Preparation and Characterization of a Sol–Gel AHEC Pore-Sealing Film Prepared on Micro Arc Oxidized AZ31 Magnesium Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Micro-Arc Oxidation (MAO) Coating Preparation
2.3. Aminated Hydroxyethyl Cellulose (AHEC) Coating Preparation
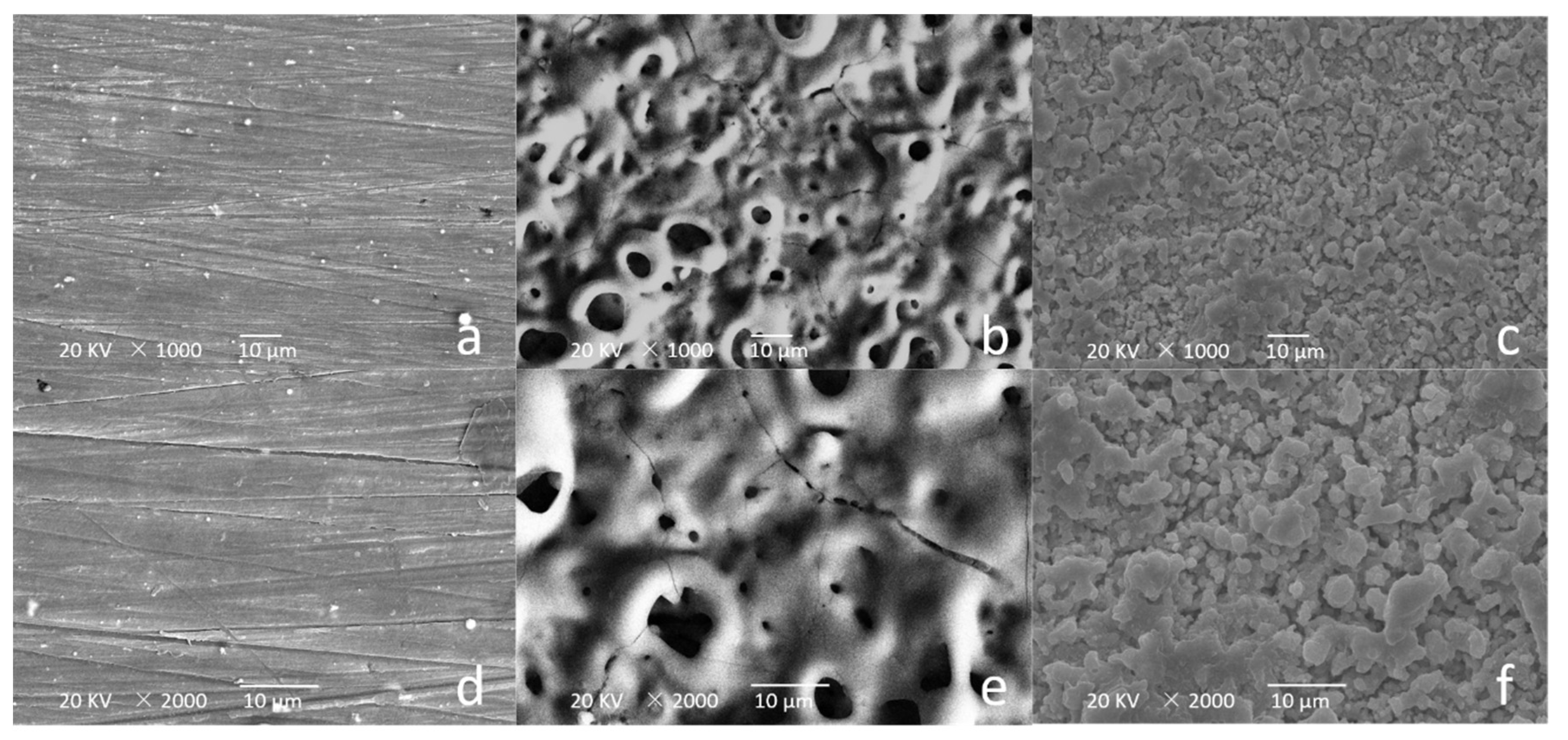
2.4. Surface Characterization
2.5. Corrosion Resistance Evaluation
2.5.1. Tafel Test
2.5.2. Hydrogen Evolution Experiment
2.5.3. Long-Term Immersion Experiment
2.6. Biocompatibility Evaluation
Cytotoxicity Test
3. Results and Discussion
3.1. Sample Characterization
3.2. Corrosion Resistance Analysis
3.2.1. Electrochemical Polarization Curve
3.2.2. Hydrogen Evolution Experiment
3.2.3. Long-Term Immersion Experiment
3.3. Cytotoxicity Test
3.3.1. CCK-8 Test
3.3.2. Observation of Cell Morphology
4. Conclusions
- Compared to the pure AZ31 magnesium alloy, the corrosion resistances of the MAO + AZ31 magnesium alloy and the AHEC + MAO + AZ31 magnesium alloy were significantly improved. The hydrogen evolution experiment and long-term immersion experiment showed that the corrosion resistance of the AHEC + MAO + AZ31 magnesium alloy was better than that of the MAO + AZ31 magnesium alloy.
- CCK-8 and cell morphology observations showed that the extract of the AZ31 magnesium alloy had no toxic effect on the growth of MC3T3-E1 cells. The biocompatibility of the MAO + AZ31 magnesium alloy could be improved by the AHEC coating.
- In summary, the application of the AHEC to seal the MAO coating could significantly improve the corrosion resistance and biocompatibility of the MAO AZ31 magnesium alloy, and thus, this material has potential application value as a medical implant material.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jung, H.D.; Jang, T.S.; Wang, L.; Kim, H.E.; Koh, Y.H.; Song, J. Novel strategy for mechanically tunable and bioactive metal implants. Biomaterials 2015, 37, 49–61. [Google Scholar] [CrossRef]
- Gawkrodger, D.J. Metal sensitivities and orthopaedic implants revisited: The potential for metal allergy with the new metal-on-metal joint prostheses. Br. J. Dermatol. 2015, 148, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Schell, H.; Zimpfer, E.; Schmidt-Bleek, K.; Jung, T.; Duda, G.N.; Ryd, L. Treatment of osteochondral defects: Chondrointegration of metal implants improves after hydroxyapatite coating. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 3575–3582. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.; Fockaert, L.; Pouran, B.; Tümer, N.; Schröder, K.-U.; Mol, J.; Weinans, H.; et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef]
- Wong, H.M.; Yeung, K.W.K.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.K.; Cheung, K.M.C. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.; Treichler, S.; Martinelli, E.; Weinberg, A.M. The influence of biodegradable magnesium implants on the growth plate. Acta Biomater. 2018, 66, 109–117. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, R.; Yongdong, X.; Song, P.; Xinbing, Z.; Ying, Z. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 1–15. [Google Scholar]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- He, R.; Liu, R.; Chen, Q.; Zhang, H.; Wang, J.; Guo, S. In vitro degradation behavior and cytocompatibility of Mg-6Zn-Mn alloy. Mater. Lett. 2018, 228, 77–80. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Song, G.L.; Song, S. A Possible Biodegradable Magnesium Implant Material. Adv. Eng. Mater. 2010, 9, 298–302. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, R.M.; Kuntal, K.K.; Gupta, P.; Das, S.; Jayaganthan, R.; Roy, P.; Lahiri, D. Sol–Gel Derived Hydroxyapatite Coating on Mg-3Zn Alloy for Orthopedic Application. JOM 2015, 67, 702–712. [Google Scholar] [CrossRef]
- Zartner, P.; Cesnjevar, R.; Singer, H.; Weyand, M. First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby. Catheter. Cardiovasc. Interv. 2010, 66, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.K.; Vince, K.; Hodgson, M.A. Biodegradable surgical implants based on magnesium alloys—A review of current research. Iop Conf. 2009, 4, 012011. [Google Scholar] [CrossRef]
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants—A Review. BioMetals 2019, 32, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Fischerauer, S.F.; Kraus, T.; Wu, X.; Tangl, S.; Sorantin, E.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J.; Weinberg, A.M. In vivo degradation performance of micro-arc-oxidized magnesium implants: A micro-CT study in rats. Acta Biomater. 2013, 9, 5411–5420. [Google Scholar] [CrossRef]
- Wan, P.; Tan, L.; Yang, K.; Metal, I.O.; Sciences, C.A.O. Surface Modification on Biodegradable Magnesium Alloys as Orthopedic Implant Materials to Improve the Bio-adaptability:A Review. J. Mater. Sci. Technol. 2016, 32, 827–834. [Google Scholar] [CrossRef]
- Editor’s Comment on: The history of biodegradable magnesium implants: A review. Acta Biomater. 2015, 23, S27. [CrossRef]
- Waizy, H.; Diekmann, J.; Weizbauer, A.; Reifenrath, J.; Bartsch, I.; Neubert, V.; Schavan, R.; Windhagen, H. In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to 12 months. J. Biomater. Appl. 2013, 28, 667–675. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G. Anodizing Treatments for Magnesium Alloys and Their Effect on Corrosion Resistance in Various Environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Gao, S.-D.; Li, P.-P.; Zeng, R.-C.; Zhang, F.; Li, S.-Q.; Han, E.-H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Dong, K.; Song, Y.; Shan, D.; Han, E.-H. Corrosion behavior of a self-sealing pore micro-arc oxidation film on AM60 magnesium alloy. Corros. Sci. 2015, 100, 275–283. [Google Scholar] [CrossRef]
- Satyanarayana, S.; Busch, E. A novel technique for sealing porous dielectrics. Solid State Technol. 2005, 48, 24–26. [Google Scholar]
- Shi, P.; Ng, W.F.; Wong, M.H.; Cheng, F.T. Improvement of corrosion resistance of pure magnesium in Hanks’ solution by microarc oxidation with sol–gel TiO2 sealing. J. Alloy. Compd. 2009, 469, 286–292. [Google Scholar] [CrossRef]
- Dou, J.; Yu, H.; Chen, C. Preparation and characterization of composite coating on Mg-1.74Zn-0.55Ca alloy by micro-arc oxidation combined with sol-gel method. Mater. Lett. 2019, 255, 126578. [Google Scholar] [CrossRef]
- Xia, W.; Li, N.; Deng, B.; Zheng, R.; Chen, Y. Corrosion behavior of a sol-gel ZrO2 pore-sealing film prepared on a micro-arc oxidized aluminum alloy. Ceram. Int. 2019, 45, 11062–11067. [Google Scholar] [CrossRef]
- Hidayat, B.J.; Felby, C.; Johansen, K.S.; Thygesen, L.G. Cellulose is not just cellulose: A review of dislocations as reactive sites in the enzymatic hydrolysis of cellulose microfibrils. Cellulose 2012, 19, 1481–1493. [Google Scholar] [CrossRef]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, J.; Songfeng, E.; Li, J.; Zhang, M. All cellulose composites prepared by hydroxyethyl cellulose and cellulose nanocrystals through the crosslink of polyisocyanate. Carbohydr. Polym. 2020, 250, 116919. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material (p3358–3393). Angew. Chem. Int. Ed. 2010, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Liesiene, J.; Kazlauske, J. Functionalization of cellulose: Synthesis of water-soluble cationic cellulose derivatives. Cellul. Chem. Technol. 2013, 47, 515–525. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Jabbarzare, S.; Iqbal, N.; Kadir, M.R.A. Deposition of nanostructured fluorine-doped hydroxyapatite-polycaprolactone duplex coating to enhance the mechanical properties and corrosion resistance of Mg alloy for biomedical applications. Mater. Eng. C Mater. Biol. Appl. 2016, 60, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Han, X.G.; Zhu, F.; Zhu, X.P.; Lei, M.K.; Xu, J.J. Electrochemical corrosion behavior of modified MAO film on magnesium alloy AZ31 irradiated by high-intensity pulsed ion beam. Surface Coat. Technol. 2013, 228, S164–S170. [Google Scholar] [CrossRef]
- Liu, S.; Qi, Y.; Peng, Z.; Liang, J. A chemical-free sealing method for Micro-arc oxidation coatings on AZ31 Mg alloy. Surf. Coat. Technol. 2020, 406, 126655. [Google Scholar] [CrossRef]
- Shang, W.; Chen, B.; Shi, X.; Chen, Y.; Xiao, X. Electrochemical corrosion behavior of composite MAO/sol–gel coatings on magnesium alloy AZ91D using combined micro-arc oxidation and sol–gel technique. J. Alloys Compd. 2009, 474, 541–545. [Google Scholar] [CrossRef]
- Li, N.; Chen, Y.; Deng, B.; Yue, J.; Qu, W.; Yang, H.; He, Y.; Xia, W.; Li, L. Low temperature UV assisted sol-gel preparation of ZrO 2 pore-sealing films on micro-arc oxidized magnesium alloy AZ91D and their electrochemical corrosion behaviors. J. Alloys Compd. 2019, 792, 1036–1044. [Google Scholar] [CrossRef]
- Tang, M.; Liu, H.; Li, W.; Zhu, L. Effect of zirconia sol in electrolyte on the characteristics of microarc oxidation coating on AZ91D magnesium. Mater. Lett. 2011, 65, 413–415. [Google Scholar] [CrossRef]
- Wang, S.; Fu, L.; Nai, Z.; Liang, J.; Cao, B. Comparison of Corrosion Resistance and Cytocompatibility of MgO and ZrO2 Coatings on AZ31 Magnesium Alloy Formed via Plasma Electrolytic Oxidation. Coatings 2018, 8, 441. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, Y.; Sun, J.; Yang, L.; Guo, C.; Liang, J.; Cao, B. Preparation and Characterization of Aminated Hydroxyethyl Cellulose-Induced Biomimetic Hydroxyapatite Coatings on the AZ31 Magnesium Alloy. Metals 2017, 7, 214. [Google Scholar] [CrossRef]
- Guo, H.; An, M. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate–fluoride solutions and evaluation of corrosion resistance. Appl. Surf. Sci. 2005, 246, 229–238. [Google Scholar] [CrossRef]
- Fu, L.; Yang, Y.; Zhang, L.; Wu, Y.; Cao, B. Preparation and Characterization of Fluoride-Incorporated Plasma Electrolytic Oxidation Coatings on the AZ31 Magnesium Alloy. Coatings 2019, 9, 826. [Google Scholar] [CrossRef]









| Samples | MAO | AHEC + MAO |
|---|---|---|
| Thickness (μm) | 23.52 ± 1.42 | 27.83 ± 0.93 |
| Wt% | C | N | 0 | F | Na | P | K |
|---|---|---|---|---|---|---|---|
| MAO + AZ31 | 15 | 3 | 33 | 24 | 7 | 16 | 2 |
| AHEC + MAO + AZ31 | 48 | 5 | 38 | 1 | 4 | 3 | 1 |
| Samples | Ecorr (V vs. Ag/AgCl) | Icorr (A/cm2) |
|---|---|---|
| AZ31 | −1.526 ± 0.002 | 1.557 ± 0.001 × 10−6 |
| MAO + AZ31 | −1.405 ± 0.005 * | 1.121 ± 0.003 × 10−9 * |
| AHEC + MAO + AZ31 | −1.072 ± 0.004 *# | 2.401 ± 0.004 × 10−10 *# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wu, Y.; Zeng, T.; Wei, Y.; Zhang, G.; Liang, J.; Cao, B. Preparation and Characterization of a Sol–Gel AHEC Pore-Sealing Film Prepared on Micro Arc Oxidized AZ31 Magnesium Alloy. Metals 2021, 11, 784. https://doi.org/10.3390/met11050784
Zhang L, Wu Y, Zeng T, Wei Y, Zhang G, Liang J, Cao B. Preparation and Characterization of a Sol–Gel AHEC Pore-Sealing Film Prepared on Micro Arc Oxidized AZ31 Magnesium Alloy. Metals. 2021; 11(5):784. https://doi.org/10.3390/met11050784
Chicago/Turabian StyleZhang, Longlong, Yuanzhi Wu, Tian Zeng, Yu Wei, Guorui Zhang, Jun Liang, and Baocheng Cao. 2021. "Preparation and Characterization of a Sol–Gel AHEC Pore-Sealing Film Prepared on Micro Arc Oxidized AZ31 Magnesium Alloy" Metals 11, no. 5: 784. https://doi.org/10.3390/met11050784
APA StyleZhang, L., Wu, Y., Zeng, T., Wei, Y., Zhang, G., Liang, J., & Cao, B. (2021). Preparation and Characterization of a Sol–Gel AHEC Pore-Sealing Film Prepared on Micro Arc Oxidized AZ31 Magnesium Alloy. Metals, 11(5), 784. https://doi.org/10.3390/met11050784






