Effect of Gas Bubbling Filtration Treatment Conditions on Melt Quality of AlSiMgCu Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Melt Quality of Al-Si-Mg-Cu Alloy after GBF Treatment
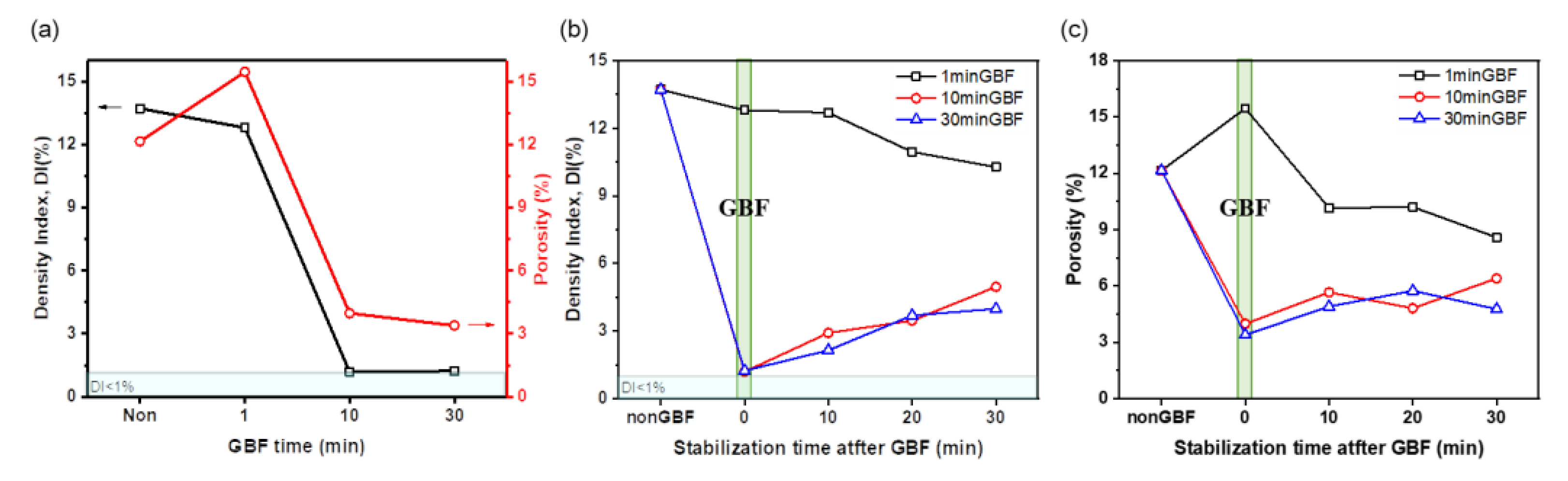
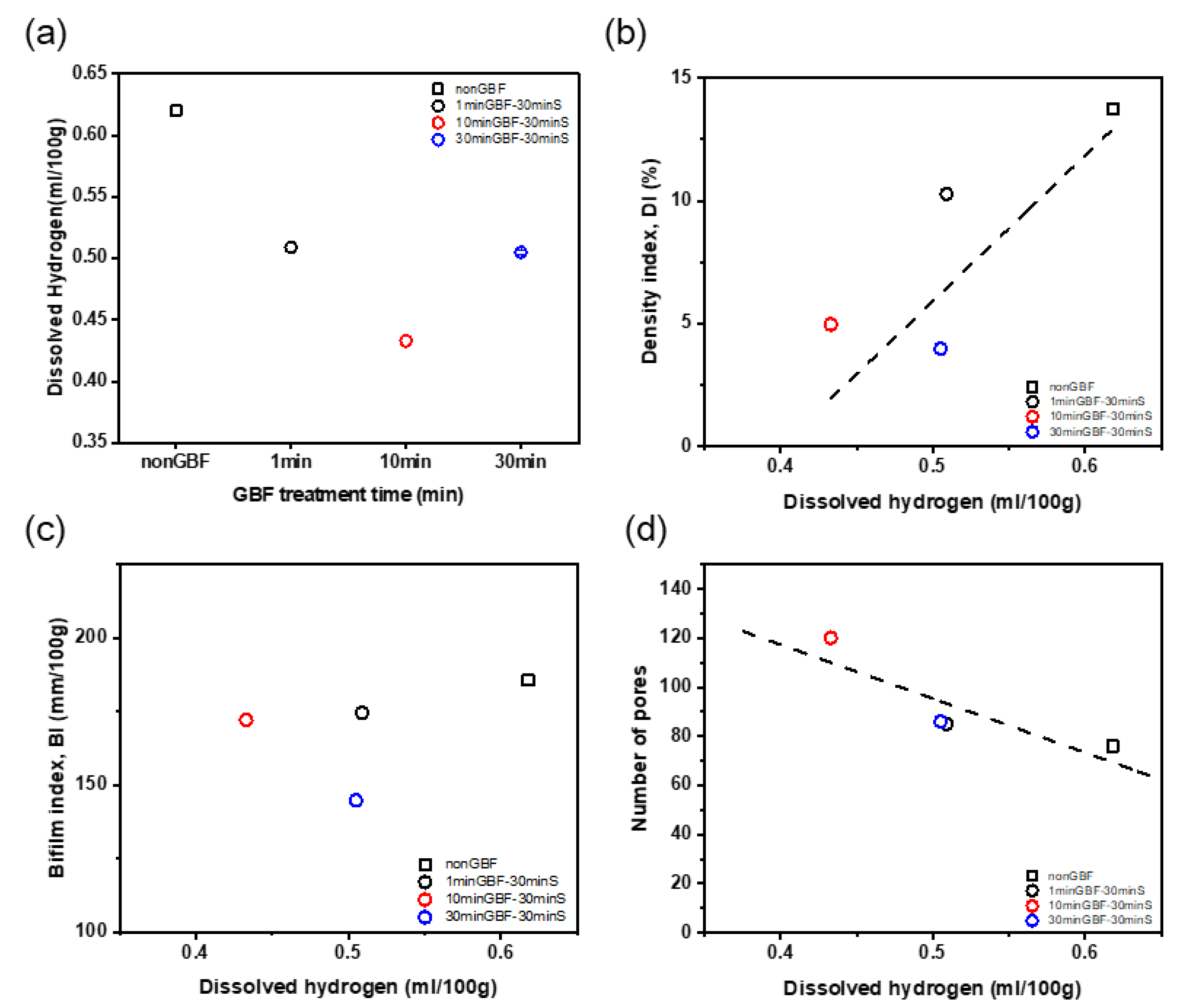
3.1.1. Effects of GBF Conditions on Density Index and Porosity
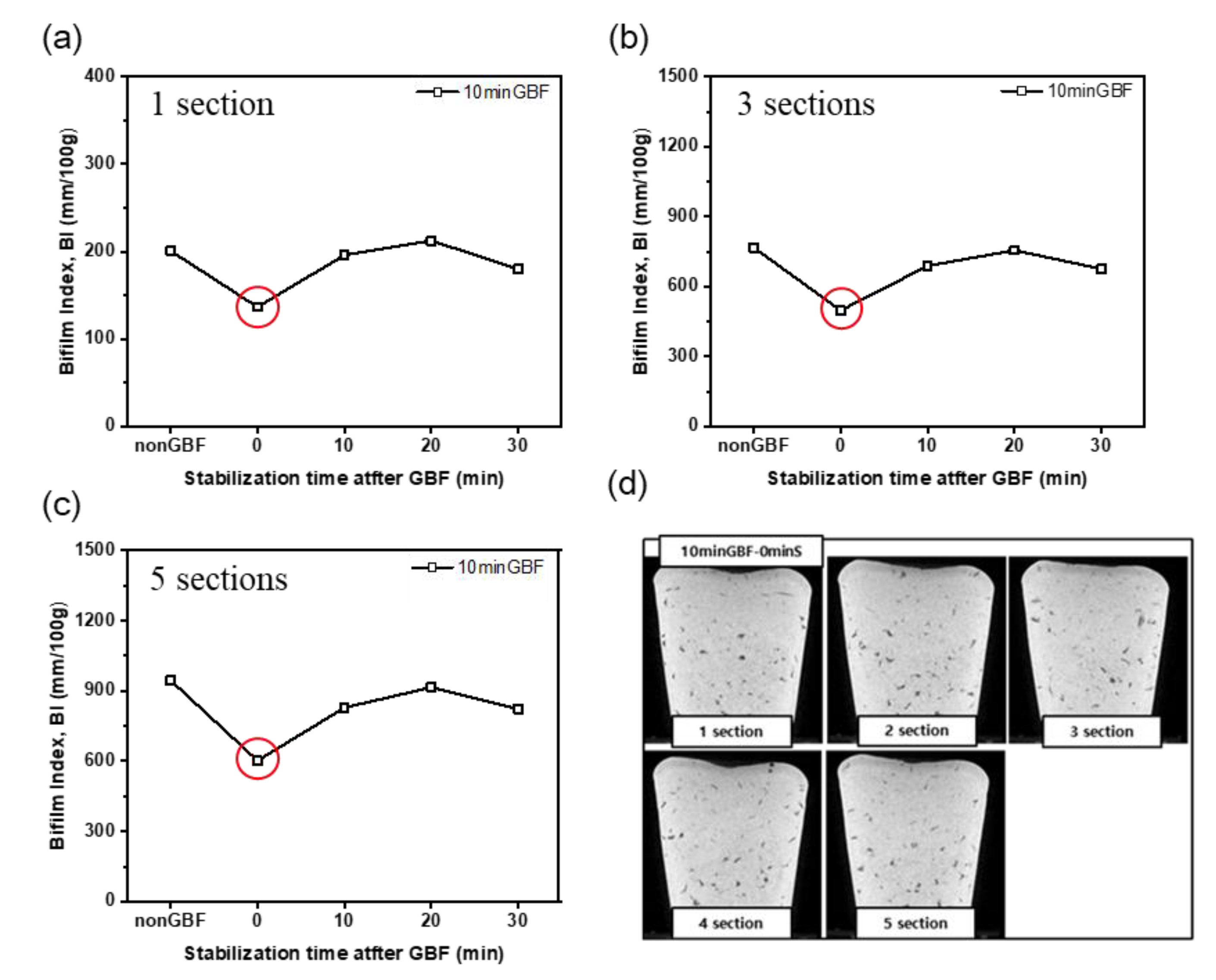
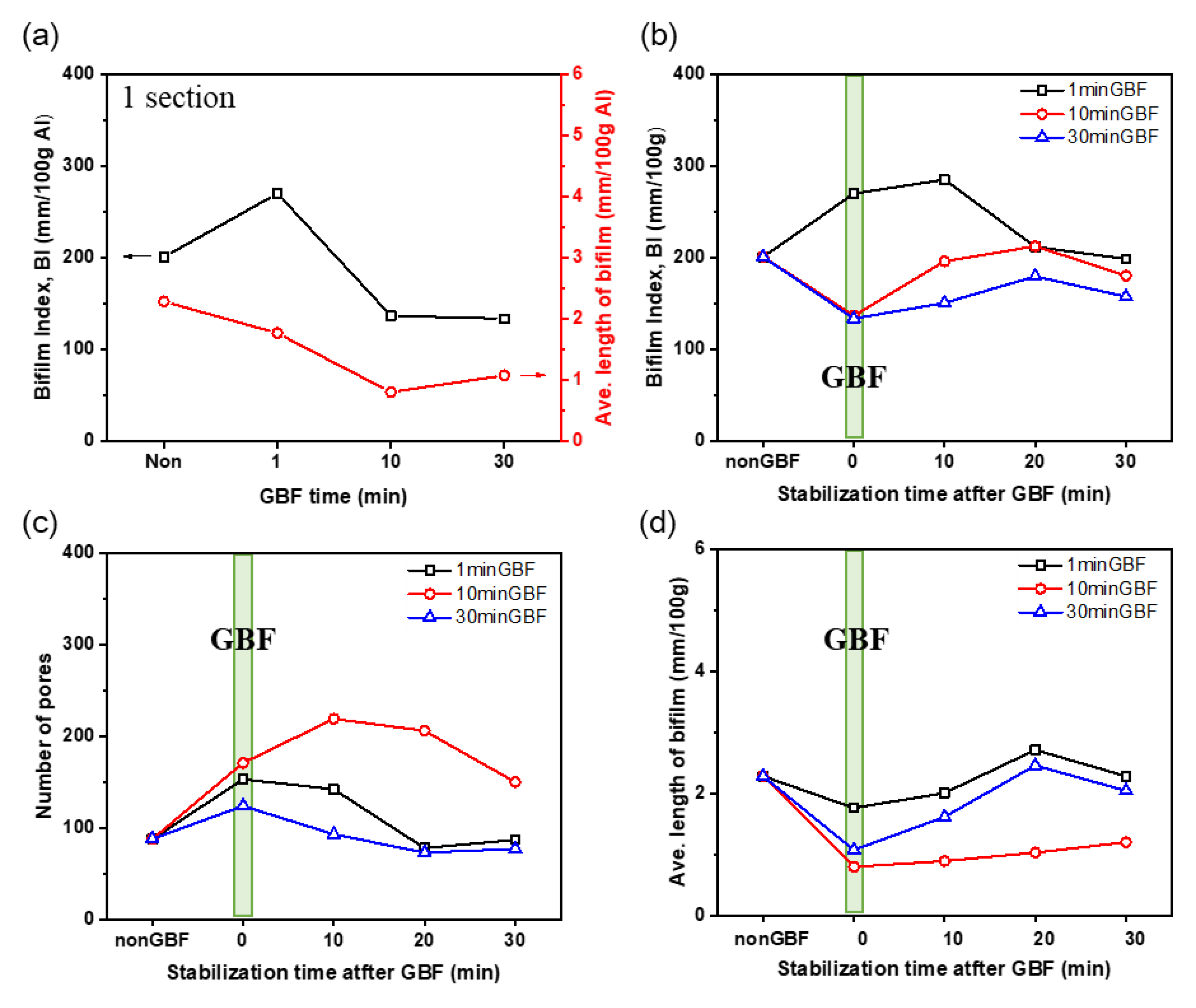
3.1.2. Effects of GBF Conditions on Bifilm Index
3.1.3. Effects of GBF Conditions on Dissolved Hydrogen Content
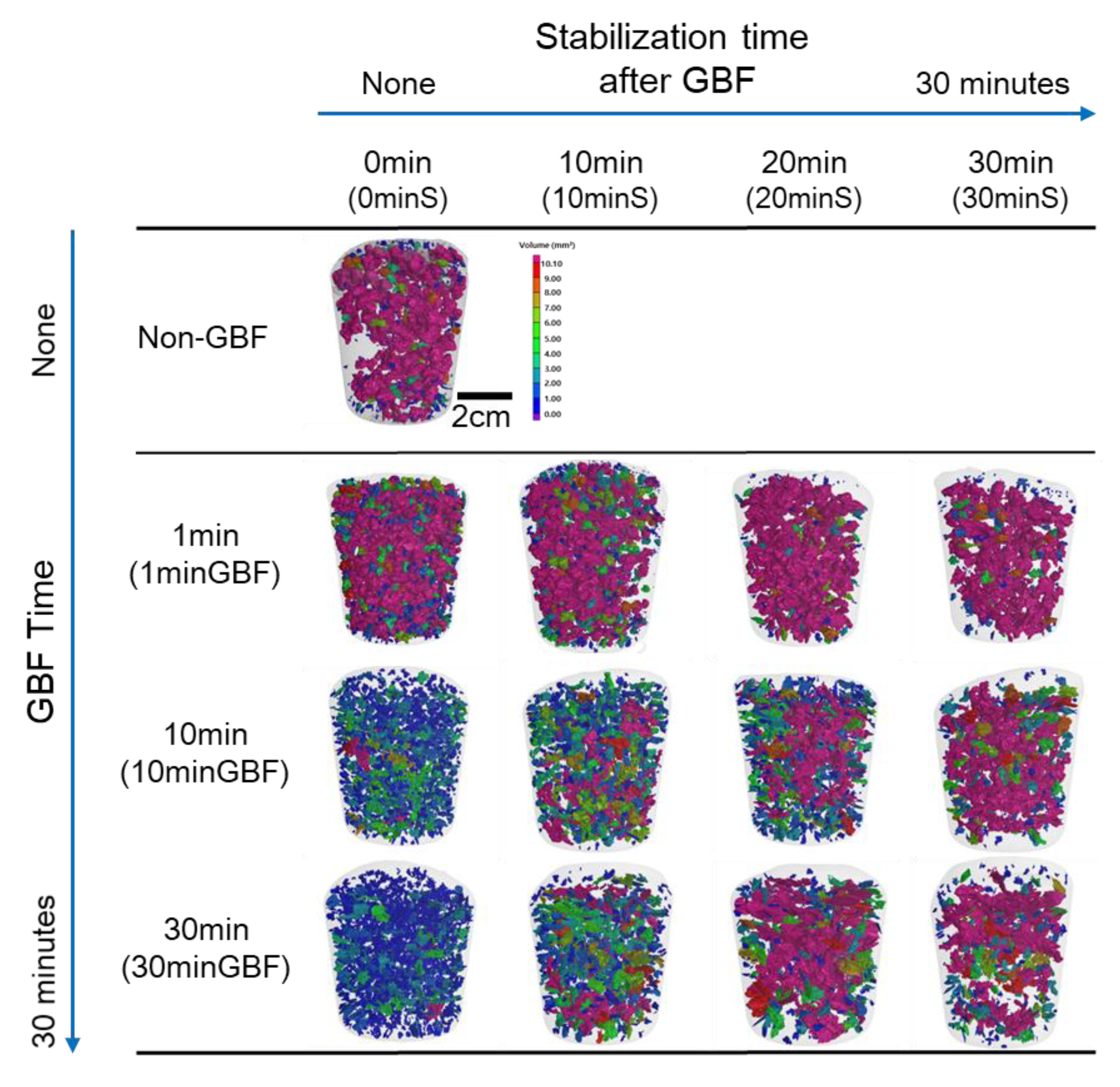
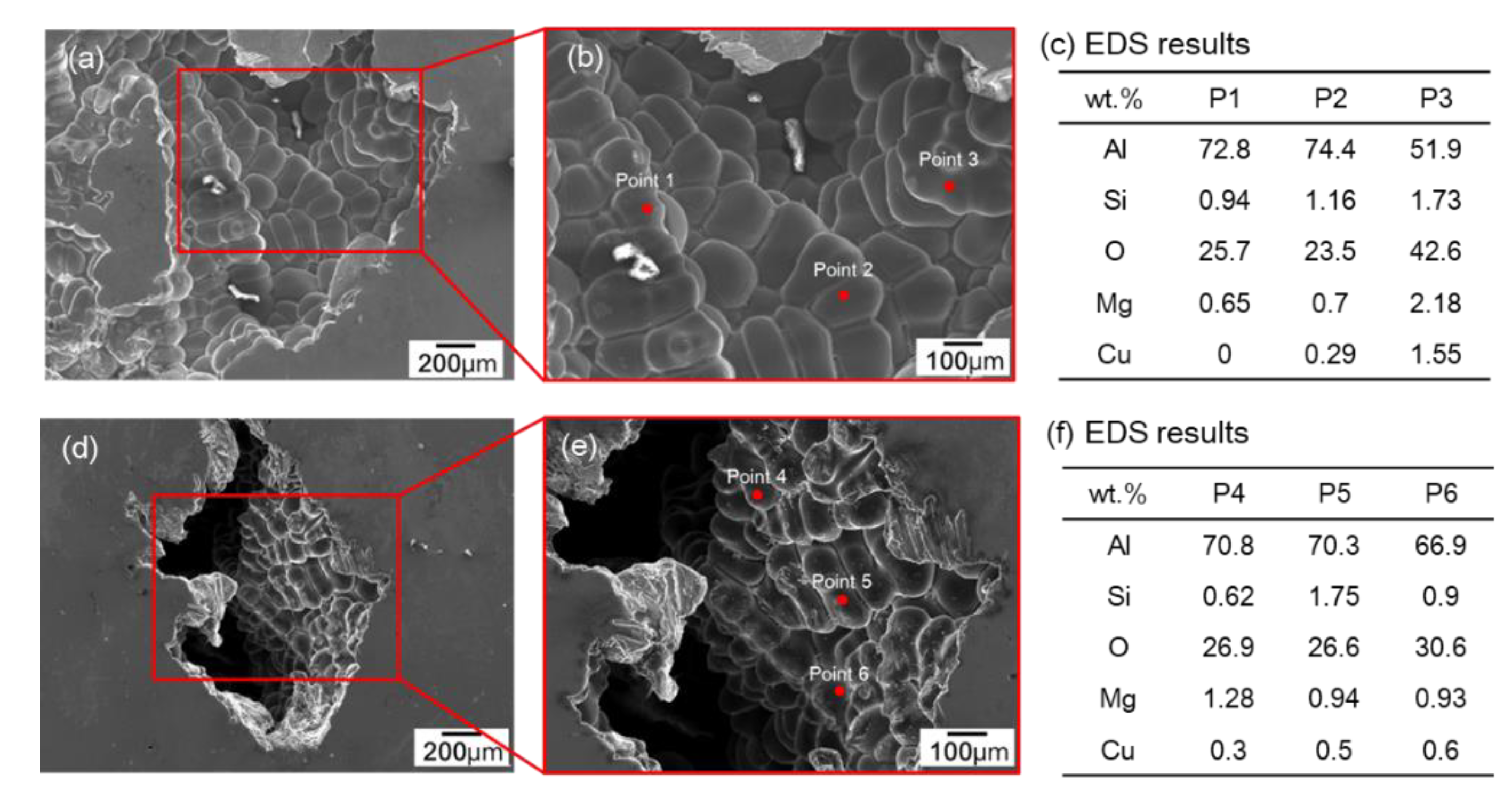
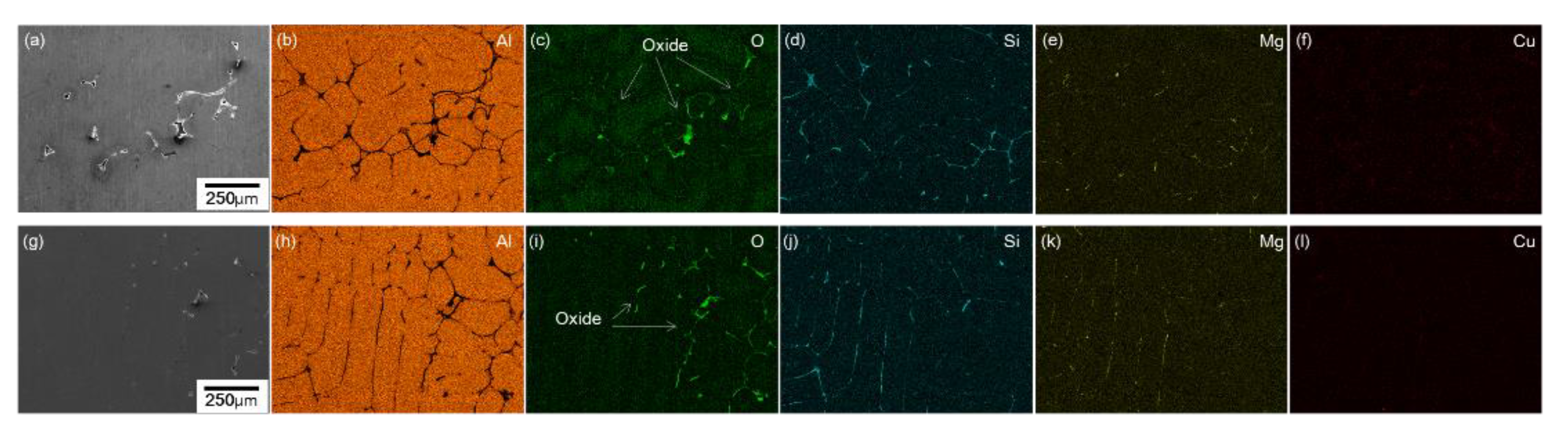
3.2. Analysis of Pores in RPT Samples by 3D X-ray CT
3.2.1. Effect of GBF Conditions on Pore Shape in RPT Samples
3.2.2. Melt Quality Parameter Comparison with the Volume of the Largest Pore
3.3. Optimization Study of GBF Time and Stabilization Time
4. Conclusions
- The molten metal quality after GBF treatment had melt quality that met the industry standard (DI< 1%), but the melt quality decreased with increase in stabilization time to 30 min, possibly because hydrogen re-dissolved during the stabilization holding time from the newly exposed surface generated after GBF treatment.
- As GBF time increased to 30 min, i.e., the amount of injected gas increased to 3 L/kg, the quality of the molten metal of Al-Si-Mg-Cu alloy improved, but the improvements of cleanliness of molten metal were similar after GBF = 10 min and 30 min.
- The shape of the pore with the largest volume by CT analysis in RPT samples can identify the different types of pores by gas, inclusion, and shrinkage. The RPT sample of Al-Si-Mg-Cu alloy without GBF had the clusters consisted of the spherical pores, which was mainly affected by the gas. After GBF treatment for 10 or 30 min, the RPT sample of Al-Si-Mg-Cu alloy had small clusters, which were formed by shrinkage. The defects became long and irregularly shaped after the stabilization of 30 min. The trend toward pore shape according to the GBF treatment conditions is generally consistent with the DI results of the samples.
- The pores in the RPT sample of Al-Si-Mg-Cu alloy were clusters, so the volume of the largest pore and shape of the largest pores with DI can be used to represent the cleanliness of the molten Al-Si-Mg-Cu alloy.
- The volume of the largest pore in RPT samples of Al-Si-Mg-Cu alloy showed a relatively strong linear relationship with the melt quality parameters of density, DI, and BI. These trends may occur because the melt quality parameters in the RPT sample are affected by cluster formation.
- These results recommend that the melt of Al-Si-Mg-Cu alloy should be treated by applying GBF for 10 min with gas flow >1 L/kg to satisfy DI < 1% for high-quality melt in the foundry industry.
- Stabilization time should be as short as possible (<30 min) after GBF treatment to maintain high molten metal cleanliness.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, X.; Campbell, J. Oxide inclusion defects in Al-Si-Mg cast alloys. Can. Metall. Q. 2005, 44, 435–448. [Google Scholar] [CrossRef]
- ASM Handbook Volume 15 Casting; ASM International: Russell Township, OH, USA, 2008; ISBN 978-0-87170-711-6.
- Jiang, G.R.; Li, Y.X.; Liu, Y. Calculation of hydrogen solubility in molten alloys. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2011, 21, 1130–1135. [Google Scholar] [CrossRef]
- Wray, P.J. Predicted volume change behavior accompanying the solidification of binary alloys. Metall. Trans. B 1976, 7, 639–646. [Google Scholar] [CrossRef]
- Lee, C.; So, T.; Shin, K. Effect of gas bubbling filtration treatment on microporosity variation in A356 aluminium alloy. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Martnez, J.L.; Ramírez-Argáez, M.A.; Juárez-Hernández, A.; González-Rivera, C.; Trápaga-Martnez, G. Novel degasification design for aluminum using an impeller degasification water physical model. Mater. Manuf. Process 2012, 27, 556–560. [Google Scholar] [CrossRef]
- Dispinar, D.; Akhtar, S.; Nordmark, A.; Di Sabatino, M.; Arnberg, L. Degassing, hydrogen and porosity phenomena in A356. Mater. Sci. Eng. A 2010, 527, 3719–3725. [Google Scholar] [CrossRef]
- Gyarmati, G.; Fegyverneki, G.; Tokár, M.; Mende, T. The Effects of Rotary Degassing Treatments on the Melt Quality of an Al-Si Casting Alloy. Int. J. Met. 2021, 15, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Qu, Z.K.; Sun, B.; Shu, D. Effects of spray degassing parameters on hydrogen content and properties of commercial purity aluminum. Mater. Sci. Eng. A 2007, 456, 386–390. [Google Scholar] [CrossRef]
- Ray, S.; Milligan, B.; Keegan, N.; Falcon, I. Measurement of Filtration Performance, Filtration Theory and Practical Applications of Ceramic Foam Filters. In Proceedings of the 9th Australasian Conference and Exhibition on Aluminium Cast House Technology, Melbourne, Australia, 12–15 September 2005; pp. 249–258. [Google Scholar]
- Damoah, L.N.W.; Zhang, L. Removal of inclusions from aluminum through filtration. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2010, 41, 886–907. [Google Scholar] [CrossRef]
- Taylor, M.B. Molten Metal Fluxing/Treatment: How Best To Achieve the Desired Quality Requirements. Aluminium 2003, 79, 44–50. [Google Scholar]
- Meidani, A.R.N.; Hasan, M. A study of hydrogen bubble growth during ultrasonic degassing of Al-Cu alloy melts. J. Mater. Process. Technol. 2004, 147, 311–320. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, X.; Torgerson, A.T.; Long, M. Removal of impurity elements from molten aluminum: A review. Miner. Process. Extr. Metall. Rev. 2011, 32, 150–228. [Google Scholar] [CrossRef]
- Hsi, R.; Tay, M.; Bukur, D.; Tatterson, G.; Morrison, G. Sound spectra of gas dispersion in an agitated tank. Chem. Eng. J. 1985, 31, 153–161. [Google Scholar] [CrossRef]
- Zhao, J.C.; Chen, J. Gas line pressure fluctuation analysis of a gas-liquid reactor. J. Therm. Sci. 2005, 14, 267–271. [Google Scholar]
- Warmoeskerken, M.M.C.G.; Smith, J.M. Flooding of disc turbines in gas-liquid dispersions: A new description of the phenomenon. Chem. Eng. Sci. 1985, 40, 2063–2071. [Google Scholar] [CrossRef]
- Saternus, M.; Merder, T. Physical modelling of aluminum refining process conducted in batch reactor with rotary impeller. Metals 2018, 8, 726. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Martínez, J.L.; Ramírez-Argáez, M.A.; Zenit-Camacho, R.; Juárez-Hernández, A.; Barceinas-Sánchez, J.D.O.; Trápaga-Martinez, G. Physical modelling of an aluminium degassing operation with rotating impellersa comparative hydrodynamic analysis. Mater. Manuf. Process. 2010, 25, 581–591. [Google Scholar] [CrossRef]
- Odenthal, H.J.; Bölling, R.; Pfeifer, H. Numerical and physical simulation of Tundish fluid flow phenomena. Steel Res. 2003, 74, 44–45. [Google Scholar] [CrossRef]
- Lee, C.; So, T.; Shin, K. Defect Susceptibility of Tensile Properties to Microporosity Variation in High-Pressure Die-Cast Aluminium Alloy Controlled by Gas Bubbling Flotation Treatment. Inter. J. Metalcast. 2019, 13, 880–889. [Google Scholar] [CrossRef]
- Lordan, E.; Lazaro-Nebreda, J.; Zhang, Y.; Fan, Z. Effective Degassing for Reduced Variability in High-Pressure Die Casting Performance. JOM 2019, 71, 824–830. [Google Scholar] [CrossRef]
- Hao, C.; Li, D.; Zeng, J. Research on porous sprayer for refining of aluminium melt. Adv. Mater. Res. 2012, 418–420, 1856–1859. [Google Scholar] [CrossRef]
- Li, Q.; He, K.; Wu, N.; Zeng, J. Purification of aluminum melt in crucibles by bubble flotation. Procedia Manuf. 2019, 37, 438–442. [Google Scholar] [CrossRef]
- Galarraga, H.; de Cortazar, M.G.; Arregi, E.; Artola, A.; Oncala, J.L.; Merchan, M. Gas blowing ultrasonic aluminium degassing assessment with the reduced pressure test (RPT) method. Arch. Foundry Eng. 2020, 20, 111–117. [Google Scholar]
- Mitrasinovic, A.; Robles Hernández, F.C.; Djurdjevic, M.; Sokolowski, J.H. On-line prediction of the melt hydrogen and casting porosity level in 319 aluminum alloy using thermal analysis. Mater. Sci. Eng. A 2006, 428, 41–46. [Google Scholar] [CrossRef]
- Dispinar, D.; Campbell, J. Critical assessment of reduced pressure test. Part 2: Quantification. Int. J. Cast Met. Res. 2004, 17, 287–294. [Google Scholar] [CrossRef]
- Uludağ, M.; Çetin, R.; Dispinar, D.; Tiryakioğlu, M. Characterization of the Effect of Melt Treatments on Melt Quality in Al-7wt. %Si-Mg Alloys. Metals 2017, 7, 157. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, J. Thermodynamic description of the Cu-Al-Si system in the copper-rich corner. Calphad Comput. Coupling Phase Diagrams Thermochem. 2007, 31, 449–456. [Google Scholar] [CrossRef]
- Opie, W.R.; Grant, N.J. Hydrogen Solubility in Aluminum and Some Aluminum Alloys. Trans. Metall. Soc. 1950, 188, 1237–1241. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Liu, Y. Hydrogen solubility in pure metals for Gasar process. ACTA Metall. Sin. Ed. 2007, 43, 113. [Google Scholar]
- Dispinar, D.; Campbell, J. Porosity, hydrogen and bifilm content in Al alloy castings. Mater. Sci. Eng. A 2011, 528, 3860–3865. [Google Scholar] [CrossRef]
- Chiti, F.; Paglianti, A.; Bujalski, W.; Work, E. A mechanistic model to estimate power. Chem. Eng. Res. Des. 2004, 82, 1105–1111. [Google Scholar] [CrossRef]
- Iguchi, M.; Nakamura, K.I.; Tsujino, R. Mixing Time and Fluid Flow Phenomena in Liquids of Varying Kinematic Viscosities Agitated by Bottom Gas Injection. Metall. Mater. Trans. B 1998, 29B, 569–575. [Google Scholar] [CrossRef]
- Saha, D.; Becker, J.S.; Gluns, L. A new in-line aluminum treatment system using nontoxic gases and a gas-permeable vessel bottom. In Proceedings of the Productivity and Technology in the Metallurgical Industries, Cologne, Germany, 17–22 September 1989; pp. 855–877. [Google Scholar]



| Element. | Si | Mg | Cu | Mn | Fe |
|---|---|---|---|---|---|
| wt. % | 1.3 | 0.34 | 0.27 | 0.2 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-S.; Kang, H.-J.; Lee, G.-H.; Yoon, P.-H.; Park, J.-Y.; Choi, Y.-S.; Shin, S. Effect of Gas Bubbling Filtration Treatment Conditions on Melt Quality of AlSiMgCu Alloy. Metals 2021, 11, 841. https://doi.org/10.3390/met11050841
Jang H-S, Kang H-J, Lee G-H, Yoon P-H, Park J-Y, Choi Y-S, Shin S. Effect of Gas Bubbling Filtration Treatment Conditions on Melt Quality of AlSiMgCu Alloy. Metals. 2021; 11(5):841. https://doi.org/10.3390/met11050841
Chicago/Turabian StyleJang, Ho-Sung, Ho-Jung Kang, Gyu-Heun Lee, Pil-Hwan Yoon, Jin-Young Park, Yoon-Suk Choi, and Sunmi Shin. 2021. "Effect of Gas Bubbling Filtration Treatment Conditions on Melt Quality of AlSiMgCu Alloy" Metals 11, no. 5: 841. https://doi.org/10.3390/met11050841
APA StyleJang, H.-S., Kang, H.-J., Lee, G.-H., Yoon, P.-H., Park, J.-Y., Choi, Y.-S., & Shin, S. (2021). Effect of Gas Bubbling Filtration Treatment Conditions on Melt Quality of AlSiMgCu Alloy. Metals, 11(5), 841. https://doi.org/10.3390/met11050841






