A Short Review on Recent Advances of Hydrogel-Based Adsorbents for Heavy Metal Ions
Abstract
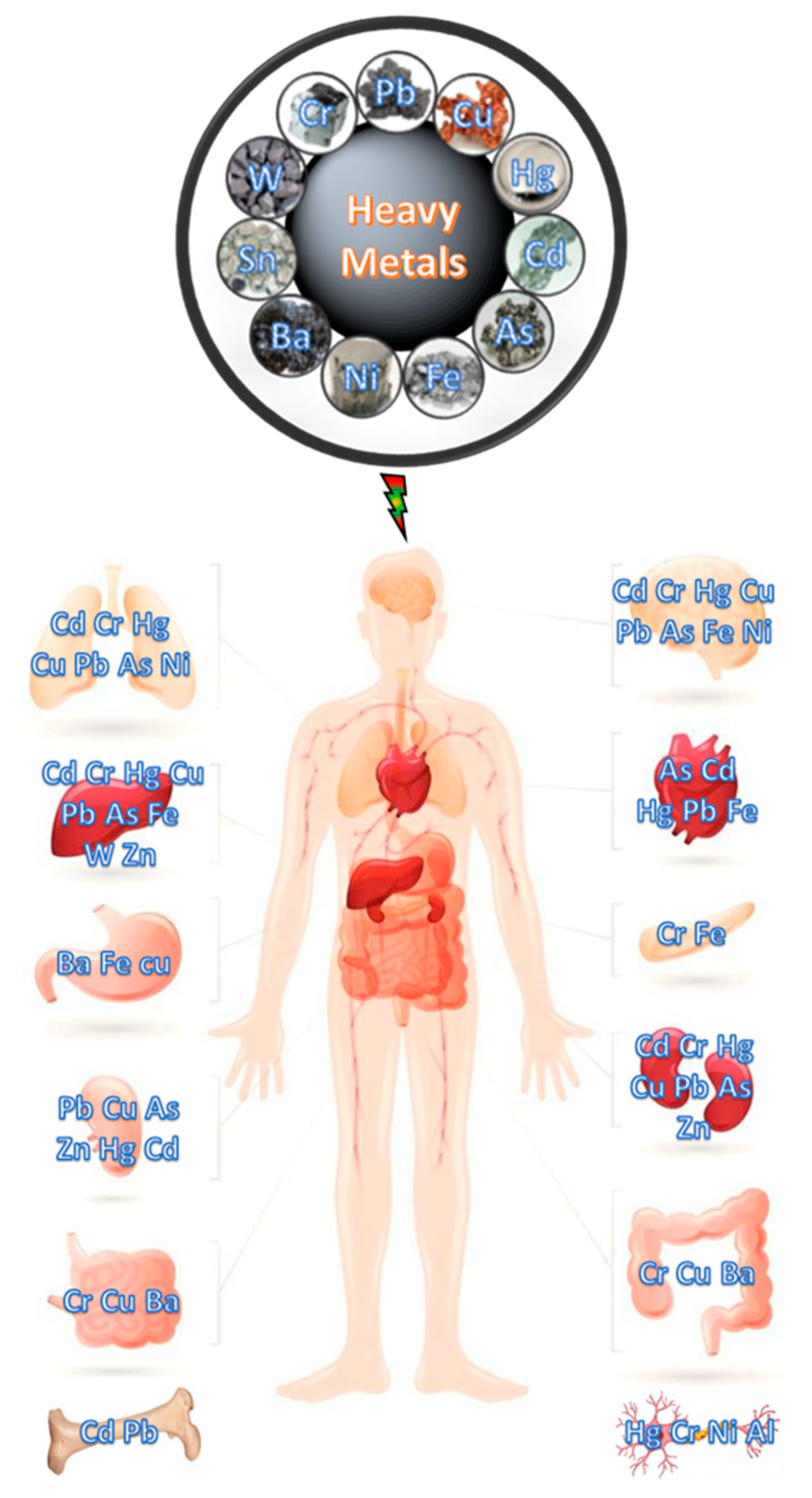
:1. Introduction
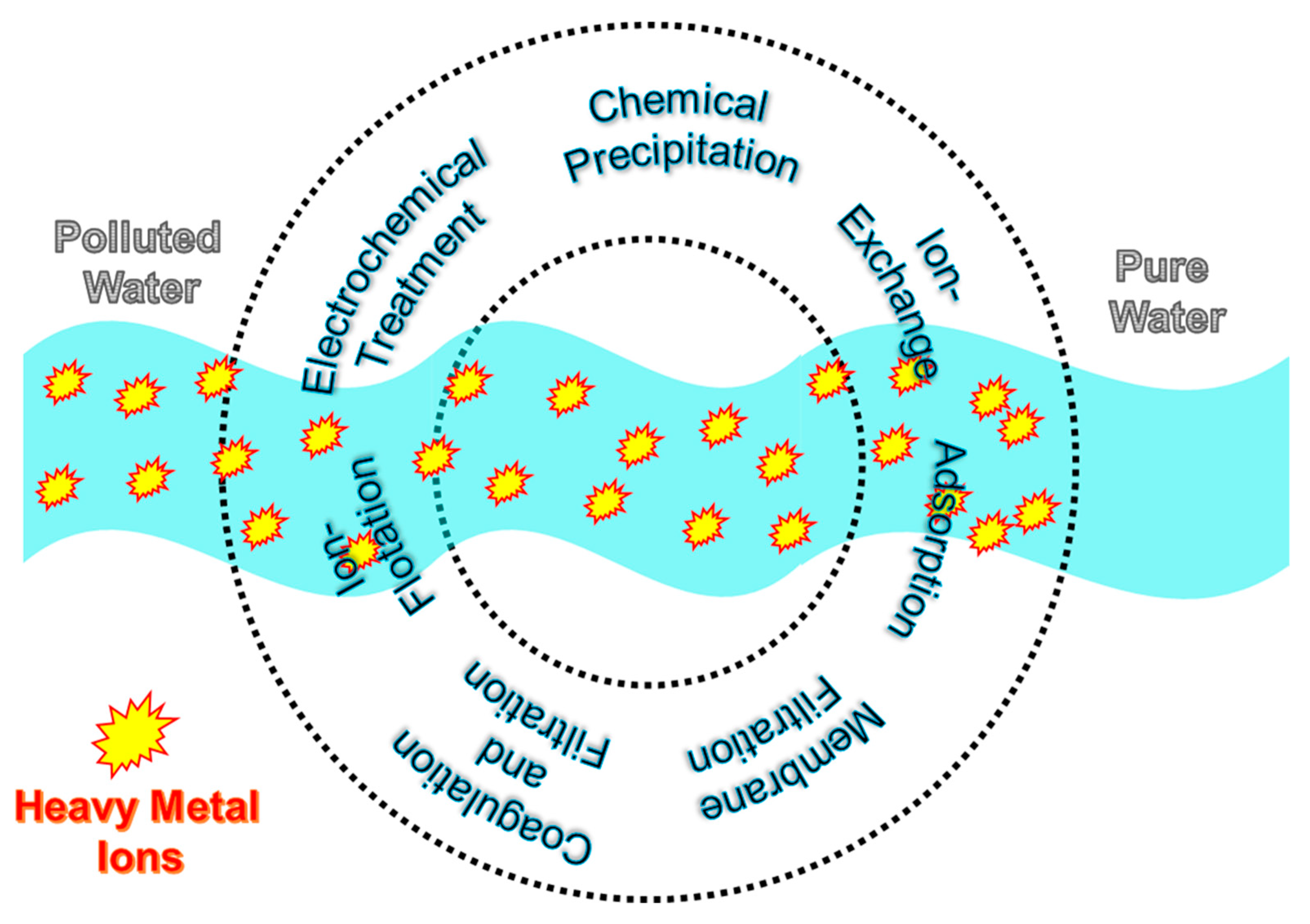
2. Chemical Precipitation
3. Coagulation and Flocculation
4. Membrane Filtration
5. Ion-Flotation
6. Ion-Exchange
7. Electrochemical Treatment
8. Adsorption
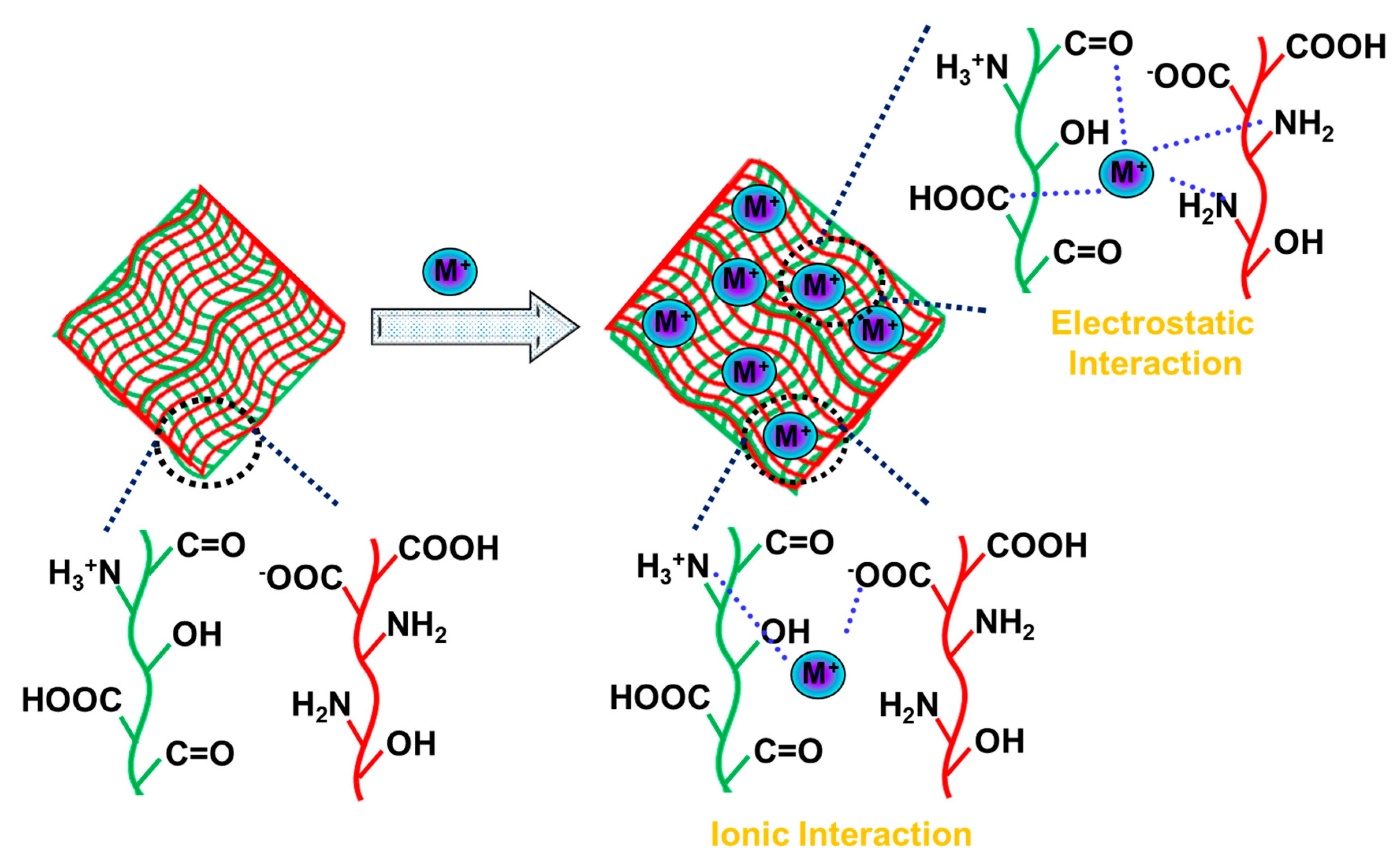
9. Hydrogels Adsorbents for Heavy Metal Ions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maret, W. The Metals in the Biological Periodic System of the Elements: Concepts and Conjectures. Int. J. Mol. Sci. 2016, 17, 66. [Google Scholar] [CrossRef] [Green Version]
- Mertz, W. The essential trace elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef] [Green Version]
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Influence of High and Low Levels of Plant-Beneficial Heavy Metal Ions on Plant Growth and Development. Front. Environ. Sci. 2016, 4, 69. [Google Scholar] [CrossRef]
- Hausen, H. Fluoride toothpaste prevents caries. Evid. Based Dent. 2003, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, Y.; Clark, S.; Ren, J.; Sreejayan, N. Molecular mechanisms of chromium in alleviating insulin resistance. J. Nutr. Biochem. 2012, 23, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar] [PubMed]
- Mertz, W. Human Requirements: Basic and Optimal. Ann. N. Y. Acad. Sci. 1972, 199, 191–201. [Google Scholar] [CrossRef]
- Bansal, S.L.; Asthana, S. Biologically Essential and Non-Essential Elements Causing Toxicity in Environment. J. Environ. Anal. Toxicol. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Chronopoulos, J.; Haidouti, C.; Chronopoulou-Sereli, A.; Massas, I. Variations in plant and soil lead and cadmium content in urban parks in Athens, Greece. Sci. Total Environ. 1997, 196, 91–98. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elements Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Uluozlu, O.D.; Tuzen, M.; Soylak, M. Speciation and separation of Cr(VI) and Cr(III) using coprecipitation with Ni2+/2-Nitroso-1-naphthol-4-sulfonic acid and determination by FAAS in water and food samples. Food Chem. Toxicol. 2009, 47, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- Richtera, L.; Nguyen, H.V.; Hynek, D.; Kudr, J.; Adam, V. Electrochemical speciation analysis for simultaneous determination of Cr(iii) and Cr(vi) using an activated glassy carbon electrode. Analyst 2016, 141, 5577–5585. [Google Scholar] [CrossRef]
- Fytianos, K. Speciation Analysis of Heavy Metals in Natural Waters: A Review. J. AOAC Int. 2001, 84, 1763–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrane, C.; Bouhidel, K.E. Analysis and speciation of heavy metals in the water, sediments, and drinking water plant sludge of a deep and sulfate-rich Algerian reservoir. Environ. Monit. Assess. 2019, 191, 73. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Fattahi, N.; Assadi, Y.; Sadeghi, M.; Sharafi, K. Speciation of As(III) and As(V) in water samples by graphite furnace atomic absorption spectrometry after solid phase extraction combined with dispersive liquid–liquid microextraction based on the solidification of floating organic drop. Talanta 2014, 130, 26–32. [Google Scholar] [CrossRef]
- Liang, P.; Peng, L.; Yan, P. Speciation of As(III) and As(V) in water samples by dispersive liquid-liquid microextraction separation and determination by graphite furnace atomic absorption spectrometry. Microchim. Acta 2009, 166, 47–52. [Google Scholar] [CrossRef]
- Smichowski, P.; Marrero, J.; Ledesma, A.; Polla, G.; Batistoni, D.A. Speciation of As(iii) and As(v) in aqueous solutions using baker’s yeast and hydride generation inductively coupled plasma atomic emission spectrometric determination. J. Anal. At. Spectrom. 2000, 15, 1493–1497. [Google Scholar] [CrossRef]
- Dvoynenko, O.; Lo, S.-L.; Chen, Y.-J.; Chen, G.W.; Tsai, H.-M.; Wang, Y.-L.; Wang, J.-K. Speciation Analysis of Cr(VI) and Cr(III) in Water with Surface-Enhanced Raman Spectroscopy. ACS Omega 2021, 6, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meshitsuka, S.; Ishizawa, M.; Nose, T. Uptake and toxic effects of heavy metal ions: Interactions among cadmium, copper and zinc in cultured cells. Experientia 1987, 43, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Lee, K.; König, R. Effects of heavy metal ions on resting and antigen-activated CD4+ T cells. Toxicology 2001, 169, 67–80. [Google Scholar] [CrossRef]
- Obasi, P.N.; Akudinobi, B.B. Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Appl. Water Sci. 2020, 10, 184. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Chapter 1 Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. In Heavy Metals in Water: Presence, Removal and Safety; Royal Society of Chemistry: London, UK, 2014; pp. 1–24. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci. Technol. 2020, 81, 1130–1136. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Z.; Hills, C.; Xue, G.; Tyrer, M. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef]
- Hargreaves, A.J.; Vale, P.; Whelan, J.; Alibardi, L.; Constantino, C.; Dotro, G.; Cartmell, E.; Campo, P. Coagulation–flocculation process with metal salts, synthetic polymers and biopolymers for the removal of trace metals (Cu, Pb, Ni, Zn) from municipal wastewater. Clean Technol. Environ. Policy 2018, 20, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Amuda, O.S.; Amoo, I.; Ipinmoroti, K.; Ajayi, O. Coagulation/flocculation process in the removal of trace metals present in industrial wastewater. J. Appl. Sci. Environ. Manag. 2006, 10, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Blöcher, C.; Dorda, J.; Mavrov, V.; Chmiel, H.; Lazaridis, N.; Matis, K. Hybrid flotation—membrane filtration process for the removal of heavy metal ions from wastewater. Water Res. 2003, 37, 4018–4026. [Google Scholar] [CrossRef]
- Cao, D.-Q.; Wang, X.; Wang, Q.-H.; Fang, X.-M.; Jin, J.-Y.; Hao, X.-D.; Iritani, E.; Katagiri, N. Removal of heavy metal ions by ultrafiltration with recovery of extracellular polymer substances from excess sludge. J. Membr. Sci. 2020, 606, 118103. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Taseidifar, M.; Makavipour, F.; Pashley, R.M.; Rahman, A.F.M.M. Removal of heavy metal ions from water using ion flotation. Environ. Technol. Innov. 2017, 8, 182–190. [Google Scholar] [CrossRef]
- Polat, H.; Erdogan, D. Heavy metal removal from waste waters by ion flotation. J. Hazard. Mater. 2007, 148, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Cao, Y.; Fan, G.; Li, C.; Peng, W. A review of the applications of ion floatation: Wastewater treatment, mineral beneficiation and hydrometallurgy. RSC Adv. 2019, 9, 20226–20239. [Google Scholar] [CrossRef]
- Zewail, T.; Yousef, N. Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alex. Eng. J. 2015, 54, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Da̧browski, A.; Hubicki, Z.; Podkoscielny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Vaaramaa, K.; Lehto, J. Removal of metals and anions from drinking water by ion exchange. Desalination 2003, 155, 157–170. [Google Scholar] [CrossRef]
- Tran, T.-K.; Leu, H.-J.; Chiu, K.-F.; Lin, C.-Y. Electrochemical Treatment of Heavy Metal-containing Wastewater with the Removal of COD and Heavy Metal Ions. J. Chin. Chem. Soc. 2017, 64, 493–502. [Google Scholar] [CrossRef]
- Tran, T.-K.; Chiu, K.-F.; Lin, C.-Y.; Leu, H.-J. Electrochemical treatment of wastewater: Selectivity of the heavy metals removal process. Int. J. Hydrog. Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Tang, Z.; Huang, Q.; Niu, F.; Wang, X.-K. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, F.; Xue, J.; Chen, S.; Wang, J.; Yang, Y. Enhanced removal of heavy metal ions from aqueous solution using manganese dioxide-loaded biochar: Behavior and mechanism. Sci. Rep. 2020, 10, 6067. [Google Scholar] [CrossRef] [Green Version]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy metal removal applications using adsorptive membranes. Nano Converg. 2020, 7, 36. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Fawzan, F.F. Adsorption study of heavy metal ions from aqueous solution by nanoparticle of wild herbs. Egypt. J. Aquat. Res. 2018, 44, 187–194. [Google Scholar] [CrossRef]
- Salam, O.E.A.; Reiad, N.A.; ElShafei, M.M. A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. J. Adv. Res. 2011, 2, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, H.N.M.E.; Huq, A.K.O.; Yahya, R.B. The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: A review. RSC Adv. 2016, 6, 14778–14791. [Google Scholar] [CrossRef]
- Deng, S.; Bai, R. Removal of trivalent and hexavalent chromium with aminated polyacrylonitrile fibers: Performance and mechanisms. Water Res. 2004, 38, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, M.; Maity, A.; Srinivasu, V.; Onyango, M.S. Removal of hexavalent chromium from aqueous solution using polypyrrole-polyaniline nanofibers. Chem. Eng. J. 2012, 181–182, 323–333. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X.; Ye, Z. Recent Progress in Heavy Metal Ion Decontamination Based on Metal–Organic Frameworks. Nanomaterials 2020, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.M.; Massadeh, A.M.; Younes, H.A. Natural Jordanian zeolite: Removal of heavy metal ions from water samples using column and batch methods. Environ. Monit. Assess. 2009, 157, 319–330. [Google Scholar] [CrossRef]
- Gebretsadik, H.; Gebrekidan, A.; Demlie, L. Removal of heavy metals from aqueous solutions using Eucalyptus Camaldulensis: An alternate low cost adsorbent. Cogent Chem. 2020, 6, 1720892. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Yoon, D.H.; Joo, J.; Cheong, I.W. Spherical Chitosan/Gelatin Hydrogel Particles for Removal of Multiple Heavy Metal Ions from Wastewater. Ind. Eng. Chem. Res. 2019, 58, 9900–9907. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Yoon, D.H.; Joo, J.; Cheong, I.W. Graphene oxide-embedded chitosan/gelatin hydrogel particles for the adsorptions of multiple heavy metal ions. J. Mater. Sci. 2020, 55, 9354–9363. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, L.; Xue, Z.; Yusef, K.K.; Hu, H.; Wu, M. Adsorption of Cu (II) and Cd (II) from Wastewater by Sodium Alginate Modified Materials. J. Chem. 2020, 2020, 5496712. [Google Scholar] [CrossRef]
- Arora, R. Adsorption of Heavy Metals–A Review. Mater. Today Proc. 2019, 18, 4745–4750. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Liao, W.; Yan, Z.; Bai, Y.; Feng, C.; Xu, Z.; Xu, D. Progress in the Research of the Toxicity Effect Mechanisms of Heavy Metals on Freshwater Organisms and Their Water Quality Criteria in China. J. Chem. 2020, 2020, 9010348. [Google Scholar] [CrossRef]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of adsorbents and recovery of heavy metals: A review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef] [Green Version]
- Ngah, W.W.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, P.P.; Prabhu, B. A Review on Removal of Heavy Metal Ions from Waste Water using Natural/Modified Bentonite. MATEC Web Conf. 2018, 144, 02021. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalination 2016, 7, 387–419. [Google Scholar] [CrossRef]
- Muya, F.N.; Sunday, C.E.; Baker, P.; Iwuoha, E. Environmental remediation of heavy metal ions from aqueous solution through hydrogel adsorption: A critical review. Water Sci. Technol. 2016, 73, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, N.; Solaiman; Roy, C.K.; Firoz, S.H.; Foyez, T.; Bin Imran, A. Role of Ionic Moieties in Hydrogel Networks to Remove Heavy Metal Ions from Water. ACS Omega 2021, 6, 836–844. [Google Scholar] [CrossRef]
- Shalla, A.H.; Yaseen, Z.; Bhat, M.A.; Rangreez, T.A.; Maswal, M. Recent review for removal of metal ions by hydrogels. Sep. Sci. Technol. 2019, 54, 89–100. [Google Scholar] [CrossRef]
- Jafari, M.; Najafi, G.R.; Sharif, M.A.; Elyasi, Z. Superabsorbent polymer composites derived from polyacrylic acid: Design and synthesis, characterization, and swelling capacities. Polym. Polym. Compos. 2020, 26, 1–7. [Google Scholar] [CrossRef]
- Thakur, S.; Arotiba, O. Synthesis, characterization and adsorption studies of an acrylic acid-grafted sodium alginate-based TiO2 hydrogel nanocomposite. Adsorpt. Sci. Technol. 2018, 36, 458–477. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Xie, X.; Zhang, X.; Zhang, J.; Wang, A. Preparation and swelling properties of pH-sensitive composite hydrogel beads based on chitosan-g-poly (acrylic acid)/vermiculite and sodium alginate for diclofenac controlled release. Int. J. Biol. Macromol. 2010, 46, 356–362. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, A. Removal of heavy metals using polyvinyl alcohol semi-IPN poly(acrylic acid)/tourmaline composite optimized with response surface methodology. Chem. Eng. J. 2010, 162, 186–193. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, X.; Yin, D.; Zhang, W. Preparation of a Hydrogel-Based Adsorbent for Metal Ions through High Internal Phase Emulsion Polymerization. ACS Omega 2020, 5, 19920–19927. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Nath, A.; Pande, P.P.; Shankar, R. Treatment of gray wastewater and heavy metal removal from aqueous medium using hydrogels based on novel crosslinkers. J. Appl. Polym. Sci. 2021, 138, 50242. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, X.; Zhang, X.; Huang, L.; Liu, Z.; Sun, G. Highly efficient removal of trace metal ions by using poly(acrylic acid) hydrogel adsorbent. Mater. Des. 2019, 181, 107934. [Google Scholar] [CrossRef]
- Anceschi, A.; Caldera, F.; Bertasa, M.; Cecone, C.; Trotta, F.; Bracco, P.; Zanetti, M.; Malandrino, M.; Mallon, P.E.; Scalarone, D. New Poly(β-Cyclodextrin)/Poly(Vinyl Alcohol) Electrospun Sub-Micrometric Fibers and Their Potential Application for Wastewater Treatments. Nanomaterials 2020, 10, 482. [Google Scholar] [CrossRef] [Green Version]
- Wahlström, N.; Steinhagen, S.; Toth, G.; Pavia, H.; Edlund, U. Ulvan dialdehyde-gelatin hydrogels for removal of heavy metals and methylene blue from aqueous solution. Carbohydr. Polym. 2020, 249, 116841. [Google Scholar] [CrossRef]
- Ozay, O.; Ekici, S.; Baran, Y.; Aktaş, N.; Sahiner, N. Removal of toxic metal ions with magnetic hydrogels. Water Res. 2009, 43, 4403–4411. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.W.P.; Batista, A.P.L.; Hammer, P.; Luz, G.H.P.; Ramalho, T.C. Removal of metal ions from aqueous solution by chelating polymeric hydrogel. Environ. Chem. Lett. 2010, 8, 343–348. [Google Scholar] [CrossRef]
- Ali, A.E.-H.; Shawky, H.; El Rehim, H.A.; Hegazy, E. Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution. Eur. Polym. J. 2003, 39, 2337–2344. [Google Scholar] [CrossRef]
- Ozay, O.; Ekici, S.; Aktas, N.; Sahiner, N. P(4-vinyl pyridine) hydrogel use for the removal of UO22+ and Th4+ from aqueous environments. J. Environ. Manag. 2011, 92, 3121–3129. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, H.; Teasdale, P.; John, R.; Zhang, S. Synthesis and characterisation of a polyacrylamide–polyacrylic acid copolymer hydrogel for environmental analysis of Cu and Cd. React. Funct. Polym. 2002, 52, 31–41. [Google Scholar] [CrossRef]
- Wang, Y.M.; Shang, D.J.; Niu, Z.W. Removal of Heavy Metals by Poly(Vinyl Pyrrolidone)/Laponite Nanocomposite Hydrogels. Adv. Mater. Res. 2013, 631–632, 291–297. [Google Scholar] [CrossRef]
- Atta, A.; Ismail, H.S.; Mohamed, H.M.; Mohamed, Z.M. Acrylonitrile/acrylamidoxime/2-acrylamido-2- methylpropane sulfonic acid-based hydrogels: Synthesis, characterization and their application in the removal of heavy metals. J. Appl. Polym. Sci. 2011, 122, 999–1011. [Google Scholar] [CrossRef]
- Chauhan, G.S.; Chauhan, S.; Sen, U.; Garg, D. Synthesis and characterization of acrylamide and 2-hydroxyethyl methacrylate hydrogels for use in metal ion uptake studies. Desalination 2009, 243, 95–108. [Google Scholar] [CrossRef]
- Ozay, O.; Ekici, S.; Baran, Y.; Kubilay, S.; Aktaş, N.; Sahiner, N. Utilization of magnetic hydrogels in the separation of toxic metal ions from aqueous environments. Desalination 2010, 260, 57–64. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Lee, S.Y.; Mafize, A.A.; Kahar, N.A.M.A.; Johari, K.; Rabat, N.E. Efficient Removal of Pb(II) from Aqueous Solutions by Using Oil Palm Bio-Waste/MWCNTs Reinforced PVA Hydrogel Composites: Kinetic, Isotherm and Thermodynamic Modeling. Polymers 2020, 12, 430. [Google Scholar] [CrossRef] [Green Version]
- Vilela, P.B.; Dalalibera, A.; Duminelli, E.C.; Becegato, V.A.; Paulino, A.T. Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ. Sci. Pollut. Res. 2019, 26, 28481–28489. [Google Scholar] [CrossRef]
- Zhang, M.; Song, L.; Jiang, H.; Li, S.; Shao, Y.; Yang, J.; Li, J. Biomass based hydrogel as an adsorbent for the fast removal of heavy metal ions from aqueous solutions. J. Mater. Chem. A 2017, 5, 3434–3446. [Google Scholar] [CrossRef]
- Pathan, S.; Bose, S. Arsenic Removal Using “Green” Renewable Feedstock-Based Hydrogels: Current and Future Perspectives. ACS Omega 2018, 3, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Dambies, L.; Guibal, E.; Roze, A. Arsenic(V) sorption on molybdate-impregnated chitosan beads. Colloids Surfaces A: Physicochem. Eng. Asp. 2000, 170, 19–31. [Google Scholar] [CrossRef]
- Lone, S.; Yoon, D.H.; Lee, H.M.; Cheong, I.W. Gelatin–chitosan hydrogel particles for efficient removal of Hg(ii) from wastewater. Environ. Sci. Water Res. Technol. 2019, 5, 83–90. [Google Scholar] [CrossRef]
- Jin, L.; Bai, R. Mechanisms of Lead Adsorption on Chitosan/PVA Hydrogel Beads. Langmuir 2002, 18, 9765–9770. [Google Scholar] [CrossRef]
- Barakat, M.; Sahiner, N. Cationic hydrogels for toxic arsenate removal from aqueous environment. J. Environ. Manag. 2008, 88, 955–961. [Google Scholar] [CrossRef]
- Morán-Quiroz, J.L.; Orozco-Guareño, E.; Manríquez, R.; Carbajal-Arízaga, G.G.; de la Cruz, W.; Gomez-Salazar, S. Polymeric hydrogels obtained using a redox initiator: Application in Cu(II) ions removal from aqueous solutions. J. Appl. Polym. Sci. 2014, 131, 39933. [Google Scholar] [CrossRef]
- Milosavljević, N.; Debeljković, A.; Krušić, M.K.; Milašinović, N.; Üzüm, Ö.B.; Karadağ, E. Application of poly(acrlymide-co-sodium methacrylate) hydrogels in copper and cadmium removal from aqueous solution. Environ. Prog. Sustain. Energy 2014, 33, 824–834. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, Q.; Ji, X.; Wang, F.; Gao, X.; Zhao, M. High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci. Rep. 2020, 10, 3285. [Google Scholar] [CrossRef]
- Ortaboy, S.; Acar, E.T.; Atun, G.; Emik, S.; İyim, T.B.; Güçlü, G.; Özgümüş, S. Performance of acrylic monomer based terpolymer/montmorillonite nanocomposite hydrogels for U(VI) removal from aqueous solutions. Chem. Eng. Res. Des. 2013, 91, 670–680. [Google Scholar] [CrossRef]
- Raj, L.; Chauhan, G.S. Uranyl ions uptake on poly(AAc/AAm)-cl-N,N-MBAAm hydrogel. Polym. Bull. 2010, 64, 363–374. [Google Scholar] [CrossRef]
- Yan, H.; Dai, J.; Yang, Z.; Yang, H.; Cheng, R. Enhanced and selective adsorption of copper(II) ions on surface carboxymethylated chitosan hydrogel beads. Chem. Eng. J. 2011, 174, 586–594. [Google Scholar] [CrossRef]
- Kaşgöz, H.; Durmus, A.; Kaşgöz, A. Enhanced swelling and adsorption properties of AAm-AMPSNa/clay hydrogel nanocomposites for heavy metal ion removal. Polym. Adv. Technol. 2008, 19, 213–220. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, D.; Wang, A. Chitosan-g-poly(acrylic acid) hydrogel with crosslinked polymeric networks for Ni2+ recovery. Anal. Chim. Acta 2011, 687, 193–200. [Google Scholar] [CrossRef]
- Evren, M.; Acar, I.; Güçlü, K.; Güçlü, G. Removal of Cu2+and Pb2+ions byN-vinyl 2-pyrrolidone/itaconic acid hydrogels from aqueous solutions. Can. J. Chem. Eng. 2014, 92, 52–59. [Google Scholar] [CrossRef]
- Döker, S.; Malcı, S.; Doğan, M.; Salih, B. New poly(N-(hydroxymethyl)methacrylamide–1-allyl-2-thiourea) hydrogels prepared by radiation-induced polymerisation: Selective adsorption, recovery and pre-concentration of Pt(II) and Pd(II). Anal. Chim. Acta 2005, 553, 73–82. [Google Scholar] [CrossRef]
- Sharaf, M.A.; Arida, H.A.; Sayed, S.A.; Younis, A.A.; Farag, A.B. Separation and preconcentration of some heavy-metal ions using new chelating polymeric hydrogels. J. Appl. Polym. Sci. 2009, 113, 1335–1344. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kim, H.J.; Ryu, H.; Oh, S.; Choi, S.-J. Three-dimensional double-network hydrogels of graphene oxide, alginate, and polyacrylonitrile for copper removal from aqueous solution. Environ. Eng. Res. 2020, 25, 924–929. [Google Scholar] [CrossRef]
- Astrini, N.; Anah, L.; Haryadi, H.R. Adsorption of Heavy Metal Ion from Aqueous Solution by Using Cellulose Based Hydrogel Composite. Macromol. Symp. 2015, 353, 191–197. [Google Scholar] [CrossRef]
- Kong, C.; Zhao, X.; Li, Y.; Yang, S.; Chen, Y.M.; Yang, Z. Ion-Induced Synthesis of Alginate Fibroid Hydrogel for Heavy Metal Ions Removal. Front. Chem. 2020, 7, 905. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, Y.; Tang, Y.; Wei, Y.; Liu, Y.; Liu, C. Efficient removal of heavy metals from melting effluent using multifunctional hydrogel adsorbents. Water Sci. Technol. 2018, 78, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Yetimoğlu, E.K.; Fırlak, M.; Kahraman, M.V.; Deniz, S. Removal of Pb2+ and Cd2+ ions from aqueous solutions using guanidine modified hydrogels. Polym. Adv. Technol. 2011, 22, 612–619. [Google Scholar] [CrossRef]
- Bai, C.; Wang, L.; Zhu, Z. Adsorption of Cr(III) and Pb(II) by graphene oxide/alginate hydrogel membrane: Characterization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2020, 147, 898–910. [Google Scholar] [CrossRef]
- Neto, J.D.O.M.; Bellato, C.R.; Milagres, J.L.; Pessoa, K.D.; De Alvarenga, E.S. Preparation and evaluation of chitosan beads immobilized with Iron(III) for the removal of As(III) and As(V) from water. J. Braz. Chem. Soc. 2013, 24, 121–132. [Google Scholar] [CrossRef] [Green Version]

| S.No | Hydrogel Adsorbents | Adsorption Capacity (Qe (mg.g−1)) | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|
| 1. | Poly(vinylpyrrolidone/acrylic acid) copolymer hydrogel | Fe(III)-20, Mn(II)-1, and Cu(II)-11 | [84] | |
| 2. | Gelatin-chitosan hydrogel particles | - | Hg(II)-98, Pb(II)-34, Cd(II)-20, and Cr(III)-16 | [96] |
| 3. | Chitosan-polyvinylalcohol hydrogel beads | Pb(II)-0.9 | - | [97] |
| 4. | Spherical chitosan-gelatin hydrogel particles | Hg(II)-47.5, Pb(II)-7.62, Cd(II)-0, Cr(III)-1.5 | Hg(II)-84.7, Pb(II)-8.7, Cd(II)-0, and Cr(III)-6.7 | [58] |
| 5. | Graphene oxide embedded chitosan-gelatin hydrogel particles | Hg(II)-54.6, Pb(II)-5.4, Cd(II)-1.67, Cr(III)-0 | Hg(II)-54.6, Pb(II)-7.3, Cd(II)-1.9, and Cr(III)-0 | [59] |
| 6. | Cationic hydrogels | - | As(V)-99.7 | [98] |
| 7. | Poly(acrlic acid-co-acrylamide) hydrogels | Cu(II)-211.7 | - | [99] |
| 8. | Poly(acrylamide-co-sodium methacrylate) hydrogel | Cu(II)-24.05 and Cd(II)-33.0 | Cu(II)-48 and Cd(II)-66 | [100] |
| 9. | Soybean hydrogel | Cd(II)-1.43 mmol.g−1 and Pb(II)-2.04 mmol.g−1 | - | [101] |
| 10. | Terpolymer/montmorillonite nanocomposite hydrogels | U(VI)-0.723 mol.g−1 | - | [102] |
| 11. | Acrylamide and acrylic acid hydrogels | U(VI)-236.6 | - | [103] |
| 12. | Chitosan hydrogel beads | Cu(II)-130 | - | [104] |
| 13. | Hydrogel-clay nanocomposites | Cu(II)-1.07 mmol.g−1, Cd(II)-1.28 mmol.g−1, and Pb(II) 1.03 mmol.g−1 | - | [105] |
| 14. | Chitosan-based hydrogel | Ni(II)-161.8 | - | [106] |
| 15. | N-vinyl-2-pyrrolidone–itaconic acid Hydrogels | Cu(II)-2.1 mmol.g−1 and Pb(II)-0.6 mmol.g−1 | - | [107] |
| 16. | Thiourea-based hydrogel | Pt(II)-477 and Pd(II)-407 | Pt(II)-96.8 | [108] |
| 17. | Polyacrylamide-based hydrogel | Cd(II)-5.3 mmol.g−1, Pb(II)-0.63 mmol.g−1, and Zn(II)-1.27 mmol.g−1 | - | [109] |
| 18. | Graphene oxide composite hydrogel | Cu(II)-5.99 | - | [110] |
| S.No | Hydrogel Adsorbents | Adsorption Capacity (Qe (mg.g−1)) | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|
| 1. | Polyacrylic acid hydrogel | Cd(II)-132.9, Cr(VI)-58.1, Fe(III)-12.4, Mn(II)-120.4, Ni(II)-128.8, Ag(I) and Ce(III)-203.5, Zn(II)-157.8 | Cd(II)-57.1, Cr(VI)-26.9, Fe(III)-5.3, Mn(II)-52.7, Ni(II)-52.5, Ag(I)-45.3, Ce(III)-70, Zn(II)-58.4 | [79] |
| 2. | Spherical chitosan-gelatin hydrogel particles | Hg(II)-42.7, Pb(II)-67.6, Cd(II)-46.8, and Cr(III)-19.2 | Hg(II)-93, Pb(II)-76.6, Cd(II)-73.4, and Cr(III)-84.2 | [58] |
| 3. | Gelatin-chitosan hydrogel | - | Hg(II)-97, Pb(II)-12, Cd(II)-2, and Cr(III)-24 | [96] |
| 4. | Cellulose-hydrogel composite | Pb(II)-146.19 and Zn(II)-286.67 | - | [111] |
| 5. | Graphene oxide embedded chitosan-gelatin hydrogel particles | Hg(II)-60.2, Pb(II)-60.1, Cd(II)-39.6, Cr(III)-14.4 | Hg(II)-92.5, Pb(II)-78.4, Cd(II)-74.0, and Cr(III)-80.0 | [59] |
| 6. | Sulfonic acid-based hydrogels | Cd(II)-0.95, Cu(II)-0.87, Fe(III)-0.83, Zn(II)-1.00, Mn(II)-0.77, and Pb(II)-0.18 | - | [88] |
| 7. | Itaconic-based hydrogels | Cu(II)-2.1 mmol.g−1 and Pb(II)-0.6 mmol.g−1 | - | [107] |
| 8. | Alginate fibroid hydrogel | Cu(II)-316.0, Cd(II)-232.35, and Pb(II)-465.2 | - | [112] |
| 9. | poly (vinyl alcohol)/poly(2-acrylamido-2-methyl-1-propanesulfonic acid) | Pb(II)-340 and Cd(II)-155.1 | Pb(II)-88.1, Cd(II)-91.4, Zn(II)-70.4, Cu(II)-77.4, Mn(II)-42.5, Ni(II)-45.1, and Fe(III)-95.4 | [113] |
| 10. | Guanidine-based hydrogel | Pb(II)-27.3 and Cd(II)-28.5 | - | [114] |
| 11. | Graphene oxide/alginate hydrogel membrane | Pb(II)-327.9 and Cr(III)-118.6 | - | [115] |
| 12. | Iron crosslinked-chitosan beads | As(III)-21.24 and As(V)-27.59 | - | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Babu, R.S.; Karpagavinayagam, P.; Vedhi, C. A Short Review on Recent Advances of Hydrogel-Based Adsorbents for Heavy Metal Ions. Metals 2021, 11, 864. https://doi.org/10.3390/met11060864
Perumal S, Atchudan R, Edison TNJI, Babu RS, Karpagavinayagam P, Vedhi C. A Short Review on Recent Advances of Hydrogel-Based Adsorbents for Heavy Metal Ions. Metals. 2021; 11(6):864. https://doi.org/10.3390/met11060864
Chicago/Turabian StylePerumal, Suguna, Raji Atchudan, Thomas Nesakumar Jebakumar Immanuel Edison, Rajendran Suresh Babu, Petchimuthu Karpagavinayagam, and Chinnapiyan Vedhi. 2021. "A Short Review on Recent Advances of Hydrogel-Based Adsorbents for Heavy Metal Ions" Metals 11, no. 6: 864. https://doi.org/10.3390/met11060864










