Acid Mine Drainage Dynamics from a Paste Tailing Deposit: Effect of Sulfate Content on the Consistency and Chemical Stability after Storage
Abstract
:1. Introduction
2. Materials and Methods
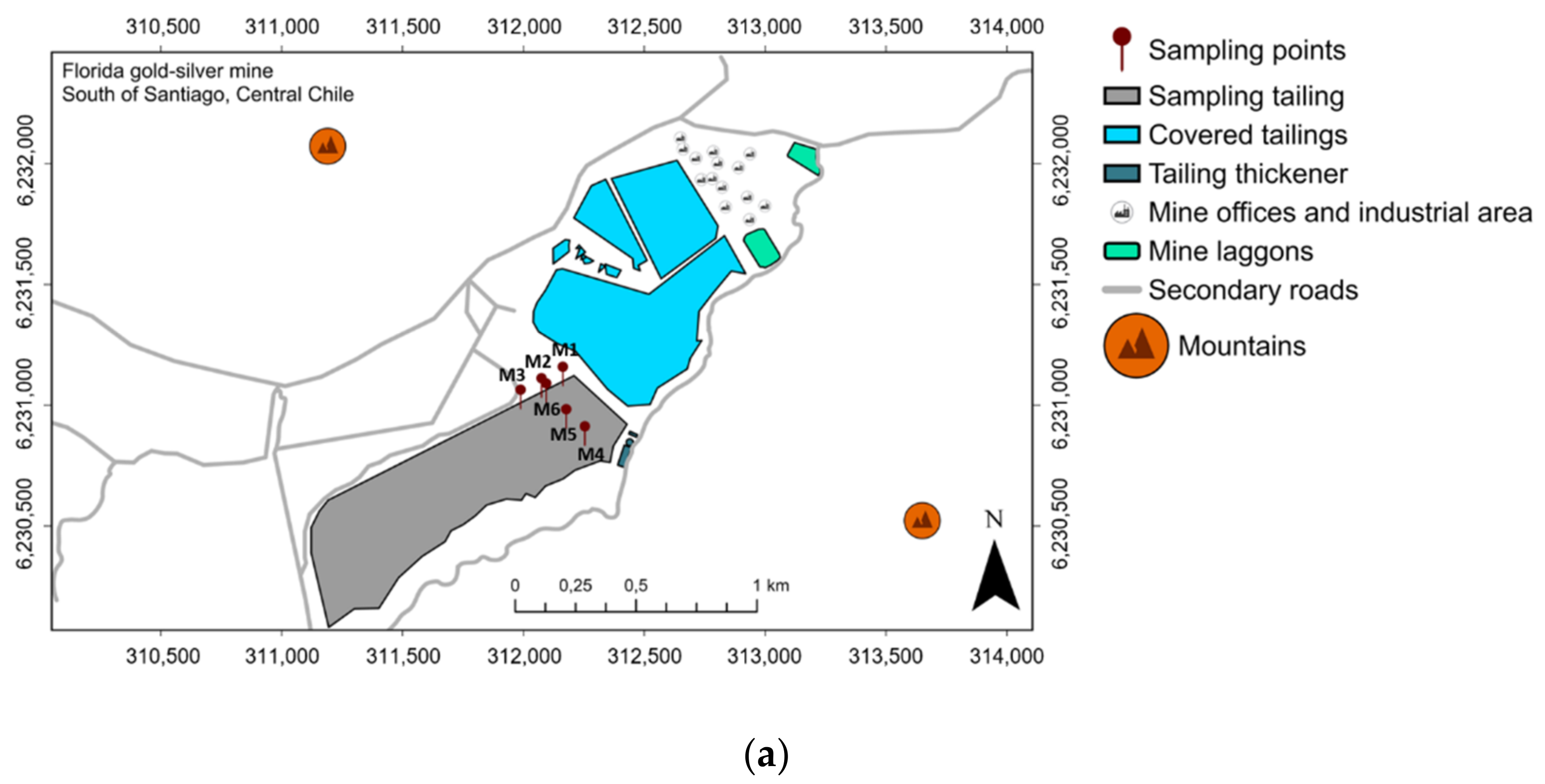
2.1. Site Description and Sampling
2.2. Tailings Analysis
2.3. Scanning Electron Microscopy
2.4. Leaching Tests: Humidity Cells
2.5. Monotonic Compression Tests
2.6. Slump Tests
2.7. Quality Assurance/Quality Control (QA/QC)
2.8. Statistical Analysis
3. Results and Discussion
3.1. General Properties of Tailings
3.2. Chemical Analysis of Tailings
3.3. Leachates Generation Rates
3.4. Effective Cohesion of the Paste-Tailing Samples
3.5. Sulfate Content Effect on the Paste Consistency and Tailings Samples Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gleisner, M. Quantification of Mineral Weathering Rates in Sulfidic Mine Tailings under Water-Saturated Conditions. Ph.D. Thesis, Institutionen för Geologi och Geokemi, Stockholms University, Stockholm, Sweden, 2005. [Google Scholar]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Crowder, J.J. Deposition, Consolidation, and Strength of a Non-Plastic Tailings Paste for Surface Disposal. Ph.D. Thesis, Department of Civil Engineering, University of Toronto, Toronto, ON, Canada, 2004. [Google Scholar]
- Newman, P.; White, R.; Cadden, A. Paste, the future of tailings disposal. In Proceedings of the 2nd International Conference on Mining and the Environment, Skelleftea, Sweden, June 25–July 1 2001; pp. 594–603. [Google Scholar]
- Cincilla, W.A.; Landriault, D.A.; Verburg, R. Application of paste technology to surface disposal of mineral wastes. In Proceedings of the Fourth International Conference on Tailings and Mine Waste; A.A. Balkema: Brookfield, The Netherlands, 1997; pp. 343–356. [Google Scholar]
- Henriquez, J.; Simms, P. Dynamic imaging and modelling of multilayer deposition of gold paste tailings. Miner. Eng. 2009, 22, 128–139. [Google Scholar] [CrossRef]
- Brackebusch, F.; Shillabeer, J. Use of paste for tailings disposal. In Proceedings of the Sixth International Symposium on Mining with Backfill, The Australasian Institute of Mining and Metallurgy, Brisbane, Australia, 14–16 April 1998; pp. 53–58. [Google Scholar]
- Newman, P.; Landriault, D. The Use of Paste Technology in the Surface Disposal of Mineral Waste; Waste Minimisation and Recycle Birmingham: Birmingham, UK, 1997.
- Meggyes, T.; Debreczeni, A. Paste technology for tailings management. Land Contam. Reclam. 2006, 14, 815. [Google Scholar] [CrossRef]
- Martin, V.; Aubertin, M.; McMullen, J. Surface disposal of paste tailings. In Proceedings of the 5th ICEG Environmental Geotechnics: Opportunities, Challenges and Responsibilities for Environmental Geotechnics; Thomas Telford Publishing: London, UK, 2006; Volume 2, pp. 1471–1478. [Google Scholar]
- Nguyen, Q.D.; Boger, D.V. Application of rheology to solving tailings disposal problems. Int. J. Miner. Process. 1998, 54, 217–233. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Belem, T.; Bussiere, B. Chemical factors that influence the performance of mine sulphidic paste backfill. Cem. Concr. Res. 2002, 32, 1133–1144. [Google Scholar] [CrossRef]
- Sofrá, F.; Boger, D.V. Environmental rheology for waste minimisation in the minerals industry. Chem. Eng. J. 2002, 86, 319–330. [Google Scholar] [CrossRef]
- Kwak, M.; James, D.F.; Klein, K.A. Flow behaviour of tailings paste for surface disposal. Int. J. Miner. Process 2005, 77, 139–153. [Google Scholar] [CrossRef]
- Pullum, L.; Graham, L.; Rudman, M.; Hamilton, R. High concentration suspension pumping. Miner. Eng. 2006, 19, 471–477. [Google Scholar] [CrossRef]
- Sofrá, F. Rheological assessment—A road map for plant designers and operators. Paste 2006. In Proceedings of the Ninth International Seminar on Paste and Thickened Tailings; Australian Centre for Geomechanics: Crawley, Autralia, 2006; pp. 13–24. [Google Scholar]
- Lamos, A.W.; Clark, I.H. The Influence of Material Composition and Sample Geometry on the Strength of Cemented Backfill. Innovation in Mining Backfill Technology; A.A. Balkema: Rotterdam, The Netherlands, 1989; pp. 89–94. [Google Scholar]
- Ouellet, J.; Bidwell, T.J.; Servant, S. Physical and mechanical characterisation of paste backfill by laboratory and in-situ testing. In Proceedings of Minefill; Australasian Institute of Mining and Metallurgy: Carlton, Australia, 1998; pp. 249–254. [Google Scholar]
- Belem, T.; Benzaazoua, M.; Bussière, B. Mechanical behaviour of cemented paste backfill. In Proceedings of the 53rd Canadian Geotechnical Conference, Montreal, QC, Canada, 15–18 October 2000. [Google Scholar]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L.; Weisener, C.G. The Geochemistry of Acid Mine Drainage. In Treatise on Geochemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2003; Volume 9, pp. 149–204. ISBN 9780080548074. [Google Scholar]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Lawrence, C.D. The influence of binder type on sulfate resistance. Cem. Concr. Res. 1992, 22, 1047–1058. [Google Scholar] [CrossRef]
- Aldhafeeri, Z.; Fall, M. Time and damage induced changes in the chemical reactivity of cemented paste backfill. J. Environ. Chem. Eng. 2016, 4, 4038–4049. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M. Modeling the effect of sulphate on strength development of paste backfill and binder mixture optimization. Cem. Concr. Res. 2005, 35, 301–314. [Google Scholar] [CrossRef]
- Fall, M.; Pokharel, M. Coupled effects of sulphate and temperature on the strength development of cemented tailings backfills: Portland cement-paste backfill. Cem. Concr. Compos. 2010, 32, 819–828. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, C.; Qi, C.; Zhang, B.; Song, K.I. A microstructural hydration model for cemented paste backfill considering internal sulfate attacks. Constr. Build. Mater. 2019, 211, 99–108. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Cui, L.; Si, Z.; Wang, H. Effect of external sulfate attack on the mechanical behavior of cemented paste backfill. Constr. Build. Mater. 2020, 263, 120968. [Google Scholar] [CrossRef]
- Cui, B.; Liu, Y.; Feng, G.; Bai, J.; Du, X.; Wang, C.; Wang, H. Experimental study on the effect of fly ash content in cemented paste backfill on its anti-sulfate erosion. Int. J. Green Energy 2020, 17, 730–774. [Google Scholar] [CrossRef]
- ASTM Standard test method for particle-size analysis of soils D 422-63. In Annual Book of ASTM Standards 04.08:117-127; American Society for Testing and Materials: West Conshohocken, PA, USA, 1972.
- USEPA. Method 3050B. Acid digestion of sediments, sludges, and soils. In Test Methods for Evaluating Solid Waste; USEPA: Washington, DC, USA, 1996. [Google Scholar]
- Sobek, A.A. Field and Laboratory Methods Applicable to Overburdens and Minesoils; Industrial Environmental Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Washington, DC, USA, 1978.
- ASTM. Standard Test Method for Accelerated Weathering of Solid Materials Using a Modified Humidity Cell; American Society for Testing and Materials: Washington, DC, USA, 1996; p. 13. [Google Scholar]
- Lawrence, R.E. Laboratory procedures for the prediction of long term weathering characteristics of mining wastes. In Proceedings of Symposium on Acid Mine Drainage; Annual Meeting Geological Association Canada and Mineralogical Association; VCH: Vancouver, BC, Canada, 1990. [Google Scholar]
- Price, W.A. Draft Guidelines and Recommended Methods for the Prediction of Metal Leaching and Acid Rock Drainage at Minesites in British Columbia; British Columbia Ministry of Employment and Investment, Energy and Minerals Division: Smithers, BC, Canada, 1997.
- White, W.W., III; Lapakko, K.A. Preliminary indications of repeatability and reproducibility of the ASTM 5744-96 kinetic test for drainage pH and sulfate release rate. In Proceedings of the 5th International Conference on Acid Rock Drainage, Denver, CO, USA, 21–24 May 2000; pp. 621–630. [Google Scholar]
- ASTM. Standard Test Method for Consolidated Undrained Triaxial Compression Test for Cohesive Soils; American Society for Testing and Materials: Washigton, DC, USA, 2004. [Google Scholar]
- Clayton, S.; Grice, T.G.; Boger, D.V. Analysis of the slump test for on-site yield stress measurement of mineral suspensions. Int. J. Min. Process. 2003, 70, 3–21. [Google Scholar] [CrossRef]
- Gawu, S.K.; Fourie, A.B. Assessment of the modified slump test as a measure of the yield stress of high-density thickened tailings. Can. Geotech. J. 2004, 41, 39–47. [Google Scholar] [CrossRef]
- Nielson, R.F.; Peterson, H.B. Treatment of Mine Tailings to Promote Vegetative Stabilisation; Agricultural Experiment Station: Logan, UT, USA, 1972. [Google Scholar]
- Evangelou, V.P.; Zhang, Y.L. A review: Pyrite oxidation mechanisms and acid mine drainage prevention. Crit. Rev. Environ. Sci. Technol. 1995, 25, 141–199. [Google Scholar] [CrossRef]
- Wild, A. Soil and the Environment: An Introduction; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Ji, Y.; Lu, Q.; Liu, Q.; Zeng, H. Effect of solution salinity on settling of mineral tailings by polymer flocculants. Colloids Surf. A Physicochem. Eng. Asp. 2013, 430, 29–38. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Durability and leaching behavior of mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 44, 743–750. [Google Scholar] [CrossRef]
- Gao, J.; Yu, Z.; Song, L.; Wang, T.; Wei, S. Durability of concrete exposed to sulfate attack under flexural loading and drying–wetting cycles. Constr. Build. Mater. 2013, 39, 33–38. [Google Scholar] [CrossRef]
- Duchesne, J.; Fournier, B. Deterioration of Concrete by the Oxidation of Sulphide Minerals in the Aggregate. J. Civ. Eng. Arch. 2013, 7, 922–931. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, T.; Benzaazoua, M.; Bussière, B.; Aubertin, M.; Belem, T. Microstructural and geochemical evolution of paste tailings in surface disposal conditions. Miner. Eng. 2008, 21, 341–353. [Google Scholar] [CrossRef]
- Fujiyasu, Y.; Fahey, M.; Newson, T. Field Investigation of Evaporation from Freshwater Tailings. J. Geotech. Geoenviron. Eng. 2000, 126, 556–567. [Google Scholar] [CrossRef]
- Dold, B.; Fontboté, L. Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. J. Geochem. Explor. 2001, 74, 3–55. [Google Scholar] [CrossRef]
- Enkhzaya, S.; Ohe, K.; Shiomori, K.; Oyuntsetseg, B.; Bayanjargal, O.; Watanabe, M. Assessment of Heavy Metals in Mining Tailing Around Boroo and Zuunkharaa Gold Mining Areas of Mongolia. J. Environ. Sci. Technol. 2016, 9, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.; Bertrand, V.; Monroy, M.; González, I. Effect of sulfide impurities on the reactivity of pyrite and pyritic concentrates: A multi-tool approach. Appl. Geochem. 2001, 16, 803–819. [Google Scholar] [CrossRef]
- Jerz, J.K.; Rimstidt, J. Pyrite oxidation in moist air. Geochim. Cosmochim. Acta 2004, 68, 701–714. [Google Scholar] [CrossRef]
- Sapsford, D.; Bowell, R.; Dey, M.; Williams, K. Humidity cell tests for the prediction of acid rock drainage. Miner. Eng. 2009, 22, 25–36. [Google Scholar] [CrossRef]
- Dold, B. Evolution of Acid Mine Drainage Formation in Sulphidic Mine Tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef] [Green Version]
- Dold, B. Acid rock drainage prediction: A critical review. J. Geochem. Explor. 2017, 172, 120–132. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid mine drainage formation and arsenic mobility under strongly acidic conditions: Importance of soluble phases, iron oxyhydroxides/oxides and nature of oxidation layer on pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef]
- Mafra, C.; Bouzahzah, H.; Stamenov, L.; Gaydardzhiev, S. Insights on the effect of pyrite liberation degree upon the acid mine drainage potential of sulfide flotation tailings. Appl. Geochem. 2020, 123, 104774. [Google Scholar] [CrossRef]
- Ódri, Á.; Becker, M.; Broadhurst, J.; Harrison, S.T.L.; Edraki, M. Stable Isotope Imprints during Pyrite Leaching: Implications for Acid Rock Drainage Characterization. Minerals 2020, 10, 982. [Google Scholar] [CrossRef]
- Chopard, A.; Plante, B.; Benzaazoua, M.; Bouzahzah, H.; Marion, P. Geochemical investigation of the galvanic effects during oxidation of pyrite and base-metals sulfides. Chemosphere 2017, 166, 281–291. [Google Scholar] [CrossRef]
- Yang, B.; Luo, W.; Wang, X.; Yu, S.; Gan, M.; Wang, J.; Liu, X.; Qiu, G. The use of biochar for controlling acid mine drainage through the inhibition of chalcopyrite biodissolution. Sci. Total Environ. 2020, 737, 139485. [Google Scholar] [CrossRef]
- Bao, Z.; Al, T.; Couillard, M.; Poirier, G.; Bain, J.; Shrimpton, H.K.; Finfrock, Y.Z.; Lanzirotti, A.; Paktunc, D.; Saurette, E.; et al. A cross scale investigation of galena oxidation and controls on mobilization of lead in mine waste rock. J. Hazard. Mater. 2021, 412, 125130. [Google Scholar] [CrossRef]
- Liao, R.; Yang, B.; Huang, X.; Hong, M.; Yu, S.; Liu, S.; Wang, J.; Qiu, G. Combined effect of silver ion and pyrite on AMD formation generated by chalcopyrite bio-dissolution. Chemosphere 2021, 279, 130516. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Nordstrom, D.K.; Dold, B.; Kirschbaum, A. Seasonal fluctuations and geochemical modeling of acid mine drainage in the semi-arid Puna region: The Pan de Azúcar Pb–Ag–Zn mine, Argentina. J. S. Am. Earth Sci. 2021, 109, 103197. [Google Scholar] [CrossRef]
- Olyphant, G.A.; Bayless, E.R.; Harper, D. Seasonal and weather-related controls on solute concentrations and acid drainage from a pyritic coal-refuse deposit in southwestern Indiana, U.S.A. J. Contam. Hydrol. 1991, 7, 219–236. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Alpers, C.N. Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc. Natl. Acad. Sci. USA 1999, 96, 3455–3462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigham, J.M.; Nordstrom, D.K. Iron and aluminum hydroxysulfates from acid sulfate waters. Rev. Miner. Geochem. 2000, 40, 351–403. [Google Scholar]
- Hawley, P.M. Site selection, characterization, and assessment. In Slope Stability in Surface Mining; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2001; pp. 267–274. [Google Scholar]
- Holtz, R.D.; Kovacs, W.D. An Introduction to Geotechnical Engineering; Pearson Education: Taipei, Taiwan, 2003; 733p. [Google Scholar]
- Wang, Y.; Akeju, O.V. Quantifying the cross-correlation between effective cohesion and friction angle of soil from limited site-specific data. Soils Found. 2016, 56, 1055–1070. [Google Scholar] [CrossRef]
- McLemore, V.T.; Fakhimi, A.; van Zyl, D.; Ayakwah, G.F.; Anim, K.; Boakye, K.; Ennin, F.; Felli, P.; Fredlund, D.; Gutierrez, L.; et al. Literature Review of Other Rock Piles: Characterization, Weathering, and Stability. Questa Rock Pile Weathering Stability Project; New Mexico Bureau of Geology and Mineral Resources: Socorro, NM, USA, 2009; p. 101. [Google Scholar]
- Aubertin, M. Waste rock disposal to improve the geotechnical and geochemical stability of piles. In Proceedings of the World Mining Congress, Montreal, QC, Canada, 11–15 August 2013. [Google Scholar]
- Das, B.M.; Sobhan, K. Principles of Geotechnical Engineering, 7th ed.; Cengage Learning: Stanford, CA, USA, 2010. [Google Scholar]
- Zhang, Q.; Yin, G.; Wei, Z.; Fan, X.; Wang, W.; Nie, W. An experimental study of the mechanical features of layered structures in dam tailings from macroscopic and microscopic points of view. Eng. Geol. 2015, 195, 142–154. [Google Scholar]
- Rankine, R.M.; Sivakugan, N. Geotechnical properties of cemented paste backfill from Cannington Mine, Australia. Geotech. Geol. Eng. 2007, 25, 383–393. [Google Scholar]
- Pierce, M.E. Laboratory and Numerical Analysis of the Strength and Deformation Behavior of Paste Backfill. Master’s Thesis, Queens University, Kingston, ON, Canada, 1997. [Google Scholar]
- Verburg, R.B. Use of paste technology for tailings disposal: Potential environmental benefits and requirements for geochemical characterization. IMWA Symp. 2001, 2001, 13. [Google Scholar]
- Kesimal, A.; Erçikdi, B.; Yilmaz, E. The effect of desliming by sedimentation on paste backfill performance. Miner. Eng. 2003, 16, 1009–1011. [Google Scholar]
- Deschamps, T.; Benzaazoua, M.; Bussière, B.; Aubertin, M. Laboratory study of surface paste disposal for sulfidic tailings: Physical model testing. Miner. Eng. 2011, 24, 794–806. [Google Scholar] [CrossRef]
- Yilmaz, E.; Benzaazoua, M.; Bussière, B.; Pouliot, S. Influence of disposal configurations on hydrogeological behaviour of sulphidic paste tailings: A field experimental study. Int. J. Miner. Process. 2014, 131, 12–25. [Google Scholar] [CrossRef]
- Theriault, J.; Frostiak, J.; Welch, D. Surface disposal of paste tailings at the Bulyanhulu gold mine. In Proceedings of the 2nd Mining Environment Conference, Sudbury, ON, Canada, 25–28 May 2003; pp. 1–8. [Google Scholar]
- Simms, P.; Grabinsky, M.W.; Zhan, G. Modelling evaporation of paste tailings from the Bulyanhulu mine. Can. Geotech. J. 2007, 44, 1417–1432. [Google Scholar] [CrossRef]







| Tailing Sample | pH | EC (mS cm−1) | Humidity (%) | Texture | ||
|---|---|---|---|---|---|---|
| Clay (%) | Silt (%) | Sand (%) | ||||
| M1 | 7.9 ± 0.1 | 2.0 ± 0.2 | 11.6 ± 0.3 | 19.0 ± 0.0 | 53.3 ± 1.2 | 27.7 ± 1.2 |
| M2 | 8.8 ± 0.1 | 1.5 ± 0.2 | 13.1 ± 0.1 | 18.3 ± 1.2 | 55.0 ± 1.0 | 26.7 ± 0.6 |
| M3 | 7.8 ± 0.2 | 1.2 ± 0.1 | 14.8 ± 0.8 | 18.0 ± 1.0 | 54.3 ± 2.1 | 27.7 ± 1.2 |
| M4 | 7.8 ± 0.2 | 11.4 ± 0.8 | 9.6 ± 0.3 | 16.3 ± 1.2 | 52.3 ± 1.5 | 31.4 ± 2.5 |
| M5 | 7.8 ± 0.2 | 1.8 ± 0.2 | 16.6 ± 0.3 | 19.0 ± 2.8 | 59.0 ± 8.5 | 22.0 ± 11.3 |
| M6 | 9.1 ± 0.2 | 1.2 ± 0.1 | 14.5 ± 0.9 | 19.0 ± 2.8 | 59.0 ± 7.1 | 22.0 ± 9.9 |
| Tailing Sample | ||||||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M6 | |
| Major and minor elements (% wt) | ||||||
| Fe | 2.9 | 2.9 | 2.9 | 3.7 | 3.9 | 3.7 |
| Al | 1.3 | 1.3 | 1.4 | 1.4 | 1.4 | 1.6 |
| Mn | 1.8 | 2.0 | 2.1 | 3.3 | 2.9 | 2.2 |
| Ca | 2.5 | 2.5 | 2.5 | 3.0 | 3.7 | 3.1 |
| S | 1.6 | 1.6 | 1.5 | 1.9 | 1.1 | 1.8 |
| Trace elements (ppm) | ||||||
| Zn | 6440 | 6227 | 6439 | 9385 | 3441 | 8218 |
| Pb | 3309 | 3194 | 3132 | 5841 | 2172 | 4610 |
| Cu | 399 | 471 | 379 | 532 | 372 | 595 |
| As | ND | ND | ND | ND | 33 | 51 |
| Cd | ND | ND | 27 | 85 | ND | 44 |
| Ag | ND | ND | ND | ND | ND | ND |
| Sample | Friction Angle φT (°) | Cohesion cT (kPa) | Effective Friction Angle φ’ (°) | Effective Cohesion c’ (kPa) |
|---|---|---|---|---|
| A | 23 | 2.9 | 10 | 6.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiva, E.; Cayazzo, M.; Dávila, L.; Torres, M.; Ledezma, C. Acid Mine Drainage Dynamics from a Paste Tailing Deposit: Effect of Sulfate Content on the Consistency and Chemical Stability after Storage. Metals 2021, 11, 860. https://doi.org/10.3390/met11060860
Leiva E, Cayazzo M, Dávila L, Torres M, Ledezma C. Acid Mine Drainage Dynamics from a Paste Tailing Deposit: Effect of Sulfate Content on the Consistency and Chemical Stability after Storage. Metals. 2021; 11(6):860. https://doi.org/10.3390/met11060860
Chicago/Turabian StyleLeiva, Eduardo, María Cayazzo, Luis Dávila, Mario Torres, and Christian Ledezma. 2021. "Acid Mine Drainage Dynamics from a Paste Tailing Deposit: Effect of Sulfate Content on the Consistency and Chemical Stability after Storage" Metals 11, no. 6: 860. https://doi.org/10.3390/met11060860
APA StyleLeiva, E., Cayazzo, M., Dávila, L., Torres, M., & Ledezma, C. (2021). Acid Mine Drainage Dynamics from a Paste Tailing Deposit: Effect of Sulfate Content on the Consistency and Chemical Stability after Storage. Metals, 11(6), 860. https://doi.org/10.3390/met11060860






