NdFeB Magnets Recycling Process: An Alternative Method to Produce Mixed Rare Earth Oxide from Scrap NdFeB Magnets
Abstract
:1. Introduction
2. Experimental
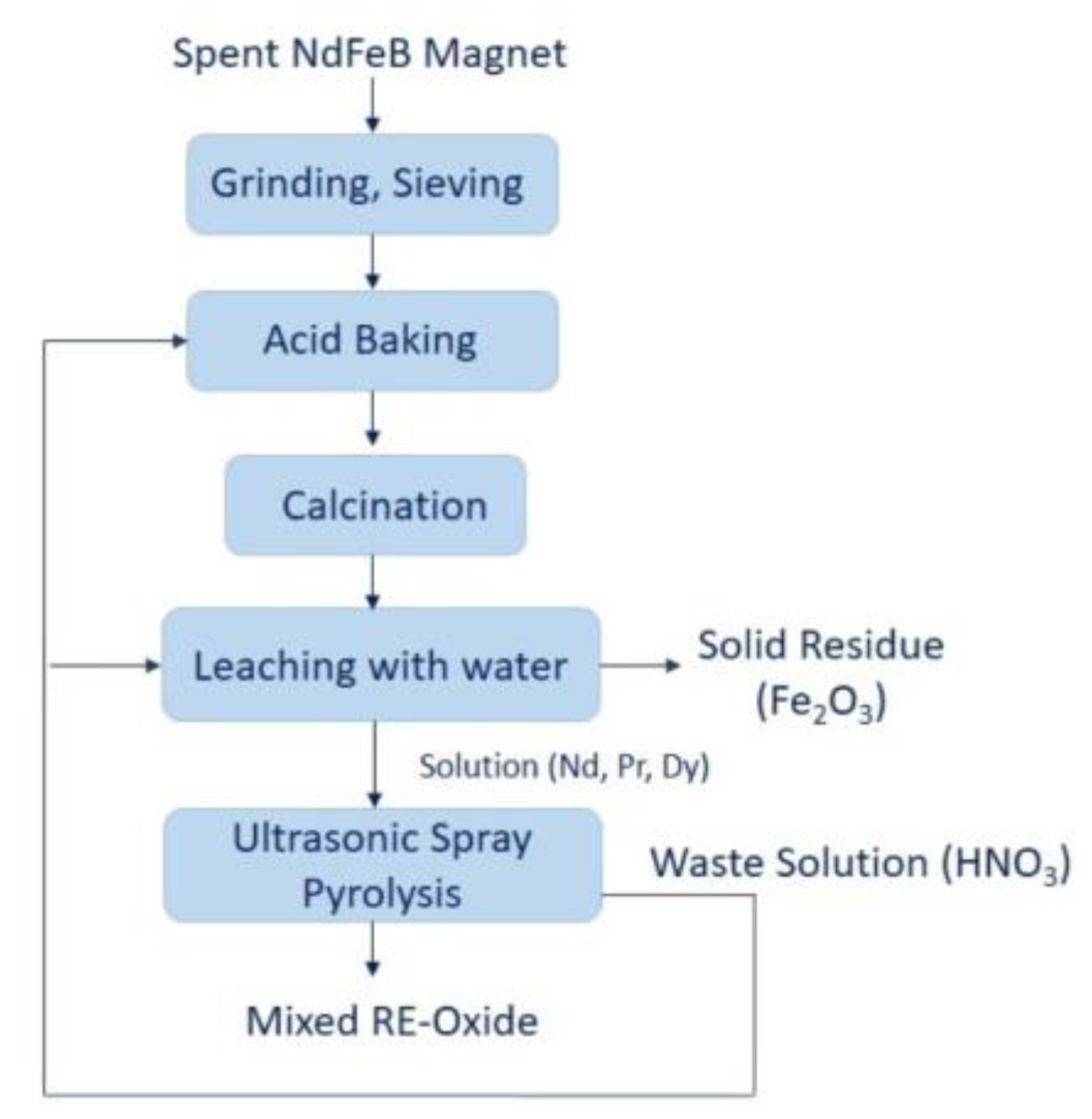
2.1. Materials, Acid Baking, and Water Leaching
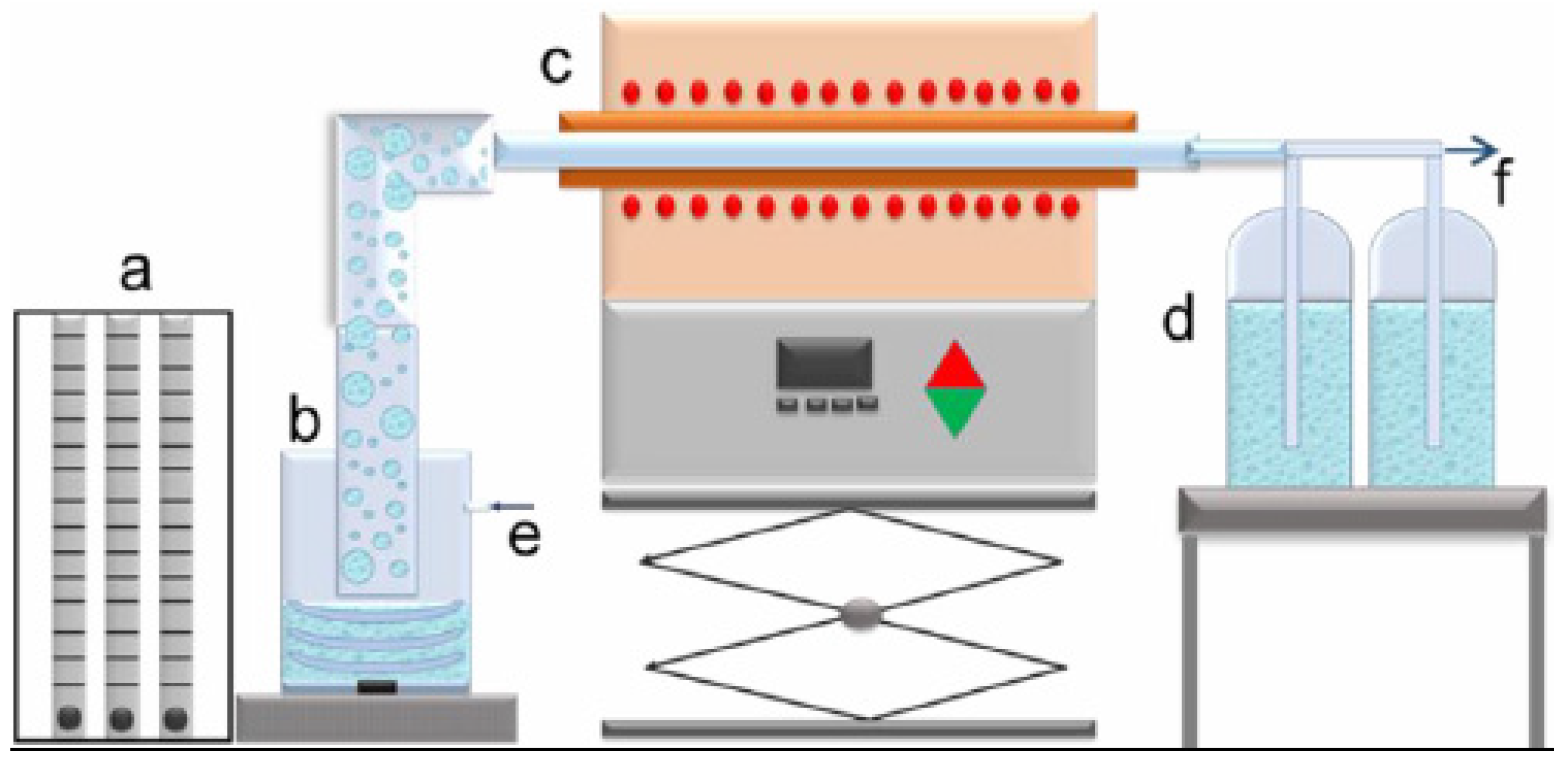
2.2. Ultrasonic Spray Pyrolysis Method for Production of RE-Oxide and Their Characterization
3. Results and Discussion
3.1. Characterization of Scrap NdFeB Magnet
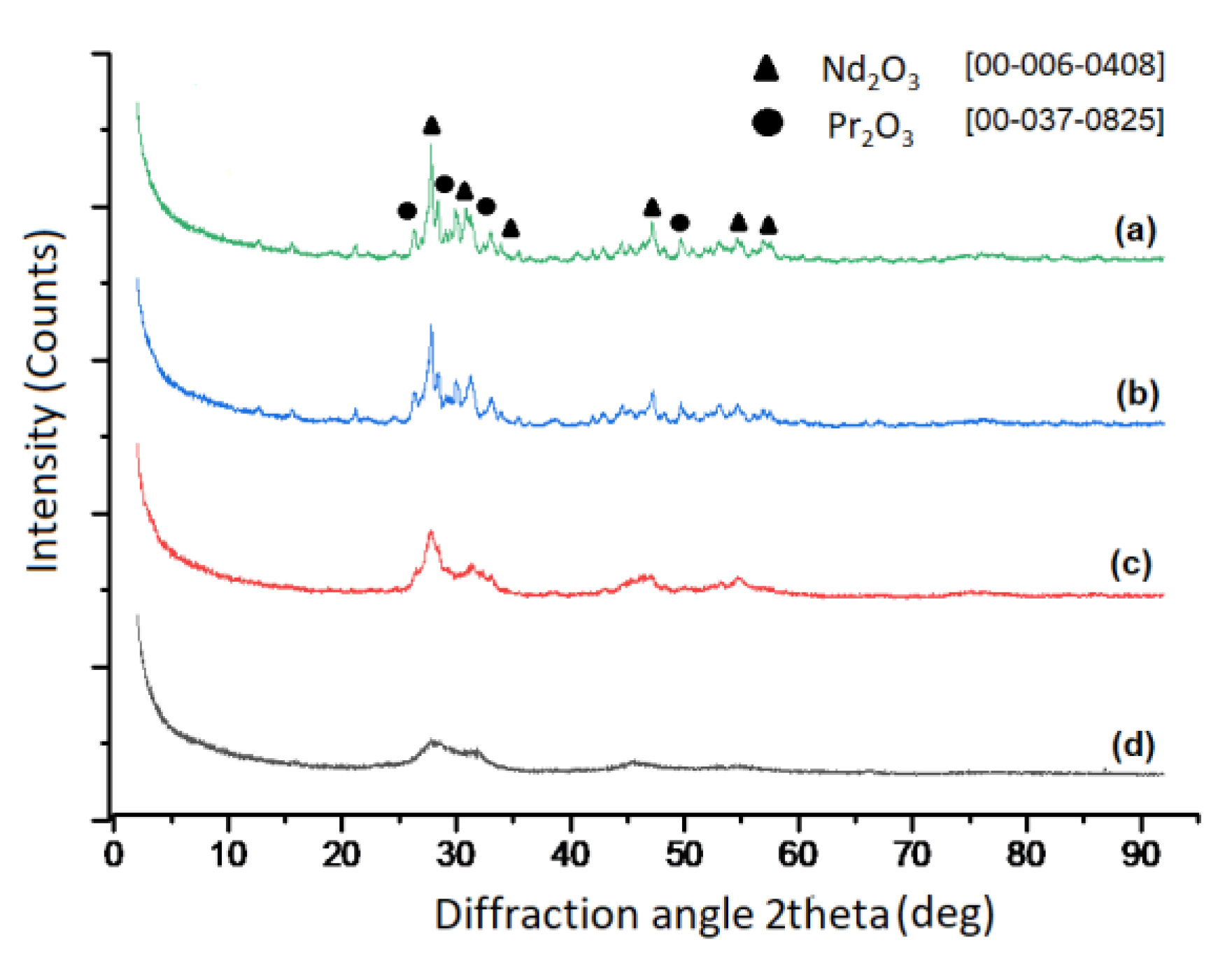
3.2. Production of REE-Oxide and Their Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuang-Taek, R. Effects of rare earth elements on the environment and human health: A literature review. Toxicol. Environ. Health Sci. 2016, 8, 189–200. [Google Scholar]
- Mancheri, N.A.; Sprecher, B.; Bailey, G.; Ge, J.; Tukker, A. Effect of Chinese policies on rare earth supply chain resilience. Resour. Conserv. Recycl. 2019, 142, 101–112. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Gronen, L.; Obradovic, S.; Milivojevic, M.; Friedrich, B. Neural network modeling for the extraction of rare earth elements from eudialyte concentrate by dry digestion and leaching. Metals 2018, 8, 267. [Google Scholar] [CrossRef] [Green Version]
- Demol, J.; Ho, E.; Senanayake, G. Sulfuric acid baking and leaching of rare earth elements, thorium and phosphate from a monazite concentrate: Effect of bake temperature from 200 to 800 °C. Hydrometallurgy 2018, 179, 254–267. [Google Scholar] [CrossRef]
- Stopic, S.; Friedrich, B. Leaching of rare earth elements from bastnasite ore (third part). Mil. Tech. Cour. 2019, 67, 561–572. [Google Scholar] [CrossRef]
- Stopic, S.; Friedrich, B. Deposition of silica in hydrometallurgical processes. Mil. Tech. Cour. 2020, 68, 65–78. [Google Scholar]
- Ma, Y.; Stopic, S.; Friedrich, B. Hydrometallurgical treatment of a eudialyte concentrate for preparation of rare earth carbonate. Johns. Matthey Technol. Rev. 2019, 63, 2–13. [Google Scholar] [CrossRef]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, S.; Gronen, L.; Stopic, S.; Friedrich, B. Novel approach for enhanced scandium and titanium leaching efficiency from bauxite residue with suppressed silica gel formation. Nat. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef]
- Alkan, G.; Yagmurlu, B.; Friedrich, B.; Ditrich, C.; Gronen, L.; Stopic, S.; Ma, Y. Selective silica gel scandium extraction from, iron –depleted red mud slags by dry digestion. Hydrometallurgy 2019, 185, 266–272. [Google Scholar] [CrossRef]
- Davris, P.; Stopic, S.; Balomenos, E.; Panias, D.; Paspaliaris, I.; Friedrich, B. Leaching of rare earth elements from Eudialyte concentrate by supressing silicon dissolution. Miner. Eng. 2017, 108, 115–122. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; van Gerven, T. Recovery of rare earths and other valuable metals from bauxite residue (red mud): A review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Ayres, R.U.; Peiró, L.T. Material efficiency: Rare and critical metals. Phylosophical Trans. R. Soc. A 2013, 371, 20110563. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Huiqing, P. Formation cause, composition analysis and comprehensive utilization of rare earth solid wastes. J. Rare Earths 2009, 27, 1096–1102. [Google Scholar]
- Sprecher, B.; Xiao, Y.; Walton, A.; Speight, J.; Harris, R.; Kleijn, R.; Kramer, G.J. Life cycle inventory of the production of rare earths and the subsequent production of NdFeB rare earth permanent magnets. Environ. Sci. Technol. 2014, 48, 3951–3958. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Önal, M.; Borra, C.; Guo, M.; Blanpain, B.; van Gerven, T. Recycling of NdFeB magnets using sulfation, selective roasting and water leaching. J. Sustain. Metall. 2015, 1, 199–215. [Google Scholar] [CrossRef]
- Önal MA, R.; Aktan, E.; Borra, C.R.; Blanpain, B.; Van Gerven, T.; Guo, M. Recycling of NdFeB magnets using nitration, calcination and water leaching for REE recovery. Hydrometallurgy 2017, 167, 115–123. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Hu, Y.; Wang, M.; Shi, Z. Preparation of large particle rare earth oxides by precipitation with oxalic acid. J. Rare Earths 2008, 26, 158–162. [Google Scholar] [CrossRef]
- Silva, R.G.; Morais, C.A.; Teixeira, L.V.; Oliveira, É.D. Selective precipitation of high-quality rare earth oxalates or carbonates from a purified sulfuric liquor containing soluble impurities. Min. Metall. Explor. 2019, 36, 967–977. [Google Scholar] [CrossRef]
- Yun, Y.; Stopic, S.; Friedrich, B. Valorization of Rare Earth Elements from a steenstrupine concentrate via a combined hydrometallurgical and pyrometallurgical method. Metals 2020, 10, 248. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Stopic, S.; Wang, X.; Forsberg, K.; Friedrich, B. Basic sulfate precipitation of zirconium from sulfuric acid leach solution. Metals 2020, 10, 1099. [Google Scholar] [CrossRef]
- Han, K.N. Characteristics of precipitation of rare earth elements with various precipitants. Minerals 2020, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Košević, M.; Stopic, S.; Cvetković, V.; Schroeder, M.; Stevanović, J.; Panic, V.; Friedrich, B. Mixed RuO2/TiO2 uniform microspheres synthesized by low-temperature ultrasonic spray pyrolysis and their advanced electrochemical performances. Appl. Surf. Sci. 2019, 464, 1–9. [Google Scholar] [CrossRef]
- Peskin, R.L.; Raco, R.J. Ultrasonic atomization of liquids. J. Acoust. Soc. Am. 1963, 35, 1378–1381. [Google Scholar] [CrossRef]
- Messing, G.; Zhang, S.; Jayanthi, G. Ceramic powder synthesis by spray pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Emil, E.; Gürmen, S. Estimation of yttrium oxide microstructural parameters using the Williamson–Hall analysis. Mater. Sci. Technol. 2018, 34, 1549–1557. [Google Scholar] [CrossRef]
- Yadav, A.A.; Lokhande, V.C.; Bulakhe, R.N.; Lokhande, C.D. Amperometric CO2 gas sensor based on interconnected web-like nanoparticles of La2O3 synthesized by ultrasonic spray pyrolysis. Microchim. Acta 2017, 184, 3713–3720. [Google Scholar] [CrossRef]
- Jung, D.S.; Hong, S.K.; Lee, H.J.; Kang, Y.C. Gd2O3: Eu phosphor particles prepared from spray solution containing boric acid flux and polymeric precursor by spray pyrolysis. Opt. Mater. 2006, 28, 530–535. [Google Scholar] [CrossRef]
- Goulart, C.; Djurado, E. Synthesis and sintering of Gd-doped CeO2 nanopowders prepared by ultrasonic spray pyrolysis. J. Eur. Ceram. Soc. 2013, 33, 769–778. [Google Scholar] [CrossRef]
- Emil, E.; Alkan, G.; Gurmen, S.; Rudolf, R.; Jenko, D.; Friedrich, B. Tuning the morphology of ZnO nanostructures with the ultrasonic spray pyrolysis process. Metals 2018, 8, 569. [Google Scholar] [CrossRef] [Green Version]
- Ardekani, S.R.; Aghdam AS, R.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A comprehensive review on ultrasonic spray pyrolysis technique: Mechanism, main parameters and applications in condensed matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. [Google Scholar] [CrossRef]
- Kaya, E.E.; Kaya, O.; Alkan, G.; Gürmen, S.; Stopic, S.; Friedrich, B. New proposal for size and size-distribution evaluation of nanoparticles synthesized via ultrasonic spray pyrolysis using search algorithm based on image-processing technique. Materials 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Oja, E.; Kultanen, P. A new curve detection method: Randomized Hough transform (RHT). Pattern Recognit. Lett. 1990, 11, 331–338. [Google Scholar] [CrossRef]
- Cvetkovic, V.; Feldhaus, D.; Vukicevic, N.; Barudzija, T.; Friedrich, B.; Jovicevic, J. Investigation on the electrochemical behaviour and deposition mechanism of neodymium in NdF3–LiF–Nd2O3 melt on Mo electrode. Metals 2020, 10, 576. [Google Scholar] [CrossRef]








| Samples Codes | Concentration of Nd(NO3)3 (g/L) | Concentration of Pr(NO3)3 (g/L) | Concentration of Dy(NO3)3 (g/L) | Reaction Temp (°C) | N2 Flow Rate (L/min) | Ultrasonic Frequency (MHz) |
|---|---|---|---|---|---|---|
| S1 | 0.458 | 0.130 | 0.010 | 700 | 1.0 | 1.75 |
| S2 | 0.458 | 0.130 | 0.010 | 800 | 1.0 | 1.75 |
| S3 | 0.458 | 0.130 | 0.010 | 900 | 1.0 | 1.75 |
| S4 | 0.458 | 0.130 | 0.010 | 1000 | 1.0 | 1.75 |
| Composition | Na2O | Al2O3 | SiO2 | MnO | Fe2O3 | Co3O4 | CuO |
| Concentration (%) | 0.34 | 0.42 | 0.24 | 1.97 | 68.1 | 0.70 | 0.14 |
| Composition | Ga2O3 | As2O3 | Nb2O5 | PdO | Pr2O3 | Nd2O3 | Tb4O7 |
| Concentration (%) | 0.20 | 0.21 | 0.12 | 0.24 | 5.72 | 20.4 | 0.70 |
| Composition | B | Co | Cr | Cu | Dy |
| Concentration (mg/L) | 278 | 245 | <1 | 32.6 | 210 |
| Composition | Fe | Mo | Nd | Ni | Pr |
| Concentration (mg/L) | 210,000 | <1 | 7580 | <1 | 2340 |
| Composition | B | Co | Cr | Cu | Dy |
| Concentration (mg/L) | 80 | 30 | <1 | <1 | 100 |
| Composition | Fe | Mo | Nd | Ni | Pr |
| Concentration (mg/L) | <1 | < 1 | 4580 | <1 | 1300 |
| REE-Nitrate | Nd(NO3)3 | Pr(NO3)3 | Dy(NO3)3 |
|---|---|---|---|
| Molar mass of REE-nitrate (g/mol) | 282.2 | 326.0 | 348.5 |
| REE-oxides | Nd2O3 | Pr2O3 | Dy2O3 |
| Density (g/cm3) | 7.2 | 6.9 | 7.8 |
| Molar mass (g/mol) | 336.5 | 329.8 | 373.0 |
| Concentration of metal in solution (g/L) | 0.458 | 0.130 | 0.010 |
| Theoretical minimal particle size (nm) | 108 | 76 | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, E.E.; Kaya, O.; Stopic, S.; Gürmen, S.; Friedrich, B. NdFeB Magnets Recycling Process: An Alternative Method to Produce Mixed Rare Earth Oxide from Scrap NdFeB Magnets. Metals 2021, 11, 716. https://doi.org/10.3390/met11050716
Kaya EE, Kaya O, Stopic S, Gürmen S, Friedrich B. NdFeB Magnets Recycling Process: An Alternative Method to Produce Mixed Rare Earth Oxide from Scrap NdFeB Magnets. Metals. 2021; 11(5):716. https://doi.org/10.3390/met11050716
Chicago/Turabian StyleKaya, Elif Emil, Ozan Kaya, Srecko Stopic, Sebahattin Gürmen, and Bernd Friedrich. 2021. "NdFeB Magnets Recycling Process: An Alternative Method to Produce Mixed Rare Earth Oxide from Scrap NdFeB Magnets" Metals 11, no. 5: 716. https://doi.org/10.3390/met11050716
APA StyleKaya, E. E., Kaya, O., Stopic, S., Gürmen, S., & Friedrich, B. (2021). NdFeB Magnets Recycling Process: An Alternative Method to Produce Mixed Rare Earth Oxide from Scrap NdFeB Magnets. Metals, 11(5), 716. https://doi.org/10.3390/met11050716








