Removal of Sb Impurities in Copper Electrolyte and Evaluation of As and Fe Species in an Electrorefining Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory-Scale and Industrial Sb/Bi Removal Plants
2.2. Analytical Techniques
3. Results and Discussion
3.1. Removal of Sb Species from Copper Electrolyte by Ion Exchange Resin
3.1.1. Laboratory-Scale Plant
3.1.2. Industrial Sb/Bi Removal Plant
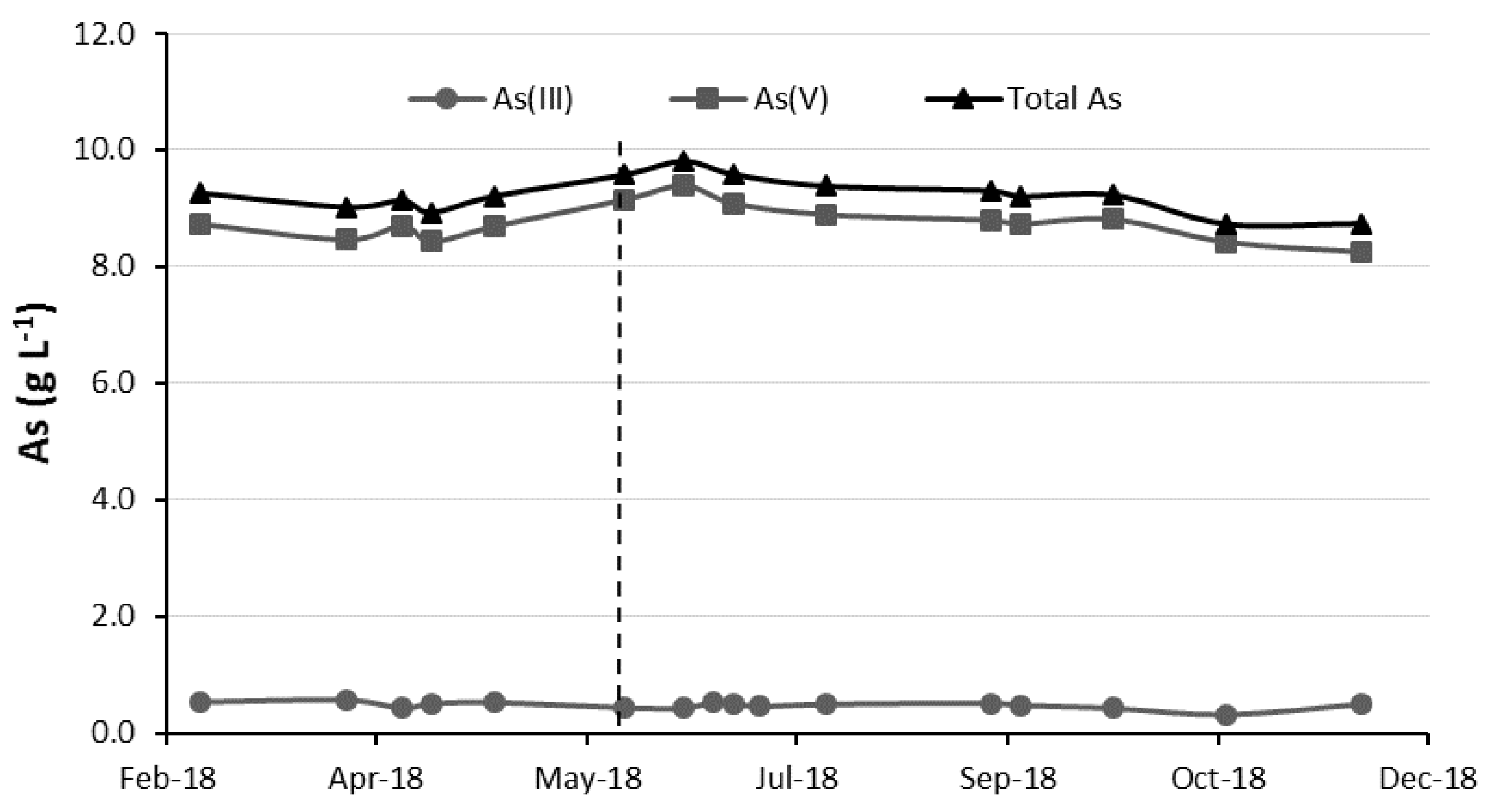
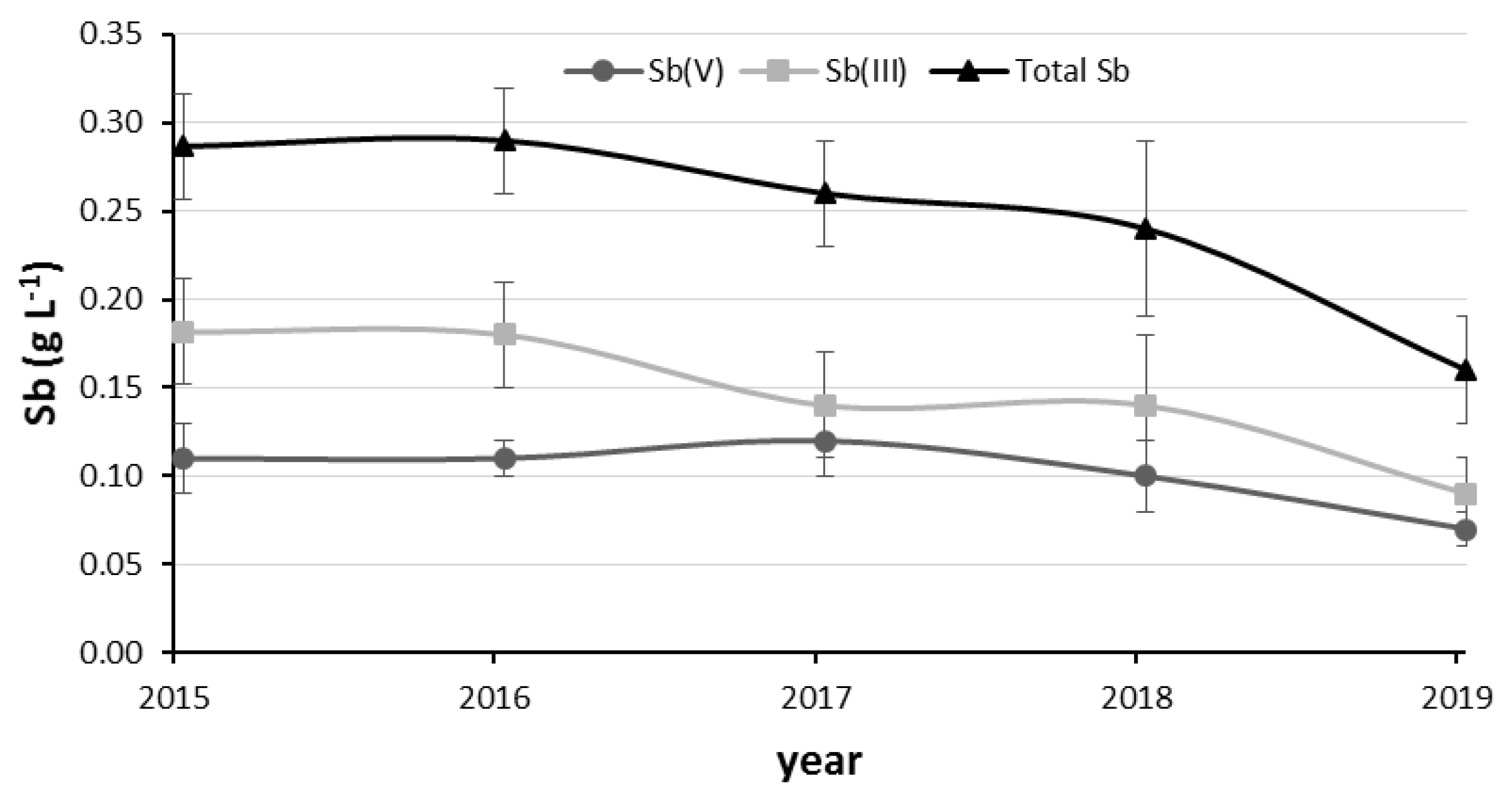
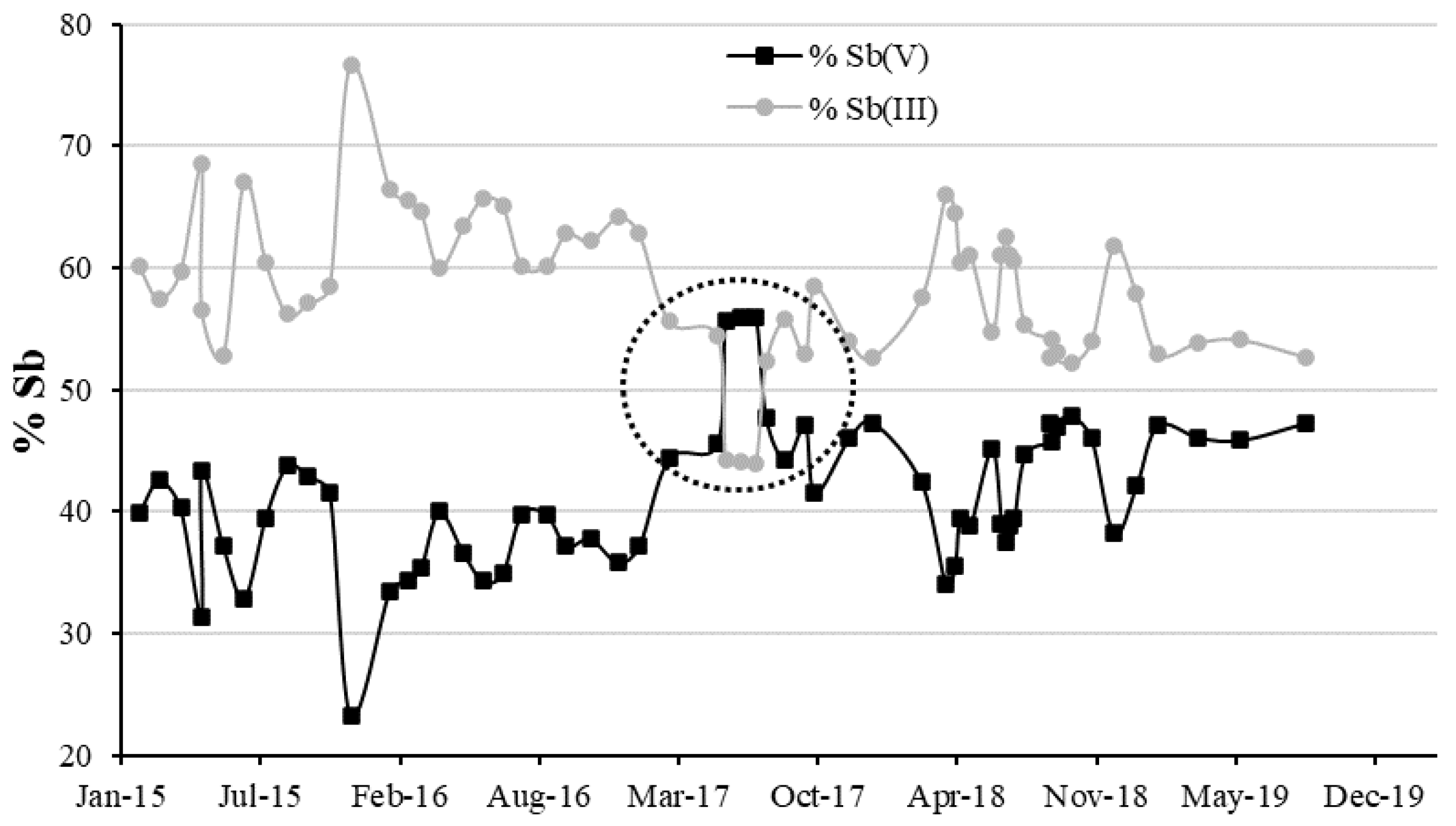
3.2. Evolution of Sb, As and Fe in the Copper Electrolyte
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Chen, Q.; Ying, Z.; Wang, M.; Xiao, B.; Zhang, F. Homogeneous precipitation of As, Sb and Bi impurities in copper electrolyte during electrorefining. Hydrometallurgy 2011, 105, 355–358. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Liu, B.; Wang, M.; Wang, H.; Liu, X.; Zhou, S. Promotion of copper electrolyte self-purification with antimonic oxides. Hydrometallurgy 2018, 175, 28–34. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. Mineralogical characterization of a copper anode and the anode slimes from the La Caridad copper refinery of Mexicana de Cobre. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2005, 36, 229–240. [Google Scholar] [CrossRef]
- Hiskey, J.B. Mechanism and thermodynamics of floating slimes formation. In Proceedings of the T.T. Chen Honorary Symposium on Hydrometallurgy, Electrometallurgy and Materials Characterization, Orlando, FL, USA, 11–15 March 2012. [Google Scholar]
- Jafari, S.; Kiviluoma, M.; Kalliomäki, T.; Klindtworth, E.; Aji, A.T.; Aromaa, J.; Wilson, B.P.; Lundström, M. Effect of typical impurities for the formation of floating slimes in copper electrorefining. Int. J. Min. Process. 2017, 168, 109–115. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Review of copper pyrometallurgical practice: Today and tomorrow. Miner. Eng. 2003, 16, 893–919. [Google Scholar] [CrossRef]
- Xiao, F.; Cao, D.; Mao, J.; Shen, X.; Ren, F. Role of Sb(V) in removal of As, Sb and Bi impurities from copper electrolyte. Trans. Nonferr. Metals Soc. China 2013, 20, 9–16. [Google Scholar] [CrossRef]
- Xiao, F.; Mao, J.; Cao, D.; Shen, X.; Ren, F. Formation of antimonate in co-precipitation reaction of As, Sb and Bi in copper electrolytes. Miner. Eng. 2012, 35, 9–15. [Google Scholar] [CrossRef]
- Abe, S.; Takasawa, Y. Prevention of floating slimes precipitation in copper electrorefining. In The Electrorefining and Winning of Copper: Proceedings of the Symposium Sponsored by TMS Copper, Nickel, Cobalt, Precious Metals, and Eletrolytic Processes Committees, Denver, CO, USA, 24–26 February 1987; Hoffmann, J.E., Bautista, R.G., Ettel, V.A., Wesely, R.J., Eds.; The Metallurgical Society: Warrendale, PA, USA, 1987; pp. 87–98. [Google Scholar]
- Beauchemin, S.; Chen, T.T.; Dutrizac, J. Behaviour of antimony and bismuth in copper electrorefining circuits. Can. Metall. Q. 2008, 47, 9–96. [Google Scholar] [CrossRef]
- Petkova, E. Mechanisms of floating slime formation and its removal with the help of sulphur dioxide during the electrorefining of anode copper. Hydrometallurgy 1997, 3, 277–286. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Yin, Z.; Xiao, L. Identification of arsenato antimonates in copper anode slimes. Hydrometallurgy 2006, 84, 211–217. [Google Scholar]
- Braun, T.B.; Rawling, J.R.; Richards, K.J. Factors Affecting the Quality of Electrorefined Cathode Copper. Extr. Metall. Copp. 1976, 1, 511–524. [Google Scholar]
- Moats, M.S.; Aslin, N.; Pranowo, A.; Alvear, F.G.R.F. Arsenic’s Behaviour and Benefits in Copper Electrorefining. CIM J. 2016, 7, 3. [Google Scholar] [CrossRef]
- Hyvarinen, O.V.J. Process for Selective Removal of Bismuth and Antimony from an Electrolyte, Especially in Electrolytic Refining of Copper. U.S. Patent 4157946, 12 June 1979. [Google Scholar]
- Navarro, P.; Alguacil, F.P. Adsorption of antimony and arsenic from a copper electrorefining solution onto activated carbon. Hydrometallurgy 2002, 66, 101–105. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Yin, Z.; Zhang, P.; Long, Z.; Su, Z. Removal of impurities from copper electrolyte with adsorbent containing antimony. Hydrometallurgy 2003, 69, 39–44. [Google Scholar] [CrossRef]
- Riveros, P.A.; Dutrizac, J.E. A study of the ion exchange removal of antimony(III) and antimony(V) from copper electrolytes. Can. Metall. Q. 2008, 47, 307–315. [Google Scholar] [CrossRef]
- Ando, K.; Tsuchida, N. Recovering Bi and Sb from Electrolyte in Copper Electrorefining. JOM 1997, 49, 49–51. [Google Scholar] [CrossRef]
- Cunningham, R.M.; Calara, J.V.; King, M.G. Removal of antimony and bismuth from copper electrolyte development of a commercial plant at Amarillo copper refinery. In EPD Congress 1997, Proceedings of Sessions and Symposia Held at the TMS Annual Meeting, Orlando, FL, USA, 9–13 February 1997; Mishra, B., Ed.; Minerals, Metals & Materials Society: Warrendale, PA, USA, 1997; pp. 453–460. [Google Scholar]
- Artzer, A.; Moats, M.; Bender, J. Removal of Antimony and Bismuth from Copper Electrorefining Electrolyte: Part II—An Investigation of Two Proprietary Solvent Extraction Extractants. JOM 2018, 70, 2856–2863. [Google Scholar] [CrossRef]
- Navarro, P.; Simpson, J.; Alguacil, F.J. Removal of antimony (III) from copper in sulphuric acid solutions by solvent extraction with LIX 1104SM. Hydrometallurgy 1999, 53, 121–131. [Google Scholar] [CrossRef]
- Arroyo-Torralvo, F.; Rodríguez-Almansa, A.; Ruiz, I.; González, I.; Ríos, G.; Fernández-Pereira, C.; Vilches-Arenas, L.F. Optimizing operating conditions in an ion exchange column treatment applied to the removal of Sb and Bi impurities from an electrolyte of a copper electro-refining plant. Hydrometallurgy 2017, 171, 285–297. [Google Scholar] [CrossRef]
- Bothelo Junior, A.B.; André de Alburquerque, V.; Espinosa, D.C.R.; Tenório, J.A.S. Effect of iron oxidation state for copper recovery from nickel laterite leach solution using chelating resin. Sep. Sci. 2020, 55, 788–798. [Google Scholar] [CrossRef]
- Nagai, T. Purification of copper electrolyte by solvent extraction and ion exchange techniques. Miner. Process. Extr. Metall. Rev. 1997, 17, 143–168. [Google Scholar] [CrossRef]
- Hoffmann, J.E. The purification of copper refinery electrolyte. JOM 2004, 56, 30–33. [Google Scholar] [CrossRef]
- McKevitt, B.R. Removal of Iron by ion Exchange from Copper Electrowinning Electrolyte Solutions Containing Antimony and Bismuth. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2007. Available online: https://open.library.ubc.ca/cIRcle/collections/24/items/1.0066602 (accessed on 7 May 2021).
- McKevitt, B.R.; Dreisinger, D.A. Comparison of various ion exchange resins for the removal of ferric ions from copper electrowinning electrolyte solutions part II: Electrolytes containing antimony and bismuth. Hydrometallurgy 2009, 98, 122–127. [Google Scholar] [CrossRef]
- de las Torres, A.I.G.; Moats, M.S.; Ríos, G.; Almansa, A.R.; Sánchez-Rodas, D. Arsenic and antimony speciation analysis in copper electrolyte by liquid chromatography coupled to hydride generation atomic fluorescence spectrometry (HPLC-HG-AFS). Anal. Methods 2020, 12, 1943–1948. [Google Scholar] [CrossRef]
- Rodier, J.; Broutin, J.P.; Chambon, P.; Champsaur, H.; Rodi, L. L’Analyse des Eaux; Dunod: Paris, France, 1996; p. 1383. [Google Scholar]
- Ahn, J.W.; Seo, J.S. A study on the removal of As, Sb, Bi from copper sulphate solutions by Ion exchange resin containing Aminophosphonic acid as a functional group. J. Korean Inst. Res. Recycl. 2012, 21, 50–57. [Google Scholar]
- Norman, N.C. Chemistry of Arsenic. Antimony and Bismuth; Blackie Academic and Professional: London, UK, 1998. [Google Scholar]
- Riveros, P.A. The removal of antimony from copper electrolytes using amino-phosphonic resins: Improving the elution of pentavalent antimony. Hydrometallurgy 2010, 105, 110–114. [Google Scholar] [CrossRef]
- Schlesinger, M.; King, M.; Sole, K.; Davenport, W. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Oxford, UK, 2011; p. 255. [Google Scholar]
- Dreisinger, D.B.; Leong, B.J.Y.; Saito, B.R.; Est-Sells, P.G. The solvent extraction and ion exchange removal of As, Sb and Bi from copper sulphate-sulphuric acid solutions. MME 1993, 49, 801–815. [Google Scholar]



| Initial Electrolyte | Electrolyte + Cu Shavings | Electrolyte + Cu Shavings + Ion Exchange Resin | |
|---|---|---|---|
| mean ± sd | mean ± sd | mean ± sd | |
| Sb(V) | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.07 ± 0.01 |
| Sb(III) | 0.20 ± 0.04 | 0.19 ± 0.07 | 0.01 ± 0.01 |
| Total Sb | 0.32 ± 0.06 | 0.32 ± 0.08 | 0.08 ± 0.03 |
| Fe(II) | 0.90 ± 0.07 | 0.92 ± 0.07 | 0.93 ± 0.07 |
| Fe(III) | 0.08 ± 0.04 | 0.05 ± 0.03 | 0.05 ± 0.04 |
| Total Fe | 0.98 ± 0.06 | 0.97 ± 0.08 | 0.98 ± 0.08 |
| Initial Electrolyte | Electrolyte + Cu Shavings | Electrolyte + Cu Shavings + Ion Exchange Resin | |
|---|---|---|---|
| mean ± sd | mean ± sd | mean ± sd | |
| Sb(V) | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.00 |
| Sb(III) | 0.12 ± 0.01 | 0.14 ± 0.04 | 0.00 ± 0.00 |
| Total Sb | 0.21 ± 0.01 | 0.21 ± 0.03 | 0.04 ± 0.00 |
| Fe(II) | 0.85 ± 0.03 | 0.86 ± 0.02 | 0.86 ± 0.05 |
| Fe(III) | 0.06 ± 0.02 | 0.05 ± 0.02 | 0.05 ± 0.01 |
| Total Fe | 0.91 ± 0.03 | 0.91 ± 0.03 | 0.91 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González de las Torres, A.I.; Moats, M.S.; Ríos, G.; Rodríguez Almansa, A.; Sánchez-Rodas, D. Removal of Sb Impurities in Copper Electrolyte and Evaluation of As and Fe Species in an Electrorefining Plant. Metals 2021, 11, 902. https://doi.org/10.3390/met11060902
González de las Torres AI, Moats MS, Ríos G, Rodríguez Almansa A, Sánchez-Rodas D. Removal of Sb Impurities in Copper Electrolyte and Evaluation of As and Fe Species in an Electrorefining Plant. Metals. 2021; 11(6):902. https://doi.org/10.3390/met11060902
Chicago/Turabian StyleGonzález de las Torres, Ana I., Michael S. Moats, Guillermo Ríos, Ana Rodríguez Almansa, and Daniel Sánchez-Rodas. 2021. "Removal of Sb Impurities in Copper Electrolyte and Evaluation of As and Fe Species in an Electrorefining Plant" Metals 11, no. 6: 902. https://doi.org/10.3390/met11060902







