Microstructure Characterization and Properties of Graphene Oxide-Reinforced TiAl Matrix Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of GO-Coated TiAl Powders
2.2. Fabrication and Characterization of GO/TiAl Composites
2.3. Properties Tests
3. Results and Discussion
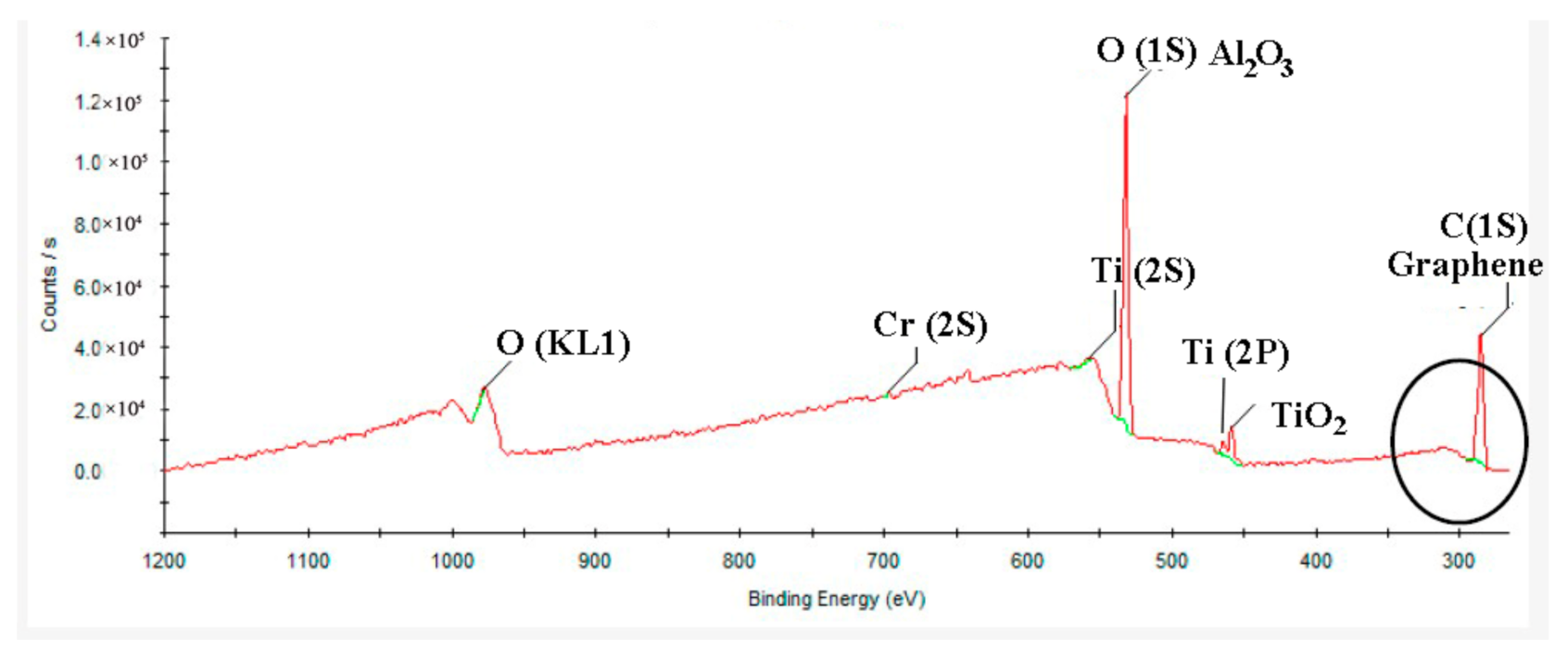
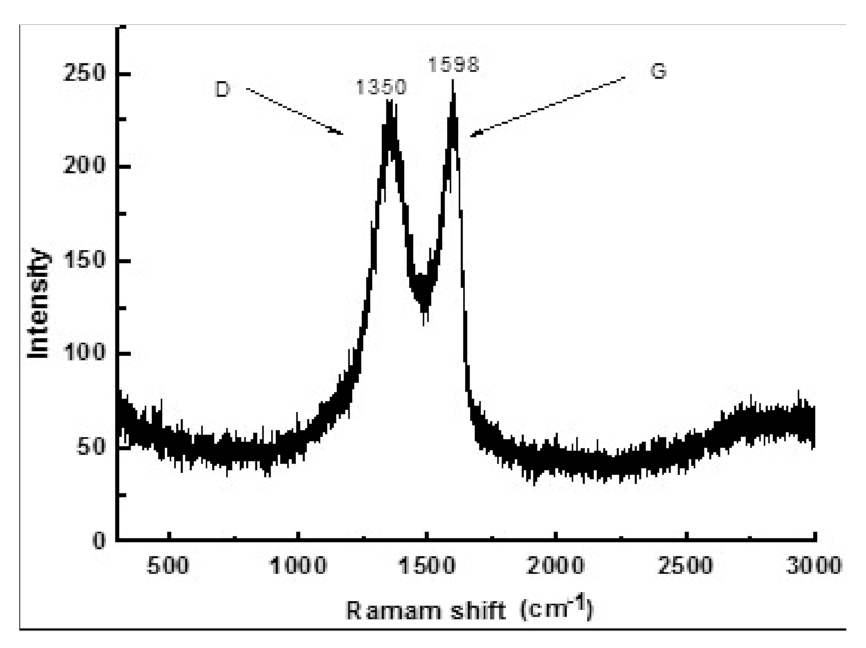
3.1. Characterization of GO-Coated TiAl Powders
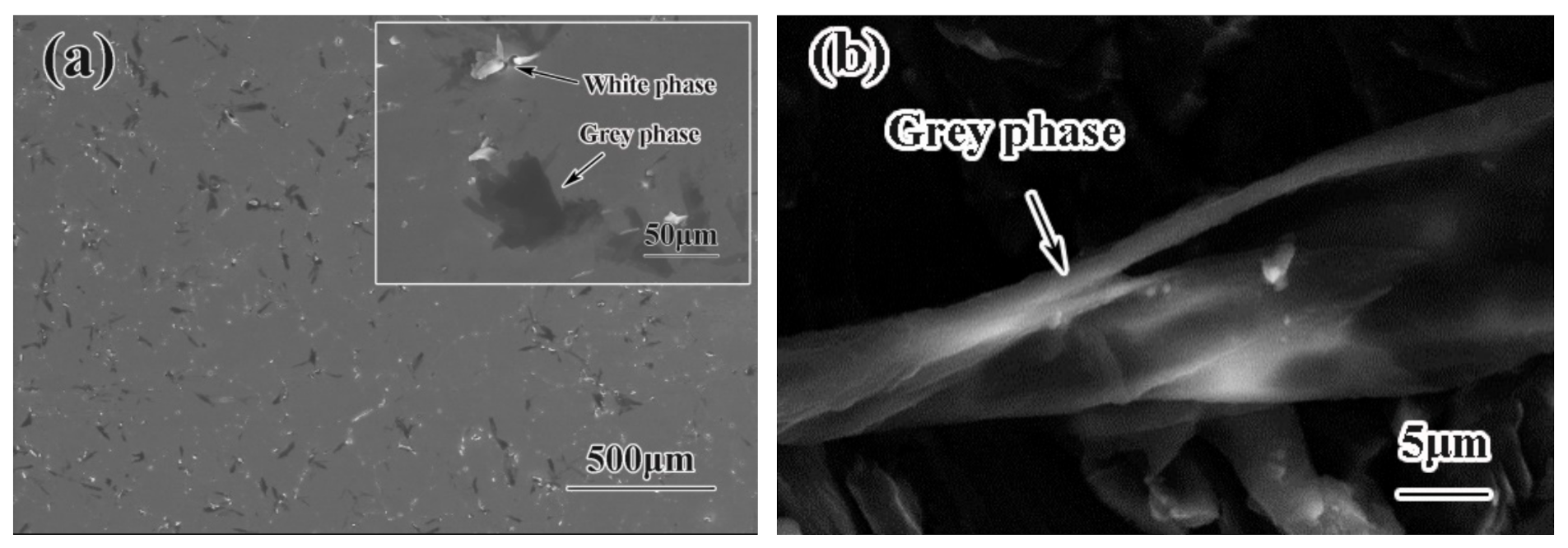
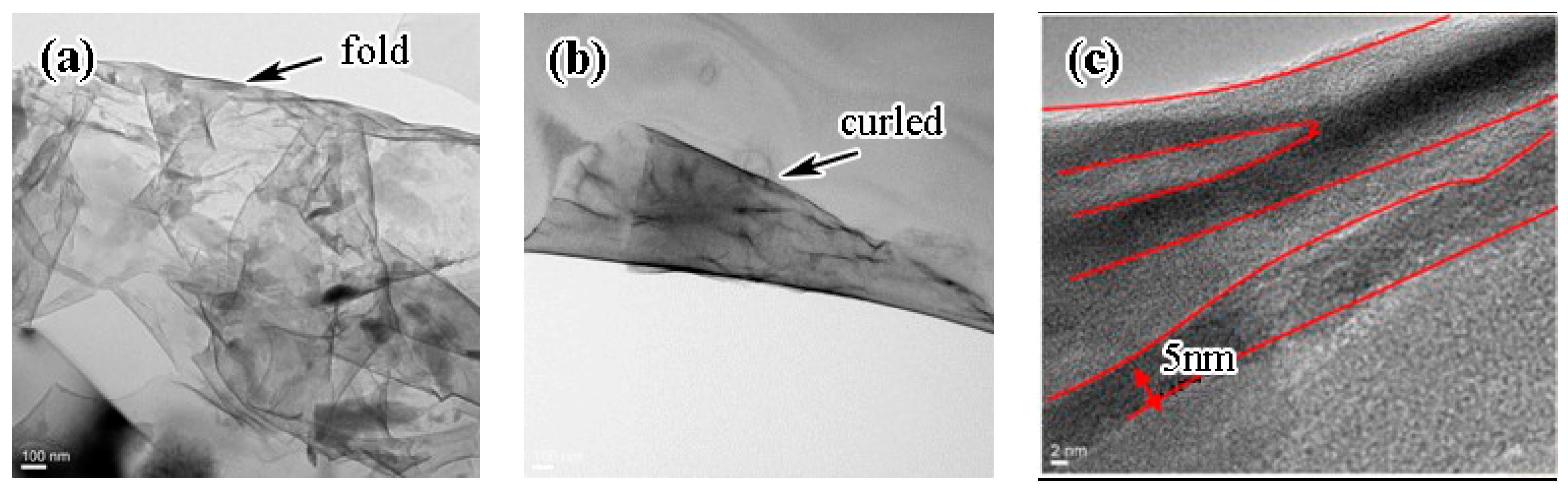
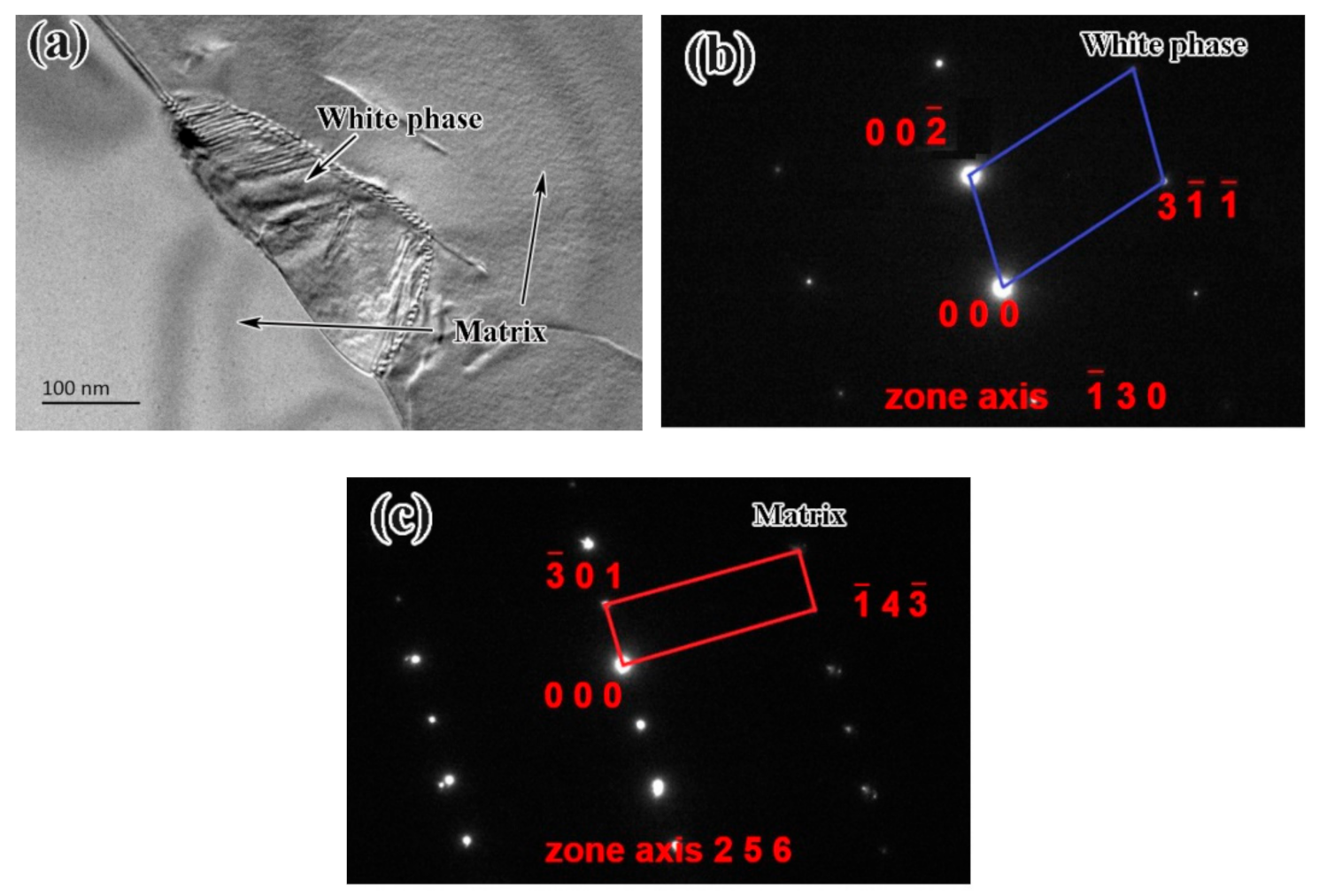
3.2. Microstructure of GO/TiAl Composites
3.3. Properties of GO/TiAl Composites
4. Discussion
5. Conclusions
- The forms of GO/TiAl powders’ bonding are C–C, C–O, and O–C=O. The defects are observed in GO, and there are more than 3 layers of GO produced in the composites.
- GO can be homogeneously dispersed into TiAl matrix in the form of random film with the diameter less than 10 μm. The minimum thickness of the GO film is about 5 nm.
- The compression strength and heat conductivity of the composites can be improved distinctly at both room temperature and high temperature. In the GO/TiAl composites, the heat conductivity coefficient can reach to above 23 W/m K, and the compressive strength can reach to 1700 MPa at room temperature.
- The GO does not well represent thermodynamically stable reinforcement at high temperatures, a certain amount of Ti3AlC is formed along the grain boundaries, and its size is about 2 μm rather than nanoscale, which can decrease the ductile reinforcement of GO in the TiAl matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.-W. Ordered intermetallic alloy, parts: Gamma titanium aluminides. JOM 1994, 46, 30–39. [Google Scholar] [CrossRef]
- Kim, Y.-W. Gamma Titanium Aluminides; TMS: Warrendale, PA, USA, 1995; p. 637. [Google Scholar]
- Thomas, M.; Raviart, J.L.; Popoff, F. Cast and PM Processing development in gamma titanium aluminides. Intermatallics 2005, 13, 944–951. [Google Scholar] [CrossRef]
- Rao, K.P.; Vyas, A. Comparison of titanium silicide and carbide reinforced in situ synthesized TiAl composites and their mechanical properties. Intermetallics 2011, 19, 1236–1242. [Google Scholar] [CrossRef]
- Lapin, L.; Štamborská, M.; Kamyshnykova, K.; Pelachová, T.; Klimová, A.; Bajana, O. Room temperature mechanical behavior of cast in-situ TiAl matrix composite reinforced with carbide particles. Intermetallics 2019, 105, 113–123. [Google Scholar] [CrossRef]
- Sun, F.; Wang, K.; Yang, H.; Shi, Y.; Yang, J.; Lou, M. Investigation of graphene reinforced titanium matrix composites preparation process and properties. Titan. Ind. Prog. 2019, 36, 8–12. [Google Scholar]
- Cao, Z.; Wang, X.; Li, J. Reinforcement with graphene nanoflakes in titanium matrix composites. J. Alloys Compd. 2017, 6, 498–502. [Google Scholar] [CrossRef]
- Zhang, L.P. Properties analysis and preparation with grapheme magnesium matrix composite in sports equipment. Hot Work. Technol. 2015, 44, 140–142. [Google Scholar]
- Luo, J.; Jiang, S.; Zhang, H. A novel non_enzymatic glu-cose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Acta 2011, 709, 47. [Google Scholar]
- Bastwros, M.; Kim, G.Y.; Zhu, C.; Zhang, K.; Wang, S.; Tang, X.; Wang, X. Effect of ball milling on graphene reinforced Al6061 composite fabricated by semi-solid sintering. Composites 2014, 60, 111–118. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, X. Study on Preparation of CNTs/TiAl Alloy. Composites by Hot Pressed Sintering; Anhui University of Technology: Maanshan, China, 2016; pp. 4–16. [Google Scholar]
- Rafieerad, A.R.; Bushroa, A.R. Graphene Oxide Modifide Anodic Temary Nanobioceramics on Ti6Al7Nb Alloy for orthopedic and dental application. Procedia Eng. 2017, 4, 409–417. [Google Scholar] [CrossRef]
- Song, X.L.; Wang, H.B.; Wu, X.L. Research and development of dispersion technique for nanoparticles. Chem. Ind. Eng. Prog. 2005, 24, 47–52. [Google Scholar]
- Yang, L.; Wang, L.; Ren, S.; Che, W. Research on mechanical of graphene reinforced TiAl self-lubricating composite materials. J. Mech. Strenghth 2019, 41, 1085–1089. [Google Scholar]
- Li, M. Effect of Boron of Carbon on the High. Temperature Feformation Microstructure of TiAl Alloy; Harbin Institude of Techonology: Harbin, China, 2019. [Google Scholar]




| Materials | Temperature (°C) | Heat Conductivity Coefficient (W/m K) | Thermal Diffusion Coefficient (m2/s) | Density (g/cm3) |
|---|---|---|---|---|
| GO/TiAl | Room Temp. | 15.65 | 6.35 | 3.982 |
| 500 °C | 23.79 | 8.07 | - | |
| TiAl | Room Temp. | 14.62 | 5.86 | 3.976 |
| 500 °C | 22.62 | 7.97 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zhu, L.; Mo, X.; Nan, H.; Ding, X. Microstructure Characterization and Properties of Graphene Oxide-Reinforced TiAl Matrix Composites. Metals 2021, 11, 883. https://doi.org/10.3390/met11060883
Sun Z, Zhu L, Mo X, Nan H, Ding X. Microstructure Characterization and Properties of Graphene Oxide-Reinforced TiAl Matrix Composites. Metals. 2021; 11(6):883. https://doi.org/10.3390/met11060883
Chicago/Turabian StyleSun, Zhiyu, Langping Zhu, Xiaofei Mo, Hai Nan, and Xianfei Ding. 2021. "Microstructure Characterization and Properties of Graphene Oxide-Reinforced TiAl Matrix Composites" Metals 11, no. 6: 883. https://doi.org/10.3390/met11060883
APA StyleSun, Z., Zhu, L., Mo, X., Nan, H., & Ding, X. (2021). Microstructure Characterization and Properties of Graphene Oxide-Reinforced TiAl Matrix Composites. Metals, 11(6), 883. https://doi.org/10.3390/met11060883






