Effect of Corrosion Time on the Synergistic Corrosion of Q235 Steel in Sodium Aluminate Solutions
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Specimens and Experimental Solutions
2.2. Immersion Experiments
2.3. Surface Analysis
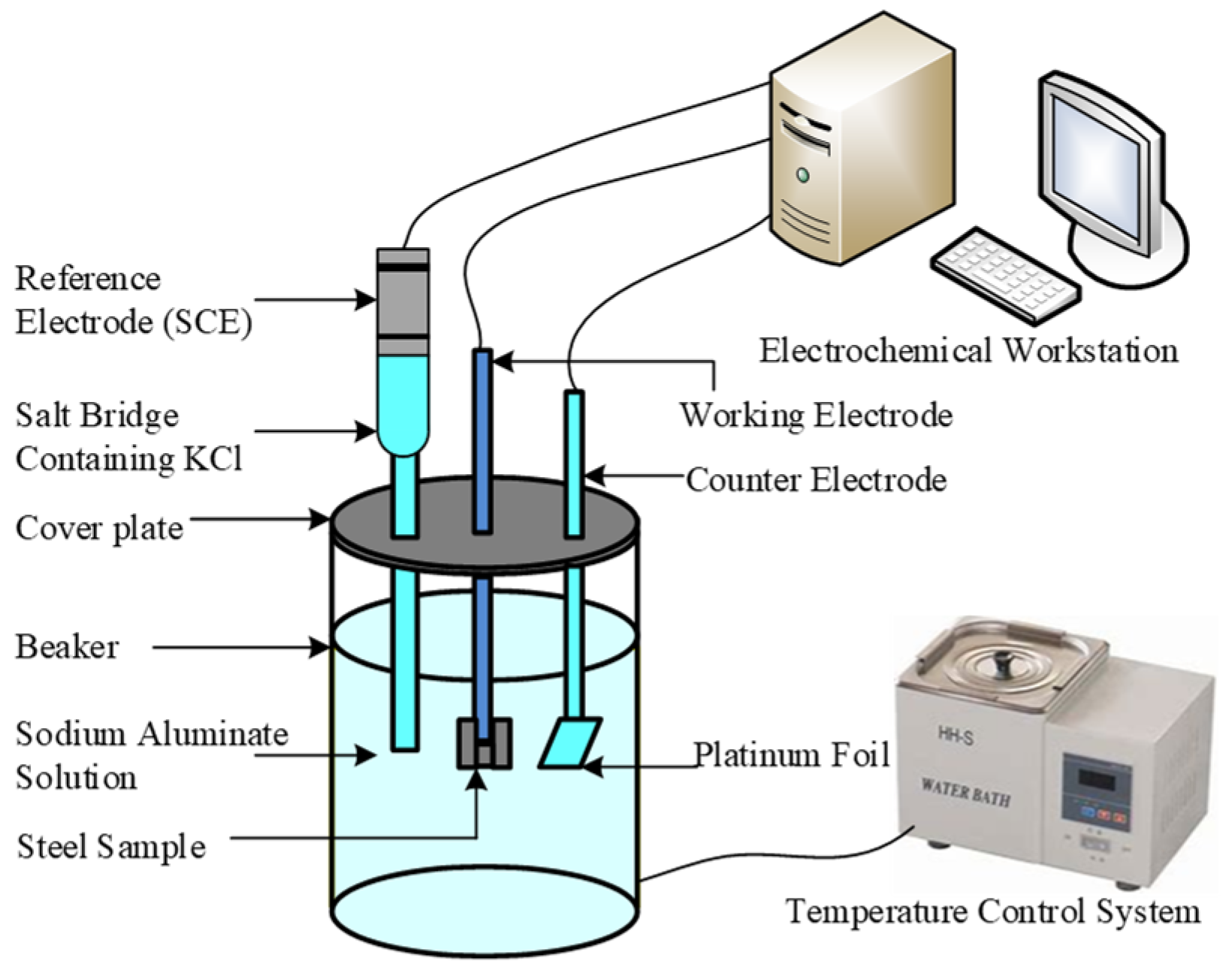
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Characteristics of Corrosion Products
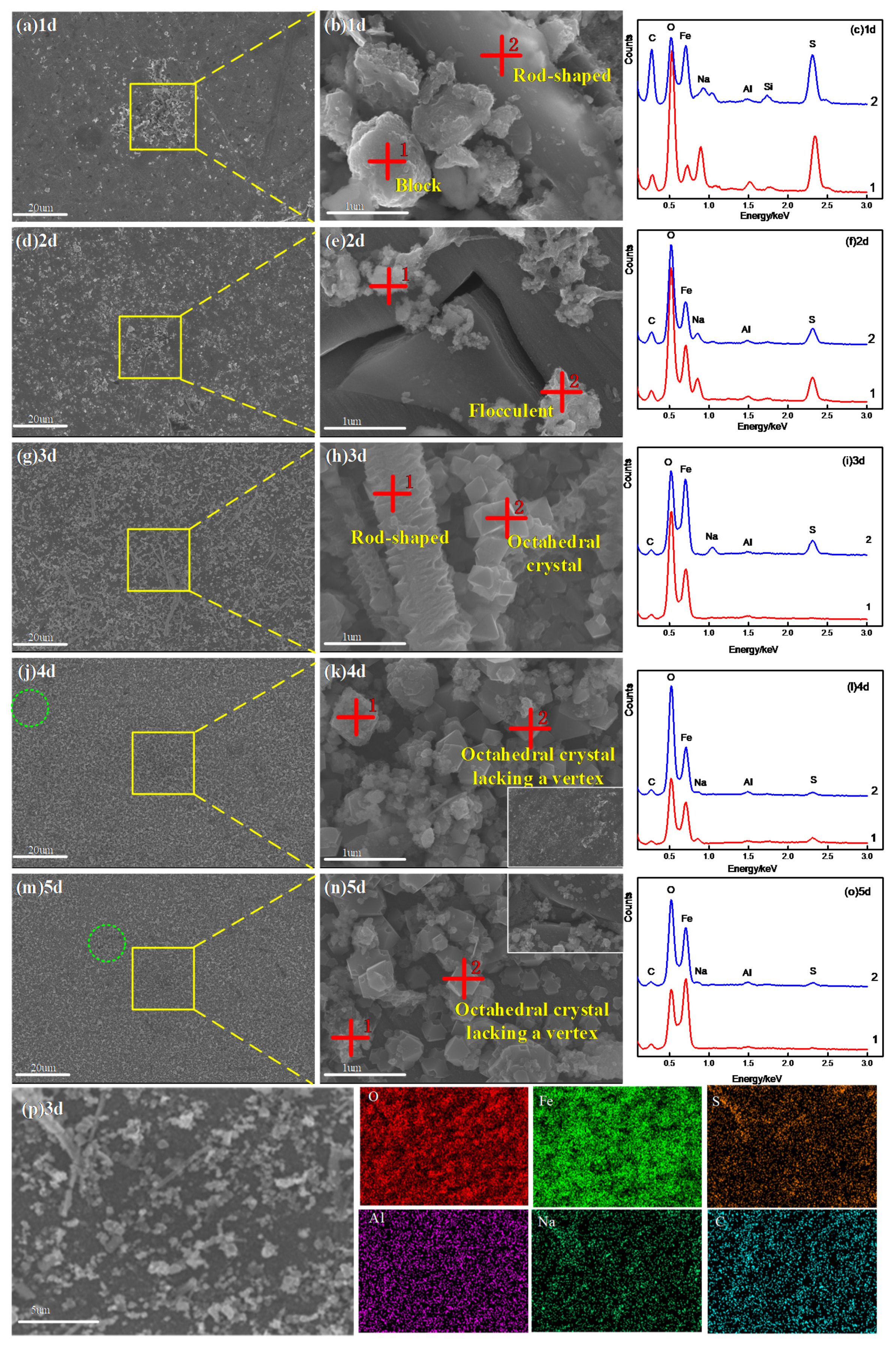
3.1.1. Morphology and Composition Analysis
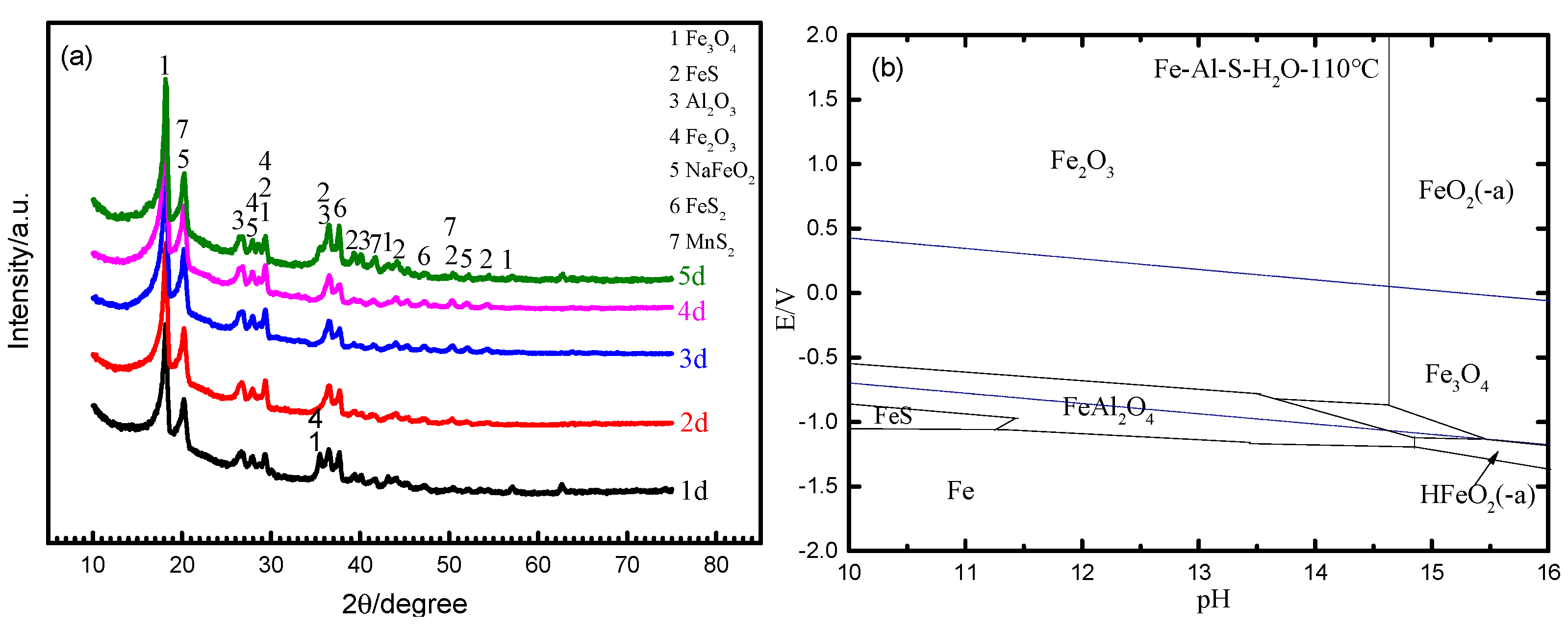
3.1.2. XRD Analysis
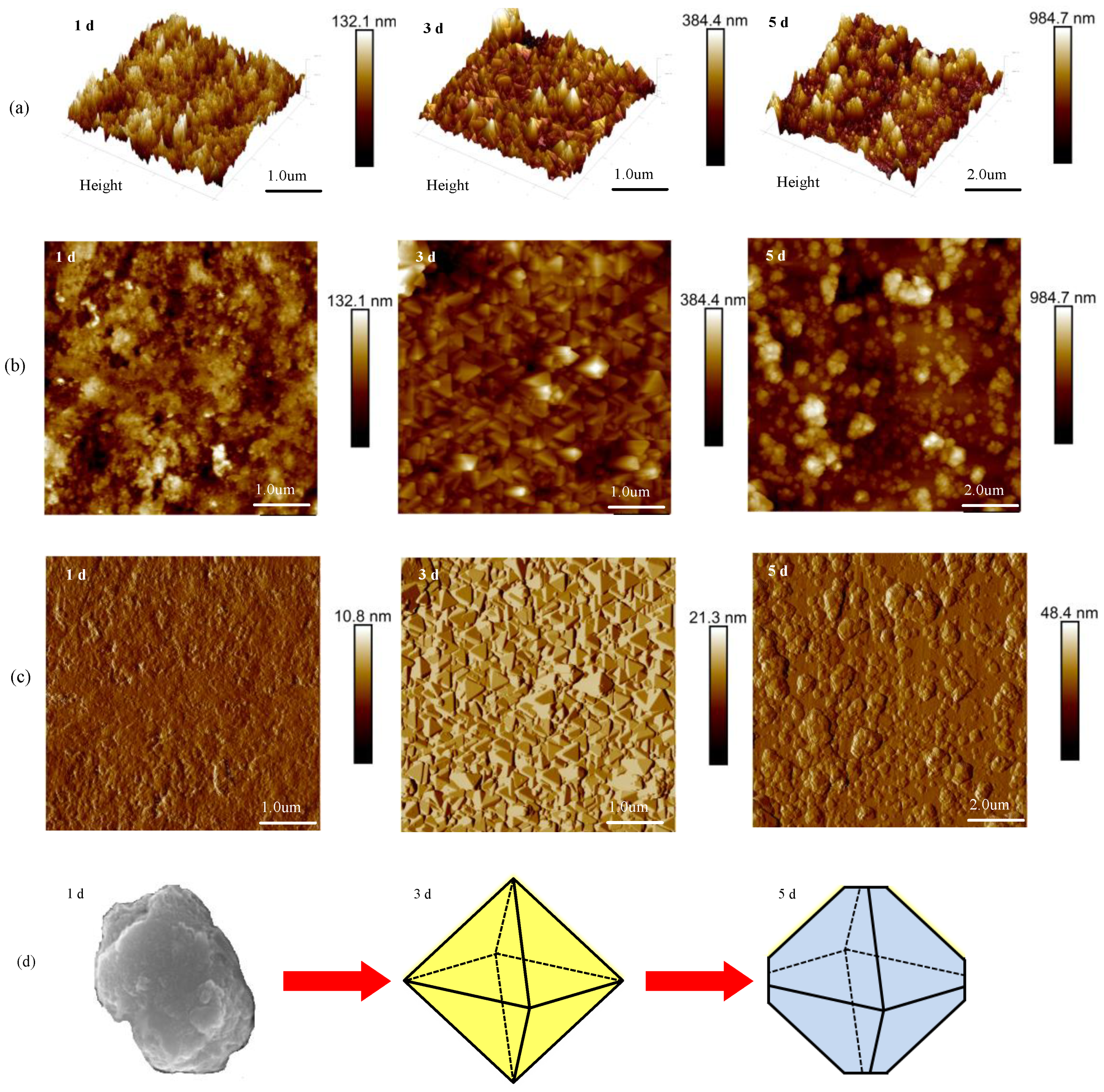
3.1.3. Surface Roughness Analysis
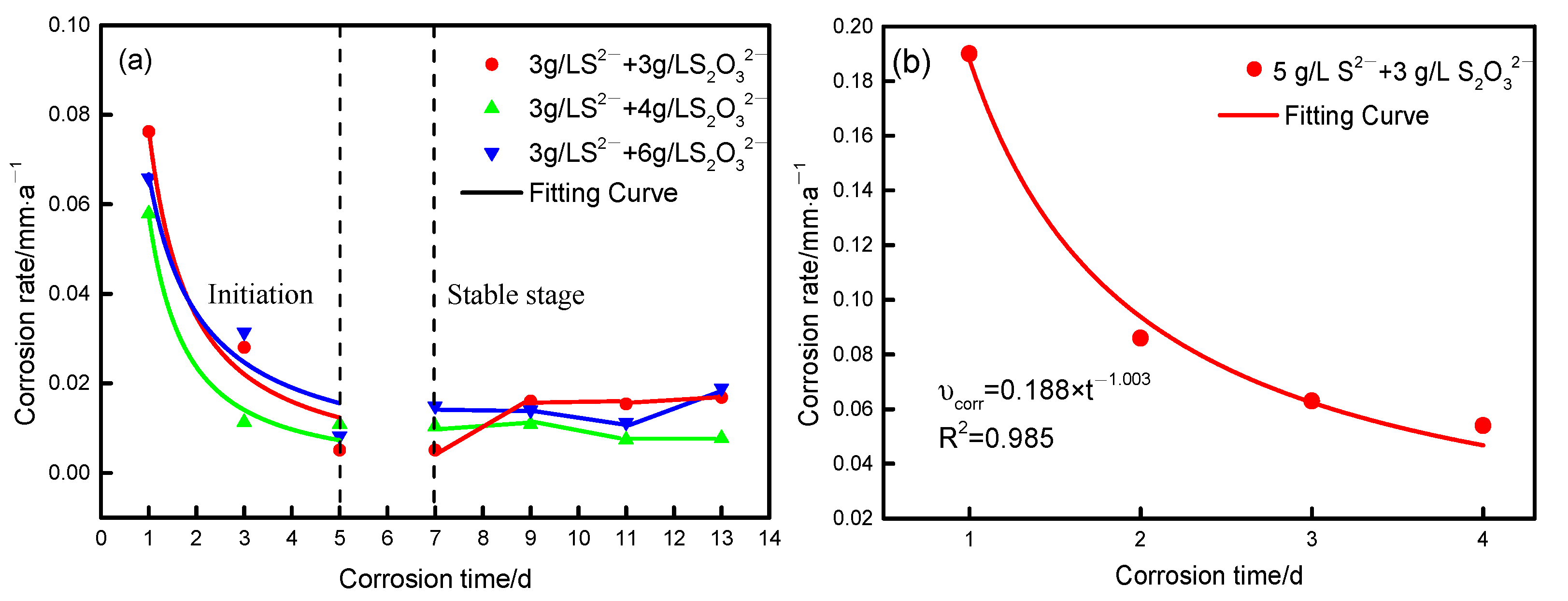
3.2. Corrosion Rate
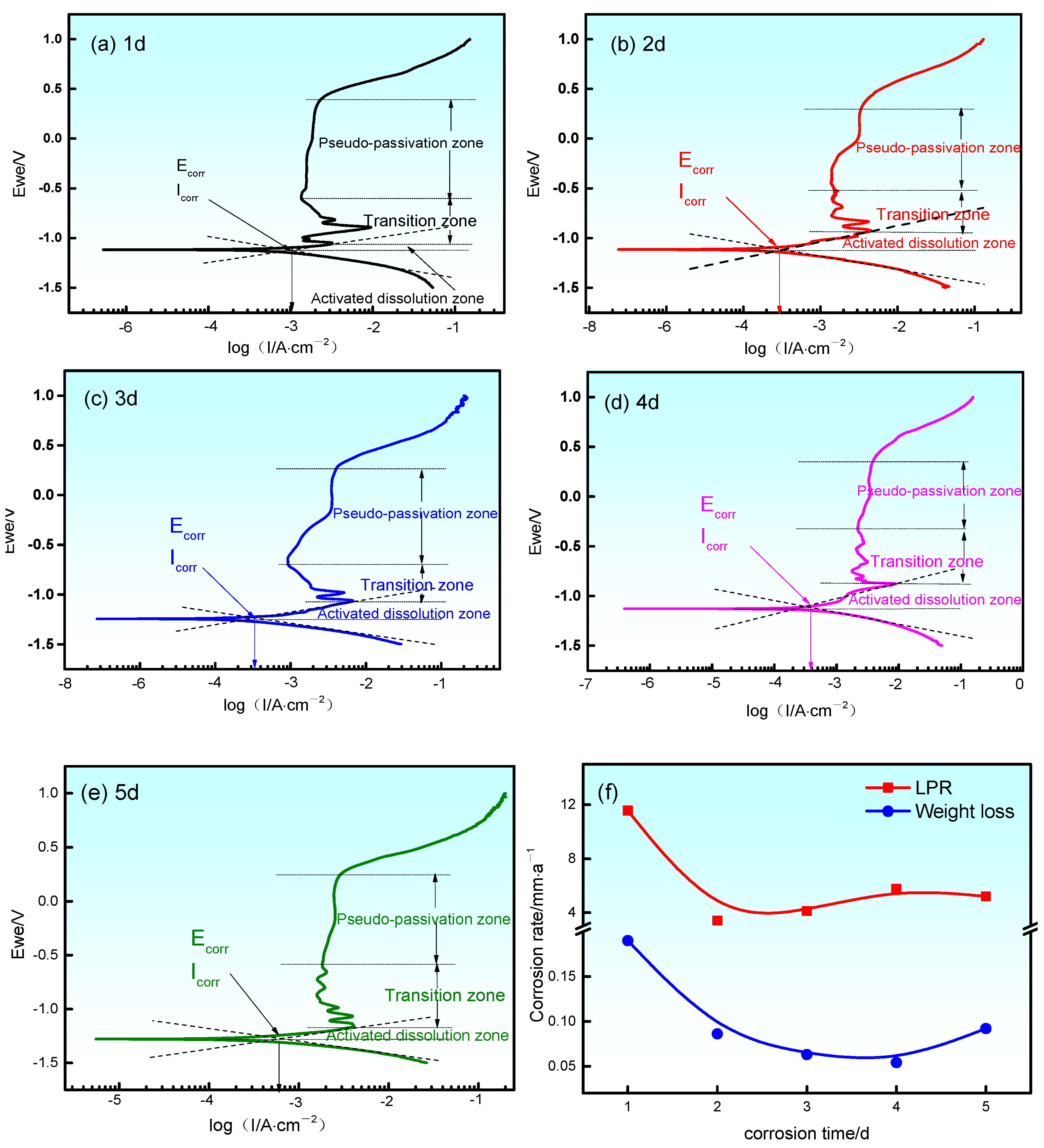
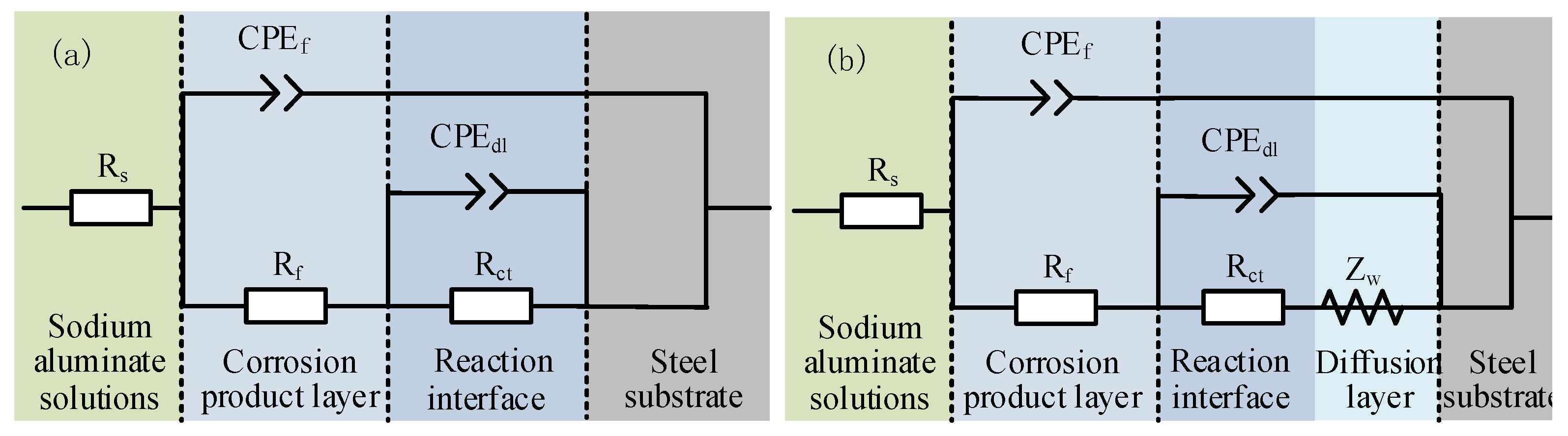
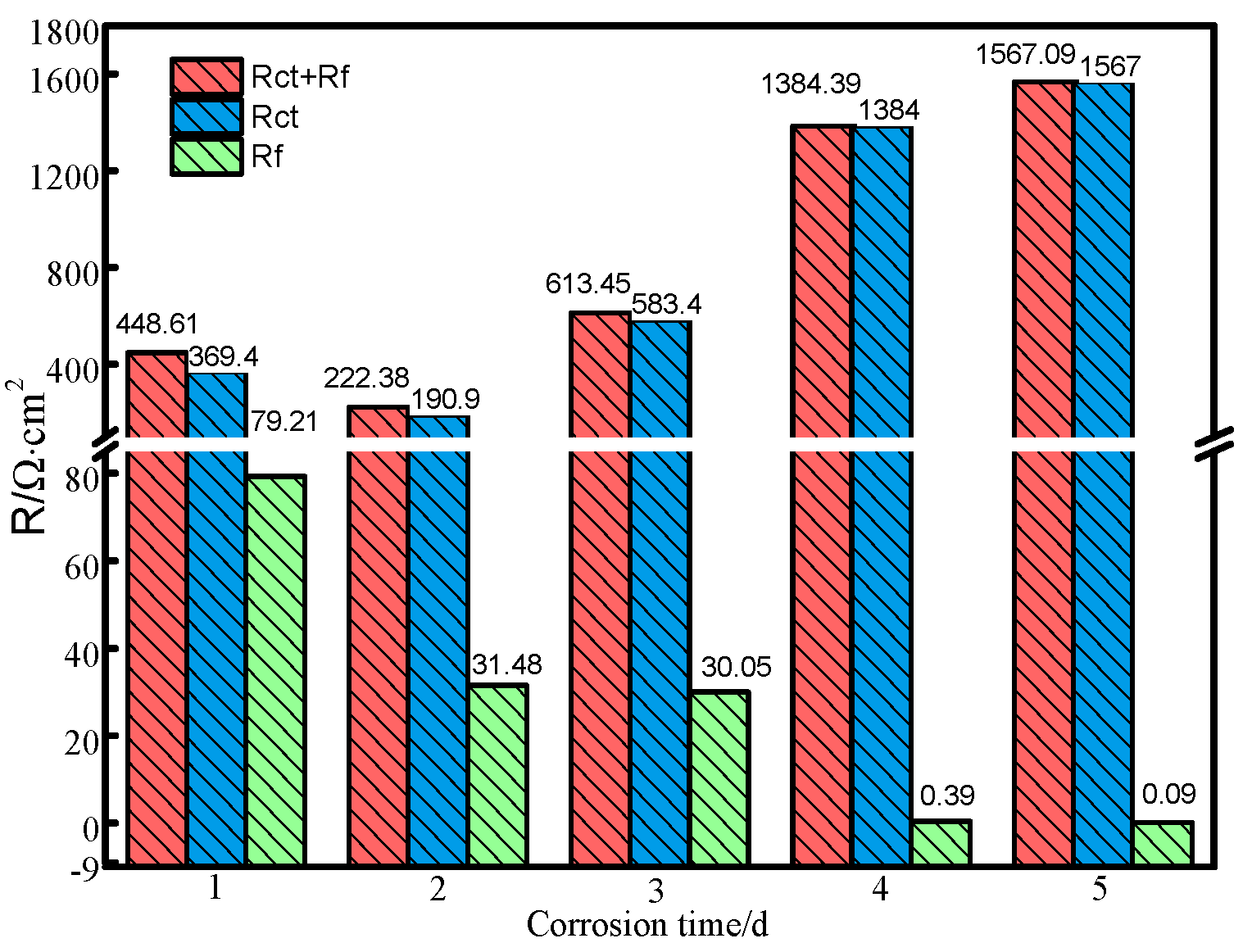
3.3. Electrochemical Impedance Spectrum
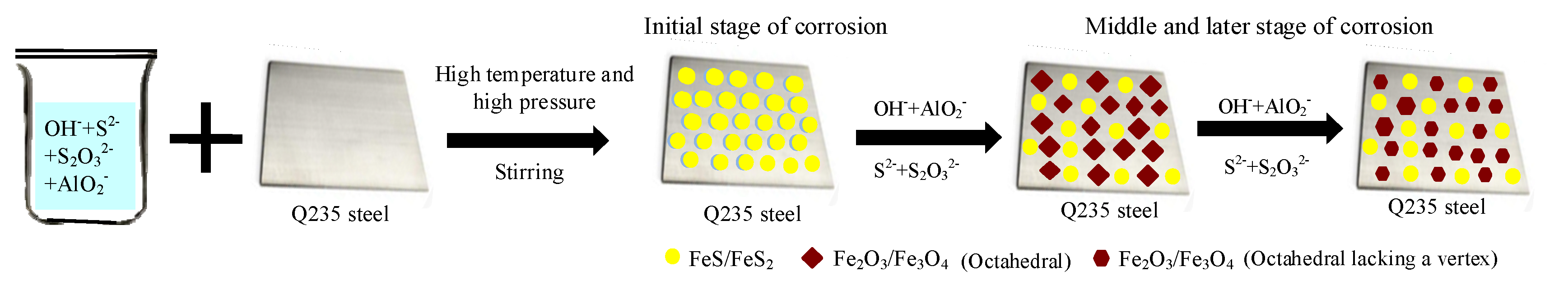
3.4. Formation Mechanism of Surface Corrosion Products
4. Conclusions
- (1)
- At the initial stage of corrosion, the corrosion rate decreases sharply and satisfies the mathematical model of the power function, and iron sulfide with an unstable structure is preferentially generated on the surface. The corrosion rate decreases slowly after 5 d;
- (2)
- The corrosion products have two morphological patterns, as follows: one is the octahedral crystal particle, which is composed of Fe2O3, Fe3O4, Al2O3 and NaFeO2, and the other is the interlayer corrosion between the surface layer and the matrix, which is composed of FeS, FeS2 and MnS2. The unstable iron sulfide is partially converted into stable iron oxide with corrosion time, which causes the anode pseudo-passivation of Q235 steel;
- (3)
- Different stages of the polarization curves correspond to different anode reactions. The dynamics of the Q235 steel electrode are controlled by charge transfer and ion diffusion for 3 d. However, at other times, the dynamics are mainly controlled by charge transfer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, W.; Huang, Y.; Peng, W.; Han, G.; Cao, Y.; Liu, J. Enhanced separation of pyrite from high-sulfur bauxite using 2-mercaptobenzimidazole as chelate collector: Flotation optimization and interaction mechanisms. Miner. Eng. 2018, 129, 93–101. [Google Scholar] [CrossRef]
- Wu, X. Study on Fluidized Roasting and Desulfurization of High-Sulfur Bauxite; Northeastern University: Shenyang, China, 2010. [Google Scholar]
- He, R.; Li, Z.; Zhang, N.; Tan, X. Study on the dissolution property of high-sulfur bauxite in Guizhou. J. Guizhou Univ. Technol. 2005, 34, 4–7. [Google Scholar]
- Liu, Z.W.; Li, D.Y.; Ma, W.H.; Yan, H.W.; Xie, K.Q.; Zheng, L.C.; Li, P.F. Sulfur removal by adding aluminum in the bayer process of high-sulfur bauxite. Miner. Eng. 2018, 119, 76–81. [Google Scholar] [CrossRef]
- Hu, X.-L.; Chen, W.-M.; Xie, Q.-L. Sulfur phase and sulfur removal in high sulfur-containing bauxite. Trans. Nonferrous Met. Soc. China 2011, 21, 1641–1647. [Google Scholar] [CrossRef]
- Lou, Z.N.; Xiong, Y.; Feng, X.D.; Shan, W.J.; Zhai, Y.C. Study on the roasting and leaching behavior of high-sulfur bauxite using ammonium bisulfate. Hydrometallurgy 2016, 165, 306–311. [Google Scholar] [CrossRef]
- Liu, Z.W.; Yan, H.W.; Ma, W.H.; Xie, K.Q.; Xu, B.Q.; Zheng, L. Digestion behavior and removal of sulfur in high-sulfur bauxite during bayer process. Miner. Eng. 2020, 149, 106237. [Google Scholar] [CrossRef]
- Zhou, X.; Yin, J.; Chen, Y.; Xia, W.; Xiang, X.; Yuan, X. Simultaneous removal of sulfur and iron by the seed precipitation of digestion solution for high-sulfur bauxite. Hydrometallurgy 2018, 181, 7–15. [Google Scholar] [CrossRef]
- Liu, Z.W.; Li, D.Y.; Ma, W.H.; Yan, H.W.; Xie, K.Q.; Zheng, L.C.; Li, P.F. Sulfur Removal by Adding Iron During the Digestion Process of High-sulfur Bauxite. Metall. Mater. Trans. B-Process. Metall. Mater. Process. Sci. 2018, 49, 509–513. [Google Scholar]
- Xie, Q.L.; Chen, W.M. Corrosion behavior of 16Mn low alloy steel in sulfide-containing Bayer solutions. Corros. Sci. 2014, 86, 252–260. [Google Scholar] [CrossRef]
- Sriram, R.; Tromans, D. The Anodic Polarization Behaviour of Carbon Steel in Hot Caustic Aluminate Solutions. Corros. Sci. 1985, 25, 79–91. [Google Scholar] [CrossRef]
- Wu, Z.; Wen, L. Stress corrosion cracking of steel in sodium aluminate solution. Chin. J. Corros. Prot. 1991, 11, 335–343. [Google Scholar]
- Xie, Q.; Chen, W. Effect of S2- on corrosion behavior of low alloy steel in sodium aluminate solution. Chin. J. Nonferrous Met. 2013, 23, 3462–3469. [Google Scholar]
- Quan, B.; Li, J.; Chen, C. Synergy Corrosion Effect of Thiosulfate and Sulfide on Q235 steel in Sodium Aluminate Solution. Mater. Res. Express 2019, 6, 025607. [Google Scholar]
- Niskanen, P.; Sanders, T.H., Jr.; Rinker, J.G.; Marek, M. Corrosion of aluminum alloys containing lithium. Corros. Sci. 1982, 283, 293–295, 304. [Google Scholar]
- Jiang, N.; Li, J.-F.; Zheng, Z.-Q.; Wei, X.-Y.; Li, Y.-F. Effect of aging on mechanical properties and localized corrosion behaviors of Al-Cu-Li alloy. Trans. Nonferrous. Met. Soc. China 2005, 15, 23–29. [Google Scholar]
- Onyeachu, I.B.; Obot, I.B.; Adesina, A.Y. Green corrosion inhibitor for oilfeld application II: The time–evolution effect on the sweet corrosion of API X60 steel in synthetic brine and the inhibition performance of 2-(2-pyridyl) benzimidazole under turbulent hydrodynamics. Corros. Sci. 2020, 168, 108589. [Google Scholar] [CrossRef]
- Chen, J.; Yang, H.Y.; Xu, G.Q.; Zhang, P.J.; Lv, J.; Sun, W.; Li, B.S.; Huang, J.; Wang, D.M.; Wu, Y.C. Phosphating passivation of vacuum evaporated Al/NdFeB magnets boosting high anti-corrosion performances. Surf. Coat. Technol. 2020, 399, 126115. [Google Scholar] [CrossRef]
- Rodrigues, J.F.Q.; Padilha, G.S.; Bortolozo, A.D.; Osório, W.R. Effect of sintering time on corrosion behavior of an Ag/Al/Nb/Ti/Zn alloy system. J. Alloys Compd. 2020, 834, 155039. [Google Scholar] [CrossRef]
- Bahmani, A.; Shin, K.S. Controlling the Microstructure and Texture Using Multidirectional Forging (MDF) to Develop a Low Corrosion Rate Mg Alloy. Corrosion 2020, 76, 750–765. [Google Scholar] [CrossRef]
- Young, J.; Reddy, R.G. Synthesis, mechanical properties, and in vitro corrosion behavior of biodegradable Zn-Li-Cu alloys. J. Alloys Compd. 2020, 844, 156257. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Meng, Y.S. Cryogenic Electron Microscopy for Characterizing and Diagnosing Batteries. Joule 2018, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Tang, W.; Wu, S.; Wang, J. Ultrasound-assisted intensified crystallization of L-glutamic acid: Crystal nucleation and polymorph transformation. Ultrason. Sonochem. 2020, 68, 105227. [Google Scholar]
- Quan, B.-L.; Li, J.-Q.; Chen, C.-Y. Effect of sulfur on corrosion behavior of Q235 and 16Mn steel in sodium aluminate solutions. Mater. Res. Express 2020, 7, 035602. [Google Scholar] [CrossRef]
- Takeda, Y.; Akagi, J.; Edagawa, A.; Inagaki, M.; Naka, S. A preparation and polymorphic relations of sodium iron oxide (nafeo2). Mater. Res. Bull. 1980, 15, 1167–1172. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Singh, P.M. Electrochemical behaviour of duplex stainless steels in caustic environment. Corros. Sci. 2011, 53, 71–81. [Google Scholar] [CrossRef]
- Lu, D.; Lv, G.; Zhang, T.-A.; Zhang, W. Roasting Pre-Treatment of High-Sulfur Bauxite for Sulfide Removal and Digestion Performance of Roasted Ore. Russ. J. Non-Ferr. Met. 2018, 59, 493–501. [Google Scholar]
- Yuan, J.; Chen, C.; Li, J.; Quan, B.; Lan, Y.; Wang, L.; Fu, H.; Gai, J. Initial Corrosion Behavior of 12Cr1MoV Steel in Thiosulfate-Containing Sodium Aluminate Solution. Metals 2020, 10, 1283. [Google Scholar] [CrossRef]
- Wei, B.; Qin, Q.; Fu, Q.; Bai, Y.; Xu, J.; Yu, C.; Sun, C.; Ke, W. X80 Steel Corrosion Induced by Alternating Current in Water-Saturated Acidic Soil. Corrosion 2020, 76, 248–267. [Google Scholar] [CrossRef]
- Wang, K.; Li, C.; Li, Y.; Lu, J.; Wang, Y.; Luo, X. A fully coupled model of hydrodynamic-chemical-electrochemical processes for CO2 uniform corrosion in multi-physics environment. J. Pet. Sci. Eng. 2020, 193, 107436. [Google Scholar] [CrossRef]
- Zakeri, M.; Naghizadeh, M.; Nakhaie, D.; Moayed, M.H. Pit Transition Potential and Repassivation Potential of Stainless Steel in Thiosulfate Solution. J. Electrochem. Soc. 2016, 163, C275–C281. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xiao, Q.; Mei, D.; Tong, Y.; Zheng, Y.; Li, L.; Zhong, Z. Microstructure, corrosion resistance and formation mechanism of alumina microarc oxidation coatings on sintered NdFeB permanent magnets. Surf. Coat. Technol. 2017, 309, 621–627. [Google Scholar] [CrossRef]
- Montgomery, E. The Corrosion Behavior of Oily Film Corrosion Preventative Compounds Applied to 1018 Steel. Ph.D. Thesis, The Pennsylvania State University, State College, PA, USA, 2008. [Google Scholar]
- Zhu, Z.; Li, S.; Zhang, R. Investigation of corrosion characteristics of Cu-10Ni-1.2Fe-xMn (x=0.53, 0.87, 1.19) alloy in 3.5% NaCl solution. Rsc Adv. 2021, 11, 11318–11328. [Google Scholar] [CrossRef]
- Diaz, B.; Guitian, B.; Novoa, X.R.; Perez, M.C. The effect of long-term atmospheric aging and temperature on the electrochemical behaviour of steel rebars in mortar. Corros. Sci. 2018, 140, 143–150. [Google Scholar] [CrossRef]
- Ahmed, I.; Islam, A.; Ali, N. Determination Of Corrosion Rate Of Mild Steel In Different Medium Measuring Current Density. In Proceedings of the International Conference on Mechanical Engineering and Renewable Energy 2017 (ICMERE2017), Chittagong, Bangladesh, 18–20 December 2017. [Google Scholar]
- Khamseh, S.; Alibakhshi, E.; Ramezanzadeh, B.; Lecomte, J.-S.; Laheurte, P.; Noirefalize, X.; Laoutid, F.; Vahabi, H. Tailoring hardness and electrochemical performance of TC4 coated Cu/a-C thin coating with introducing second metal Zr. Corros. Sci. 2020, 172, 108713. [Google Scholar] [CrossRef]
- Zhang, G.A.; Lu, M.X.; Qiu, Y.B.; Guo, X.P.; Chen, Z.Y. The Relationship between the Formation Process of Corrosion Scales and the Electrochemical Mechanism of Carbon Steel in High Pressure CO2-Containing Formation Water. J. Electrochem. Soc. 2012, 159, C393–C402. [Google Scholar] [CrossRef]
- Tang, S.K.; Dai, Z.X.; Tan, G.L.; Gong, S.; Liu, B.; Xie, G.L.; Peng, L.J.; Guo, J.L.; Li, Z. High-strength, ductility and corrosion-resistant in a novel Cu20Ni20Mn0.3Cr0.3Al alloy. Mater. Chem. Phys. 2020, 252, 123177. [Google Scholar] [CrossRef]
- He, B.; Lu, C.-H.; Han, P.-J.; Bai, X.-H. Short-term electrochemical corrosion behavior of pipeline steel in saline sandy environments. Eng. Fail. Anal. 2016, 59, 410–418. [Google Scholar] [CrossRef]
- De Motte, R.; Basilico, E.; Mingant, R.; Kittel, J.; Ropital, F.; Combrade, P.; Necib, S.; Deydier, V.; Crusset, D.; Marcelin, S. A study by electrochemical impedance spectroscopy and surface analysis of corrosion product layers formed during CO2 corrosion of low alloy steel. Corros. Sci. 2020, 172, 108666. [Google Scholar] [CrossRef]
- Mythreyi, O.V.; Raja, A.; Nagesha, B.K.; Jayaganthan, R. Corrosion Study of Selective Laser Melted IN718 Alloy upon Post Heat Treatment and Shot Peening. Metals 2020, 10, 19. [Google Scholar] [CrossRef]
- Shi, J.; Ming, J.; Sun, W.; Zhang, Y. Corrosion performance of reinforcing steel in concrete under simultaneous flexural load and chlorides attack. Constr. Build. Mater. 2017, 149, 315–326. [Google Scholar] [CrossRef]
- Guo, F.; Duan, S.; Wu, D.; Matsuda, K.; Wang, T.; Zou, Y. Facile etching fabrication of superhydrophobic 7055 aluminum alloy surface towards chloride environment anticorrosion. Corros. Sci. 2021, 182, 109262. [Google Scholar] [CrossRef]
- Fu, H.; Chen, C.-Y.; Li, J.-Q.; Lan, Y.-P.; Zhang, X.-Q.; Yuan, J.-J. Effect of Time on the Corrosion of Q345 Steel in Thiosulfate Sodium Aluminate Solution. Surf. Technol. 2018, 47, 166–172. [Google Scholar]
- Zou, J.Y.; Chin, D.T. Anodic Behavior Of Carbon-Steel In Concentrated Naoh Solutions. Electrochim. Acta 1988, 33, 477–485. [Google Scholar] [CrossRef]
- Vladimirov, M.G.; Ryzhkov, Y.F.; Alekseev, V.A.; Bogdanovskaya, V.A.; Otroshchenko, V.A. Electrochemical reduction of carbon dioxide on pyrite as a pathway for abiogenic formation of organic molecules. Orig. Life Evol. Biosph. 2004, 34, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Crowe, D.C. The High Temperature Electrochemical Behavior Of Carbon Steel In Alkaline Sulfide Solutions. Ph.D. Thesis, The University Of British Columbia, Vancouver, BC, Canada, 1985. [Google Scholar] [CrossRef]


| No | NaOH/g·L−1 | Al2O3/g·L−1 | S2−/g·L−1 | S2O32−/g·L−1 |
|---|---|---|---|---|
| 1 | 255 | 110 | 5 | 3 |
| 2 | 110 | 90 | 3 | 3 |
| 3 | 110 | 90 | 3 | 4 |
| 4 | 110 | 90 | 3 | 6 |
| Concentration of Sulfide/(g∙L−1) | Rate Equation/(mm·a−1) | R2 |
| 3 g·L−1 S2− and 3 g·L−1 S2O32− | 0.933 | |
| 3 g·L−1 S2− and 4 g·L−1 S2O32− | 0.972 | |
| 5 g·L−1 S2− and 3 g·L−1 S2O32− | 0.985 | |
| 3 g·L−1 S2− and 6 g·L−1 S2O32− | 0.882 |
| Corrosion Time /d | Ecorr /V | Icorr /uA·cm−2 | βa /mV | βc /mV | Rp /Ω·cm2 | Corrosion Rate /mm·a−1 |
|---|---|---|---|---|---|---|
| 1 | −1.12 | 983.40 | 309.00 | 209.88 | 26.53 | 11.57 |
| 2 | −1.11 | 315.71 | 153.26 | 128.17 | 90.06 | 3.41 |
| 3 | −1.24 | 350.28 | 127.16 | 118.50 | 75.96 | 4.12 |
| 4 | −1.13 | 491.31 | 217.61 | 170.76 | 53.10 | 5.78 |
| 5 | −1.28 | 443.31 | 143.23 | 107.22 | 58.84 | 5.21 |
| Impedance Parameters | Corrosion Time/d | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Rs/ Ω·cm2 | 1.42 | 2.38 | 1.27 | 0.98 | 1.22 |
| Rct/ Ω·cm2 | 369.4 | 190.9 | 583.4 | 1384.0 | 1567.0 |
| CPEdl(Y0)/Ω−1·cm−2·sn | 0.07 | 0.01 | 0.08 | 0.25 | 0.48 |
| n1 | 0.79 | 0.55 | 0.96 | 0.95 | 0.89 |
| Rf/Ω·cm2 | 79.21 | 31.48 | 30.05 | 0.39 | 0.09 |
| CPEf(Y0)/Ω−1·cm−2·sn | 0.06 | 0.004 | 0.002 | 0.00003 | 0.02 |
| n2 | 0.85 | 0.81 | 0.85 | 0.98 | 0.92 |
| WR/Ω·cm2 | – | – | 428.50 | – | – |
| WT | – | – | 132.50 | – | – |
| WP | – | – | 0.39 | – | – |
| X2 (10−4) | 4.27 | 9.15 | 8.31 | 3.38 | 2.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, B.; Li, J.; Chen, C. Effect of Corrosion Time on the Synergistic Corrosion of Q235 Steel in Sodium Aluminate Solutions. Metals 2021, 11, 753. https://doi.org/10.3390/met11050753
Quan B, Li J, Chen C. Effect of Corrosion Time on the Synergistic Corrosion of Q235 Steel in Sodium Aluminate Solutions. Metals. 2021; 11(5):753. https://doi.org/10.3390/met11050753
Chicago/Turabian StyleQuan, Bianli, Junqi Li, and Chaoyi Chen. 2021. "Effect of Corrosion Time on the Synergistic Corrosion of Q235 Steel in Sodium Aluminate Solutions" Metals 11, no. 5: 753. https://doi.org/10.3390/met11050753





