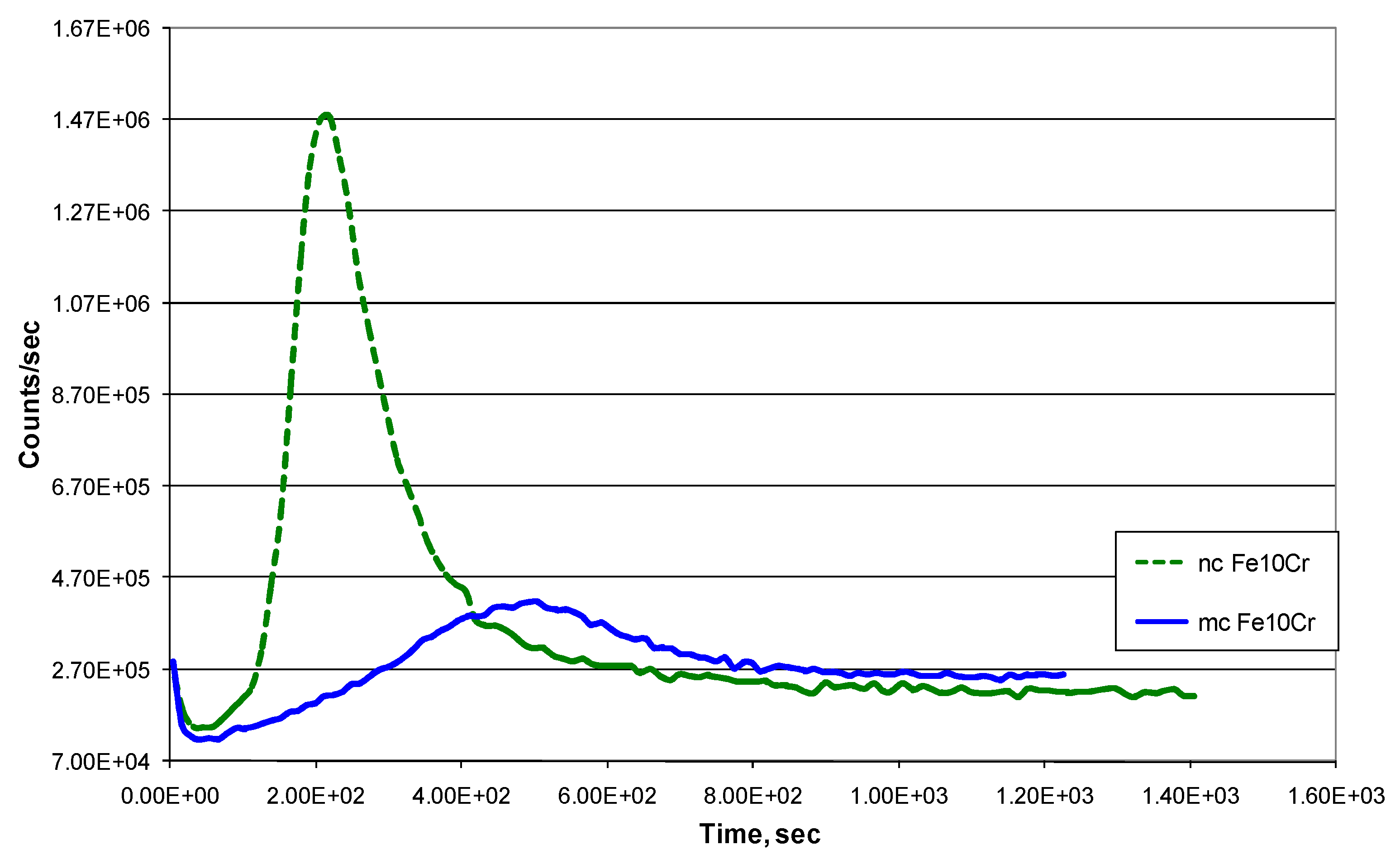

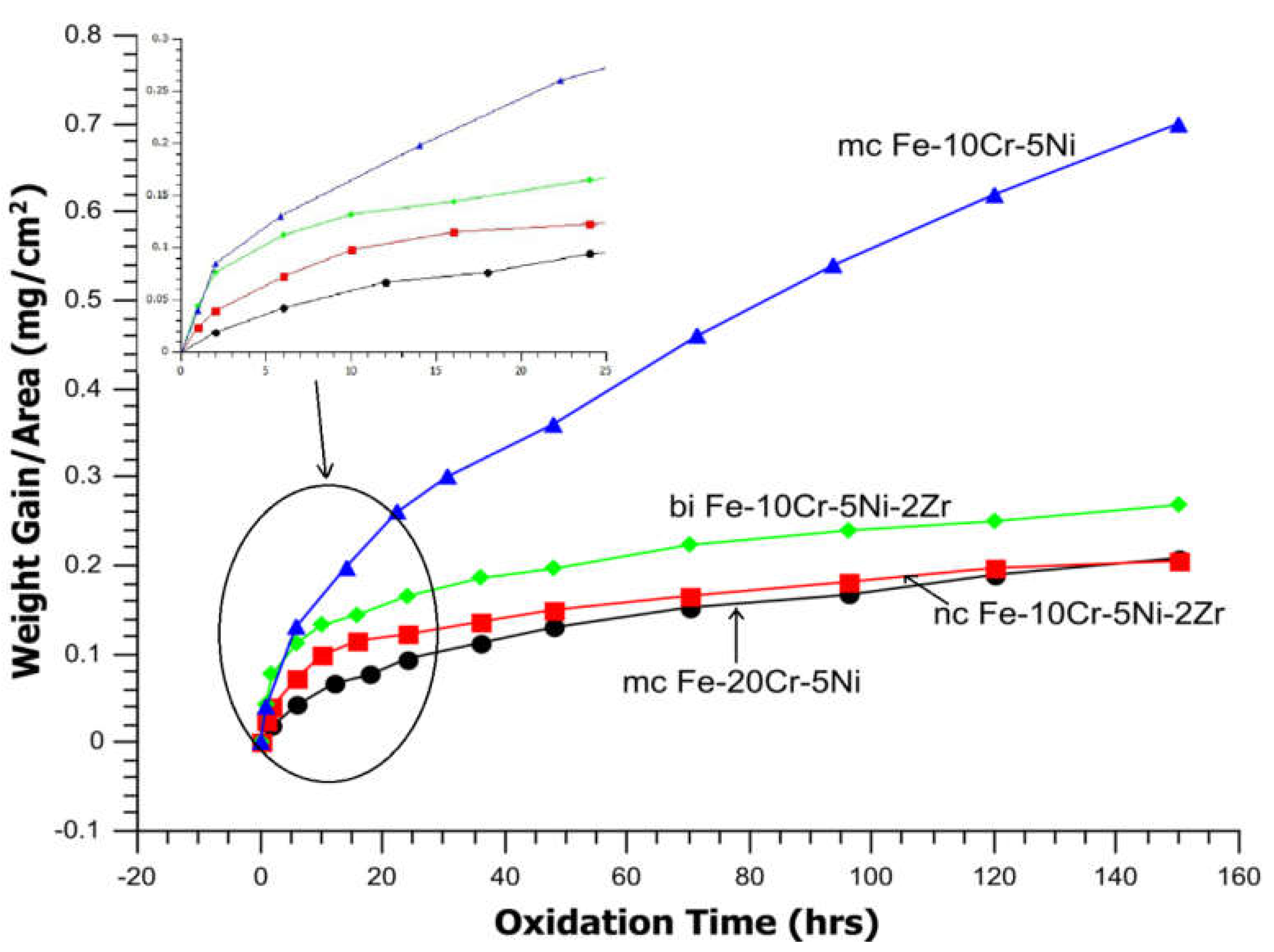
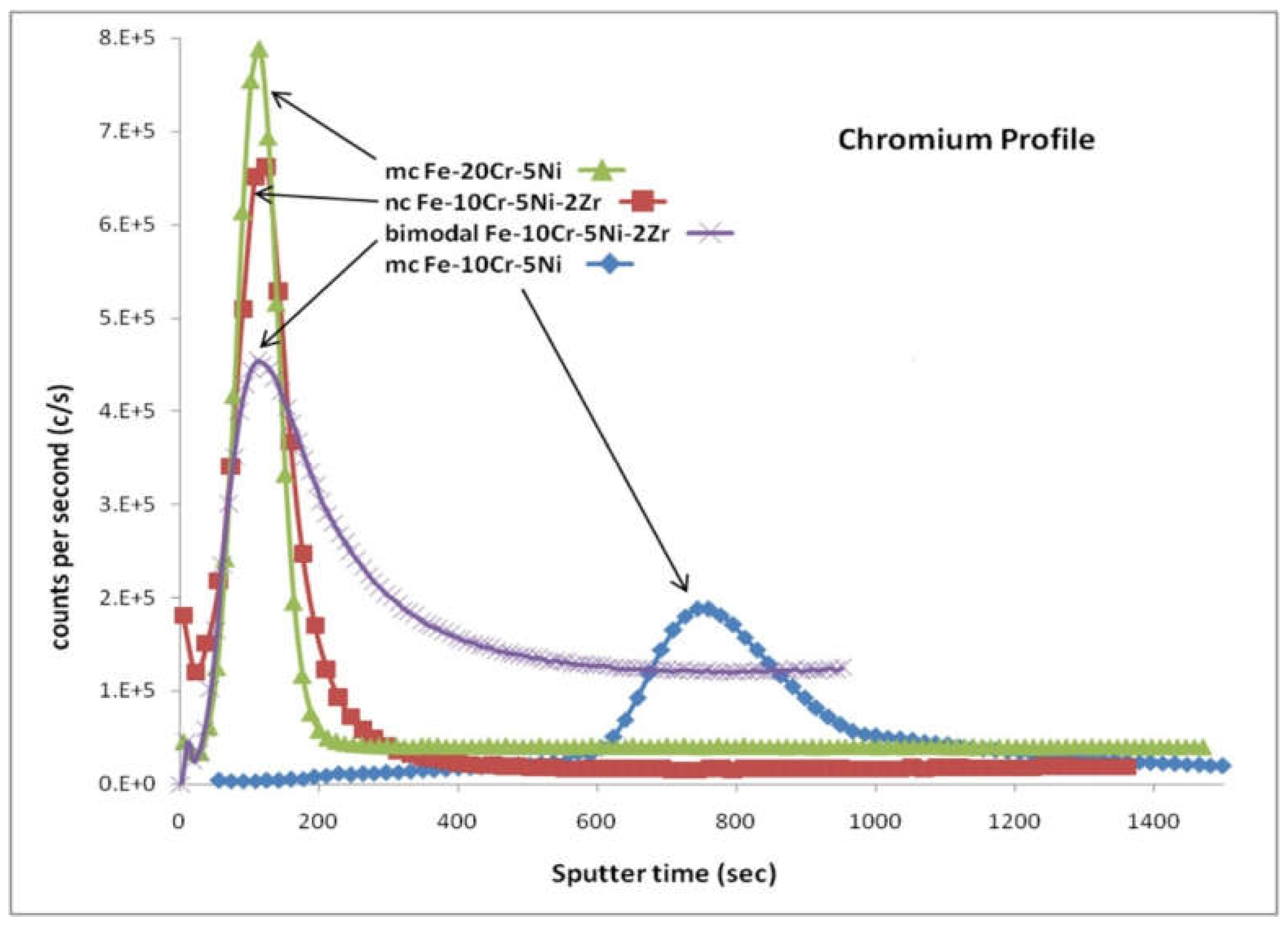
| Temperature | Lattice (Db) m2/s | GB (Db) m2/s | NC Iron (Dn) m2/s | MC Iron (Dm) m2/s |
|---|---|---|---|---|
| 300 °C | 1.2 × 10−26 | 8.6 × 10−22 | 1.7 × 10−17 | -- |
| 340 °C | 3.7 × 10−25 | 1.7 × 10−20 | 1.6× 10−16 | -- |
| 380 °C | 7.6 × 10−24 | 2.3 × 10−19 | 2.8 × 10−15 | 3.6 × 10−19 |
| 840 °C | 1.5 × 10−15 | 3.7 × 10−12 | -- | 2.2 × 10−15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh Raman, R.K. Mechanical Alloying of Elemental Powders into Nanocrystalline (NC) Fe-Cr Alloys: Remarkable Oxidation Resistance of NC Alloys. Metals 2021, 11, 695. https://doi.org/10.3390/met11050695
Singh Raman RK. Mechanical Alloying of Elemental Powders into Nanocrystalline (NC) Fe-Cr Alloys: Remarkable Oxidation Resistance of NC Alloys. Metals. 2021; 11(5):695. https://doi.org/10.3390/met11050695
Chicago/Turabian StyleSingh Raman, R. K. 2021. "Mechanical Alloying of Elemental Powders into Nanocrystalline (NC) Fe-Cr Alloys: Remarkable Oxidation Resistance of NC Alloys" Metals 11, no. 5: 695. https://doi.org/10.3390/met11050695
APA StyleSingh Raman, R. K. (2021). Mechanical Alloying of Elemental Powders into Nanocrystalline (NC) Fe-Cr Alloys: Remarkable Oxidation Resistance of NC Alloys. Metals, 11(5), 695. https://doi.org/10.3390/met11050695






