Study on the Correlation between the Microstructure Characteristics and Corrosion Behaviors of 2A12-T4 Aluminum Alloy under Thermal Strain
Abstract
:1. Introduction
2. Materials and Methods
3. Results
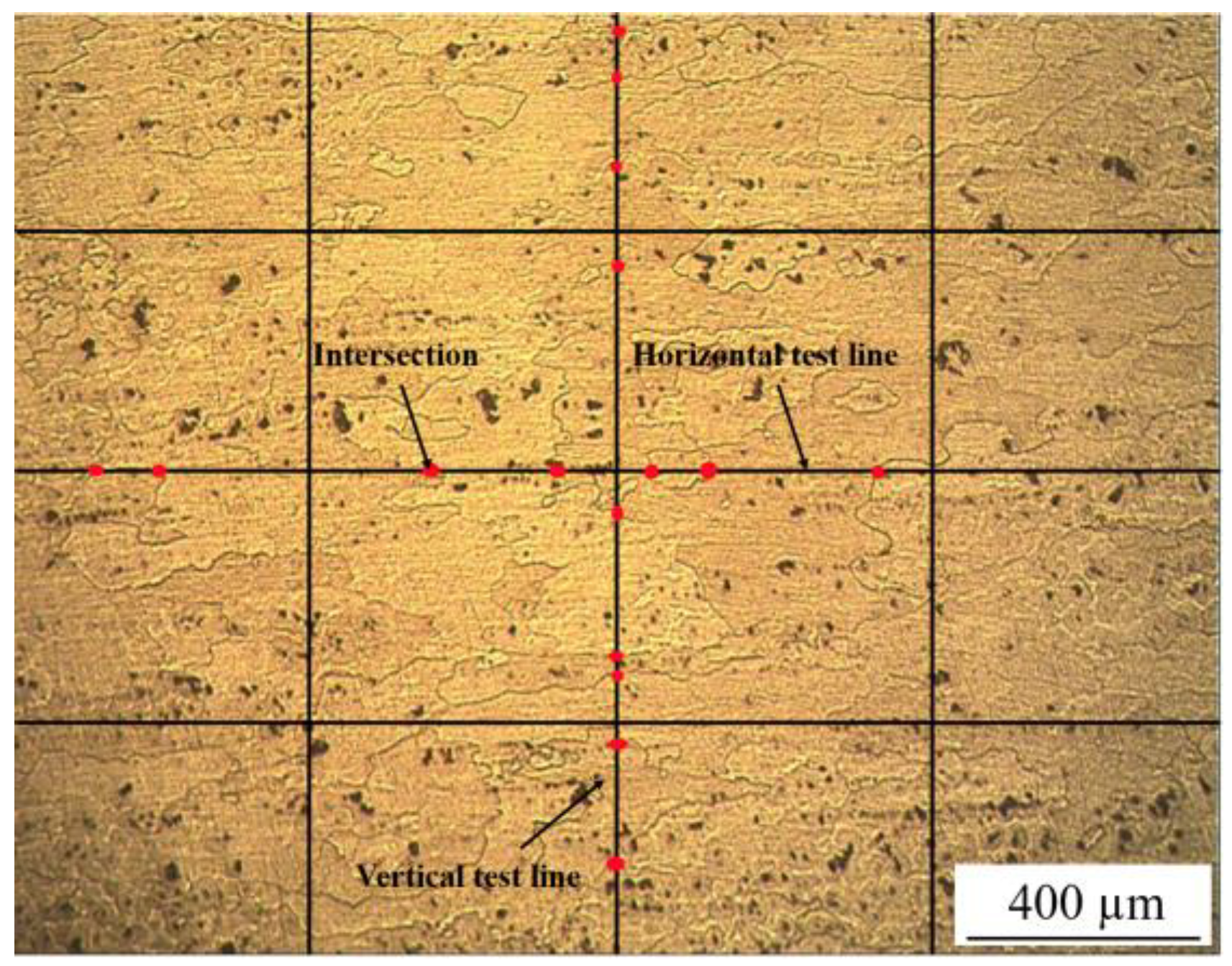
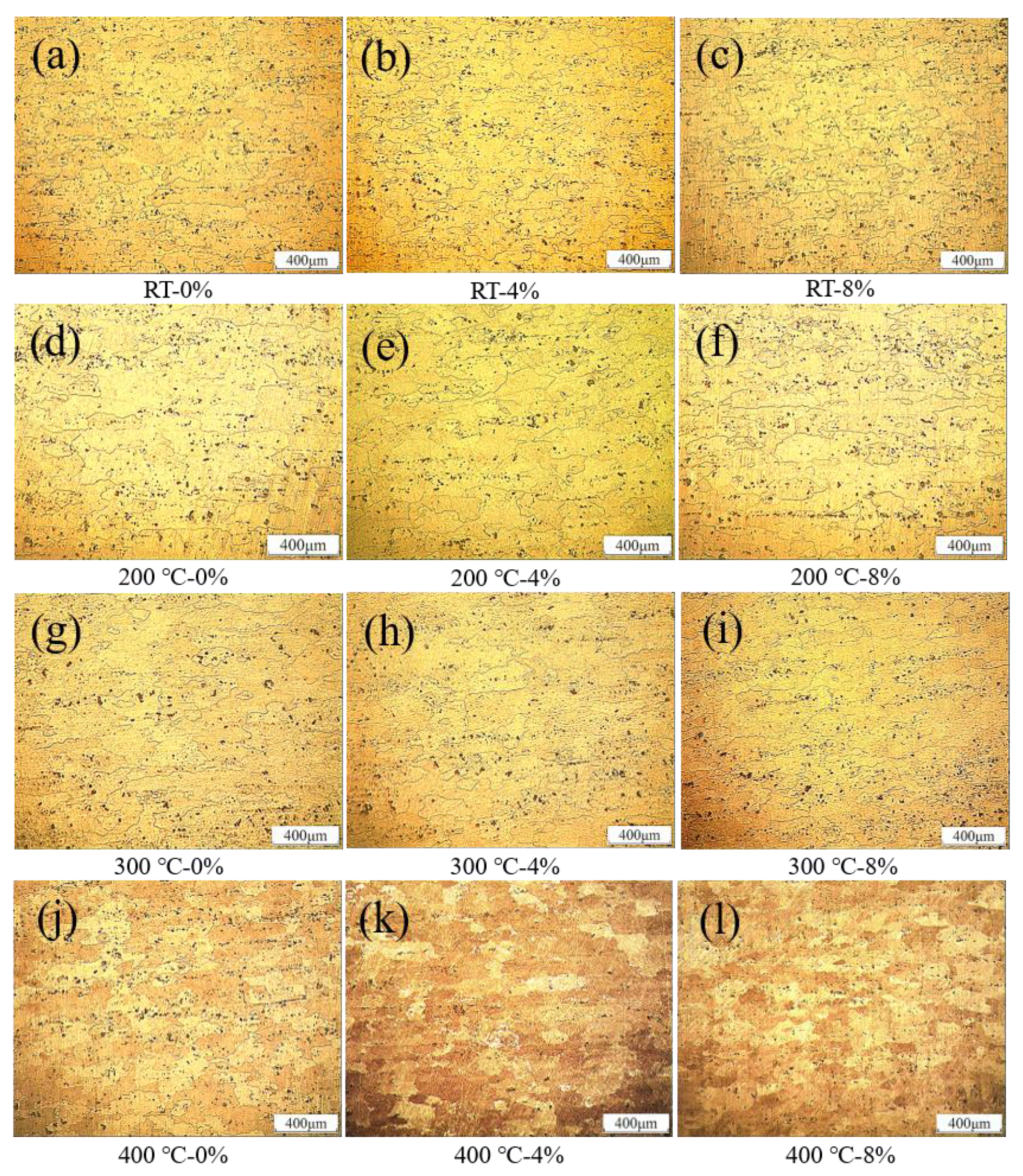
3.1. Microscopic Observation
3.1.1. Metallographic Observation
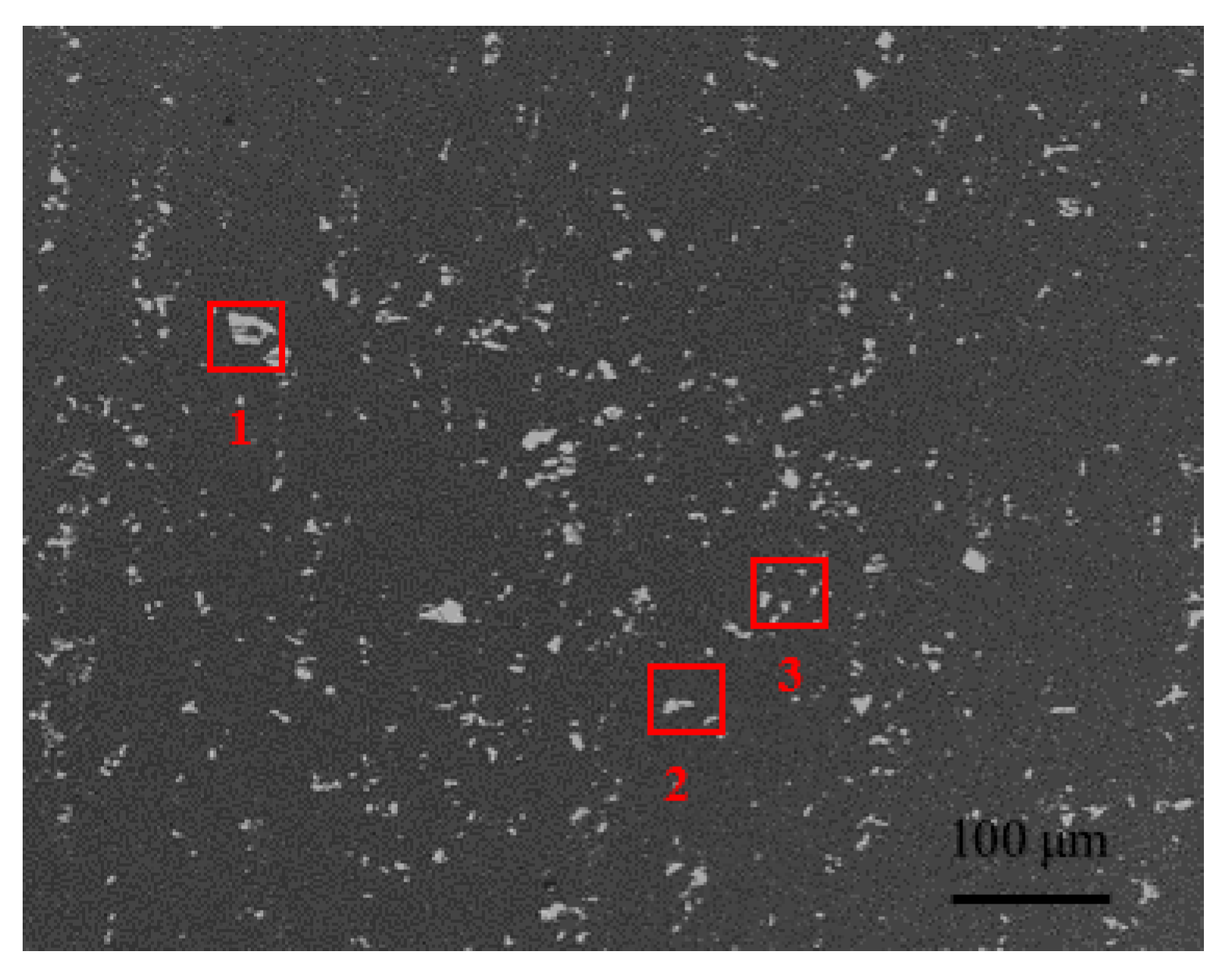
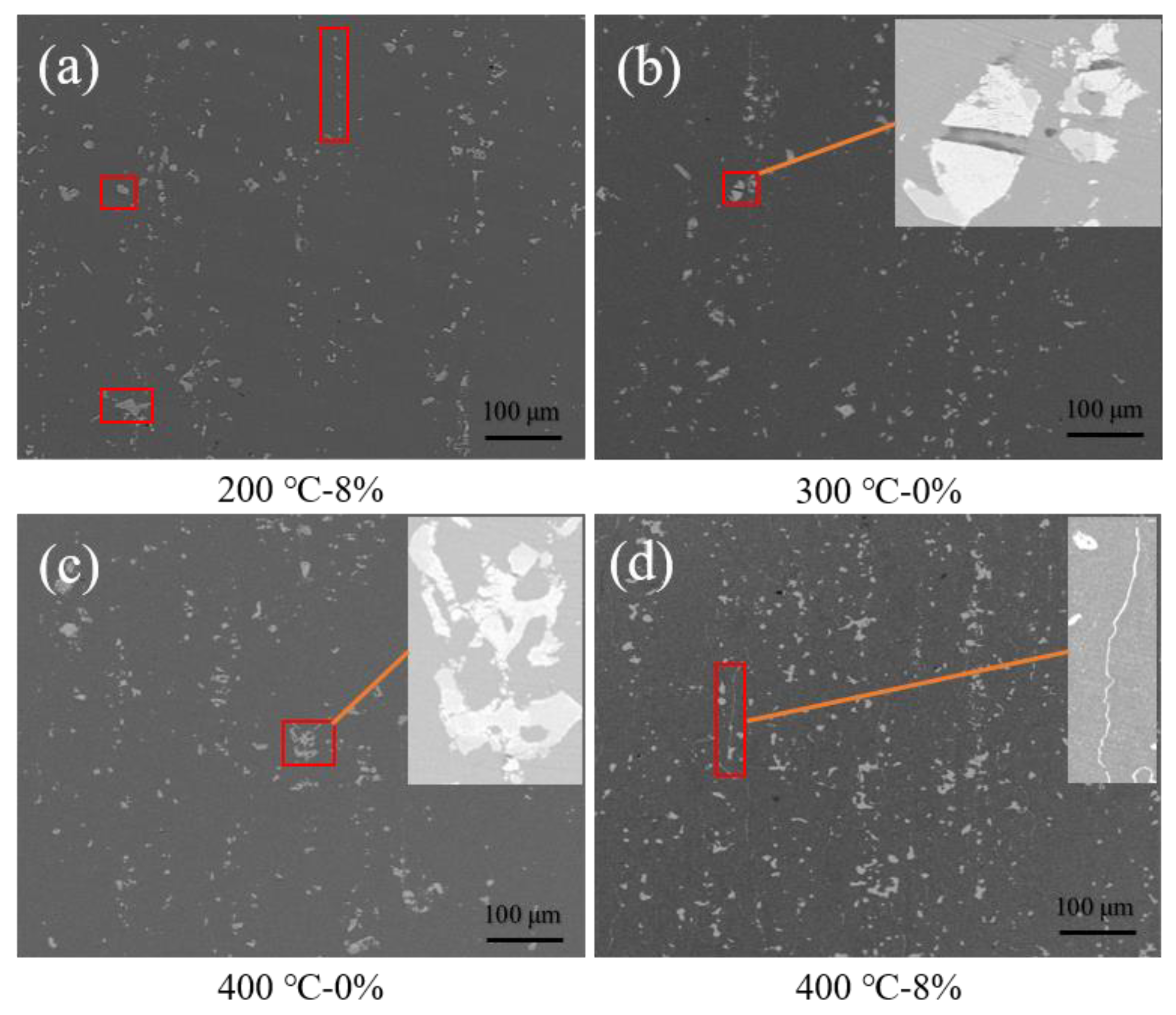
3.1.2. Precipitated Phase Observation
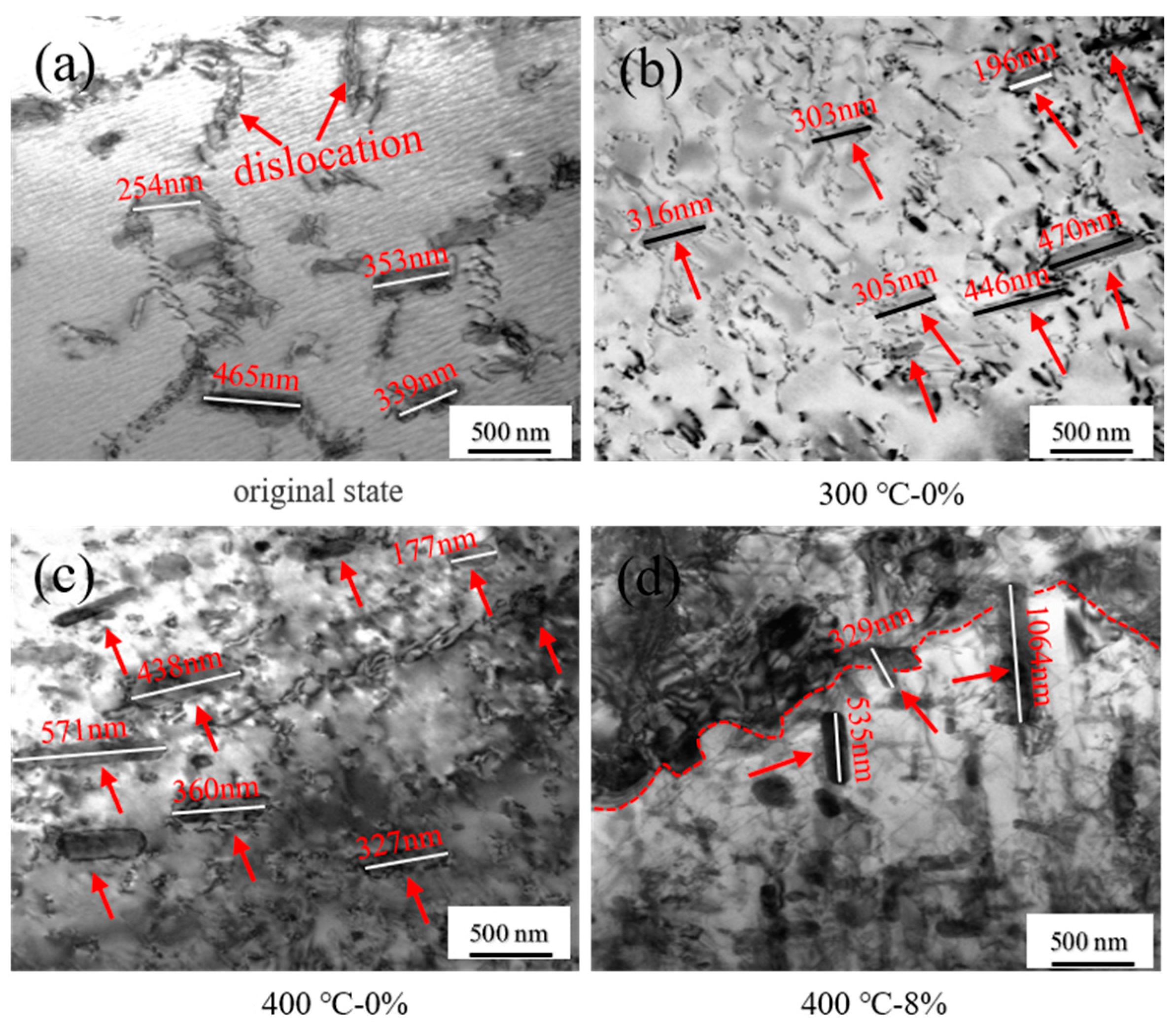
3.1.3. TEM Observation
3.2. Corrosion Behavior Study
3.2.1. Polarization Curve Test
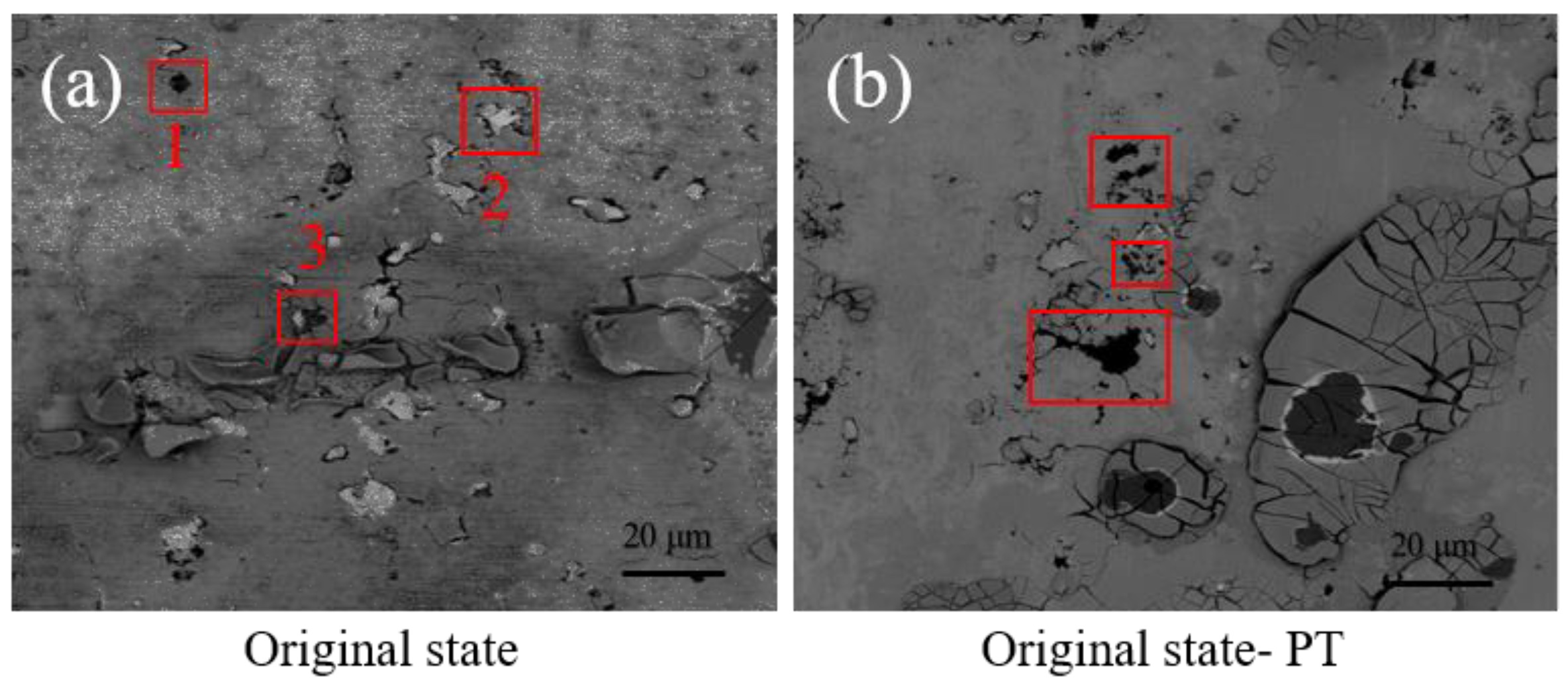
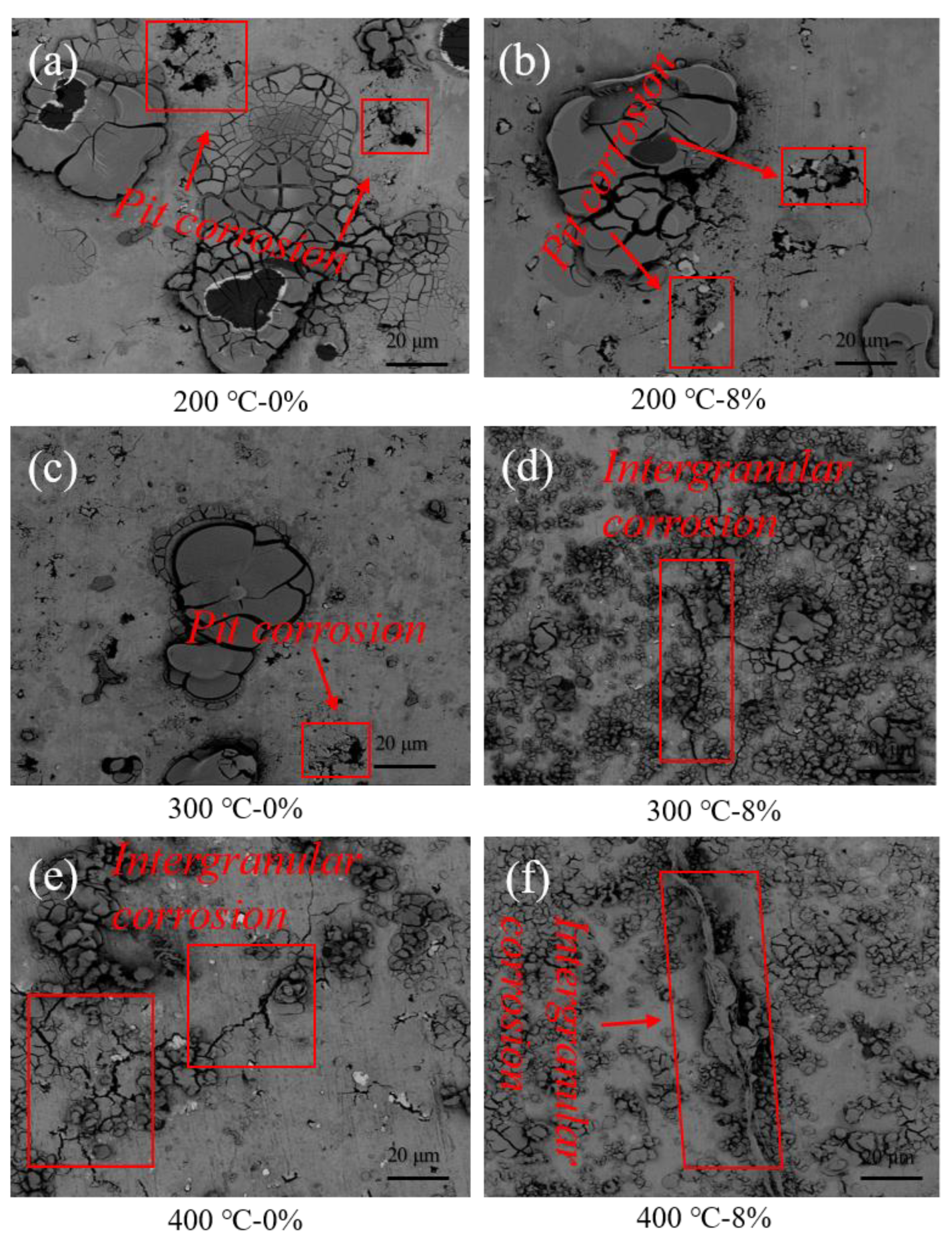
3.2.2. Corrosion Morphology Observation
4. Conclusions
- The precipitated phase was distributed along the direction of tensile strain, and the grain size coarsened from 152 to 260 µm (even more than 280 µm), after experiencing peak temperatures of 300 and 400 °C.
- The risk of corrosion for the samples that experienced thermal strain increased compared to those that did not undergo tensile strain. The samples that experienced a peak temperature of 300 °C presented the best corrosion resistance, as the precipitated phase was evenly distributed in the matrix. When the peak temperature was 400 °C and the strain was 8%, the number and density of the precipitated phase increased due to the dynamic recrystallization, and the worsening corrosion resistance of this sample.
- Dynamic recrystallization occurred in the sample with a peak temperature of 400 °C, and the precipitated phase was mainly distributed along the grain boundaries. This led to the decrease of the corrosion resistance of the grain boundaries, and corrosion developed from pitting corrosion to intergranular corrosion.
Author Contributions
Funding
Conflicts of Interest
References
- Xu, W.-F.; Ma, J.; Wang, M.; Lu, H.-J.; Luo, Y.-X. Effect of cooling conditions on corrosion resistance of friction stir welded 2219-T62 aluminum alloy thick plate joint. Trans. Nonferrous Met. Soc. China 2020, 30, 1491–1499. [Google Scholar] [CrossRef]
- Li, G.; Zhou, L.; Luo, L.; Wu, X.; Guo, N. Microstructural evolution and mechanical properties of refill friction stir spot welded alclad 2A12-T4 aluminum alloy. J. Mater. Res. Technol. 2019, 8, 4115–4129. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Wang, Y.; Xiao, G.; Liu, Y.; Zeng, L. Microstructure and mechanical properties of thixoforged complex box-type component of 2A12 aluminum alloy. Mater. Des. 2020, 193, 108859. [Google Scholar] [CrossRef]
- Du, C.; Zhao, H.; Dai, Z.; Tian, Z.; Wang, J.; Wang, Z. The preparation and properties of black coating by micro arc oxidation on 2A12 aluminum alloy. Mater. Lett. 2018, 236, 723–726. [Google Scholar] [CrossRef]
- Prapas, M.; Jennarong, N.; Woraphot, P. Effect of post-weld heat treatment on microstructure and mechanical properties of friction stir welded SSM7075 aluminum alloy. J. Wuhan Univ. Technol. 2017, 32, 1420–1425. [Google Scholar] [CrossRef]
- Sadoun, A.M.; Meselhy, A.F.; Deabs, A.W. Improved strength and ductility of friction stir tailor-welded blanks of base metal AA2024 reinforced with interlayer strip of AA7075. Results Phys. 2020, 16, 102911. [Google Scholar] [CrossRef]
- Rajendran, C.; Srinivasan, K.; Balasubramanian, V.; Balaji, H.; Selvaraj, P. Effect of tool tilt angle on strength and microstructural characteristics of friction stir welded lap joints of AA2014-T6 aluminum alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 1824–1835. [Google Scholar] [CrossRef]
- Iqbal Perwej, M.; Jain, R.; Pal, S.K. Numerical and experimental study on friction stir welding of aluminum alloy pipe. J. Mater. Process. Technol. 2019, 274, 116258. [Google Scholar] [CrossRef]
- Deng, H.; Chen, Y.; Jia, Y.; Pang, Y.; Zhang, T.; Wang, S.; Yin, L. Microstructure and mechanical properties of dissimilar NiTi/Ti6Al4V joints via back-heating assisted friction stir welding. J. Manuf. Process. 2021, 64, 379–391. [Google Scholar] [CrossRef]
- Sen, M.; Shankar, S.; Chattopadhyaya, S. Investigations into FSW joints of dissimilar aluminum alloys. Mater. Today Proc 2020, 27, 2455–2462. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, Y.; Kang, J.; Fu, R.-D.; Guo, X.-D.; Li, Y.-J. Effect of precipitate evolution on corrosion behavior of friction stir welded joints of AA2060-T8 alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 701–709. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Zhou, L.; Meng, G.; Liu, B.; Wang, J.; Shao, Y.; Jiang, J. Macro-galvanic effect and its influence on corrosion behaviors of friction stir welding joint of 7050-T76 Al alloy. Corros. Sci. 2019, 164, 108360. [Google Scholar] [CrossRef]
- Bocchi, S.; Cabrini, M.; D’Urso, G.; Giardini, C.; Lorenzi, S.; Pastore, T. Stress enhanced intergranular corrosion of friction stir welded AA2024-T3. Eng. Fail. Anal. 2020, 111, 104483. [Google Scholar] [CrossRef]
- Bousquet, E.; Poulon-Quintin, A.; Puiggali, M.; Devos, O.; Touzet, M. Relationship between microstructure, microhardness and corrosion sensitivity of an AA 2024-T3 friction stir welded joint. Corros. Sci. 2011, 53, 3026–3034. [Google Scholar] [CrossRef]
- Wadeson, D.A.; Zhou, X.; Thompson, G.E.; Skeldon, P.; Djapic Oosterkamp, L.; Scamans, G. Corrosion behaviour of friction stir welded AA7108 T79 aluminium alloy. Corros. Sci. 2006, 48, 887–897. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Wang, K.; Cai, J.; Qiao, K. Effect of Travel Speed on the Stress Corrosion Behavior of Friction Stir Welded 2024-T4 Aluminum Alloy. J. Mater. Eng. Perform. 2016, 25, 1820–1828. [Google Scholar] [CrossRef]
- Yan, J.; Zeng, X.; Gao, M.; Lai, J.; Lin, T. Effect of welding wires on microstructure and mechanical properties of 2A12 aluminum alloy in CO2 laser-MIG hybrid welding. Appl. Surf. Sci. 2009, 255, 7307–7313. [Google Scholar] [CrossRef]
- Chen, G.; Fu, X.; Zhao, F.; Zhou, W. Microstructure and mechanical properties of 2A12 aluminum alloy after age forming. Trans. Nonferrous Met. Soc. China 2012, 22, 1975–1980. [Google Scholar] [CrossRef]
- Cui, Q.; Yi, D.; Wang, H.; Zhang, J.; Xu, J.; Wang, B. Effects of grain size and secondary phase on corrosion behavior and electrochemical performance of Mg-3Al-5Pb-1Ga-Y sacrificial anode. J. Rare Earths 2019, 37, 1341–1350. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, T.; He, Y.; Liu, D.; Wang, J.; Du, X.; Ma, B. Long-term atmospheric corrosion of aluminum alloy 2024-T4 in coastal environment: Surface and sectional corrosion behavior. J. Alloys Compd. 2019, 789, 460–471. [Google Scholar] [CrossRef]
- Chen, M.; Deng, Y.; Tang, J.; Fan, S.; Zhang, X. A study of the crystallographic pitting behavior of Al-0.54Mg-0.66Si aluminum alloy in acidic chloride solutions. Mater. Charact. 2019, 148, 259–265. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; de Wit, J.H.W. Effect of solution heat treatment on galvanic coupling between intermetallics and matrix in AA7075-T6. Corros. Sci. 2003, 45, 1733–1746. [Google Scholar] [CrossRef]

| Peak Temperature (°C) | Strain (%) | Heating Rate (°C/s) | Cooling Rate (°C/s) | Peak Temperature Residence Time (s) |
|---|---|---|---|---|
| RT | 0, 4,8 | - | - | - |
| 200 | 0, 4, 8 | 50 | 5 | 1 |
| 300 | 0, 4, 8 | 50 | 5 | 1 |
| 400 | 0, 4, 8 | 50 | 5 | 1 |
| Peak Temperature (°C) | Grain Size (µm) |
|---|---|
| RT—0% | 152.04 ± 1 |
| RT—4% | 171.05 ± 1 |
| RT—8% | 177.09 ± 1 |
| 200 °C—0% | 211.84 ± 1 |
| 200 °C—4% | 233.03 ± 1 |
| 200 °C—8% | 245.01 ± 1 |
| 300 °C—0% | 261.04 ± 1 |
| 300 °C—4% | 269.46 ± 1 |
| 300 °C—8% | 275.21 ± 1 |
| 400 °C—0% | 271.55 ± 1 |
| 400 °C—4% | 279.61 ± 1 |
| 400 °C—8% | 282.09 ± 1 |
| Element | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| w/% | x/% | w/% | x/% | w/% | x/% | |
| Mg | 0.67 | 0.88 | 8.57 | 10.90 | 8.34 | 10.70 |
| Al | 60.46 | 71.90 | 56.11 | 71.20 | 63.24 | 75.36 |
| Si | 4.23 | 4.83 | 1.16 | 1.28 | - | - |
| Fe | 14.41 | 8.28 | - | - | - | - |
| Mn | 10.19 | 5.95 | - | - | - | - |
| Cu | 10.04 | 8.16 | 34.16 | 16.63 | 28.42 | 13.94 |
| Peak Temperature/°C | Potential, Ecorr (E/V) | Current Density, Icorr (mA·cm−2) |
|---|---|---|
| RT—0% | −0.5740 | 12.27 |
| RT—0% | −0.5772 | 12.51 |
| RT—0% | −0.5797 | 12.77 |
| 200 °C—0% | −0.5541 | 12.13 |
| 200 °C—4% | −0.5871 | 13.02 |
| 200 °C—8% | −0.5723 | 12.24 |
| 300 °C—0% | −0.5495 | 10.04 |
| 300 °C—4% | −0.5689 | 11.61 |
| 300 °C—8% | −0.5656 | 11.21 |
| 400 °C—0% | −0.6119 | 13.45 |
| 400 °C—4% | −0.6248 | 14.46 |
| 400 °C—8% | −0.6267 | 14.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, S.; Zhang, T.; Chen, Y.; Li, H.; Xie, J.; He, P. Study on the Correlation between the Microstructure Characteristics and Corrosion Behaviors of 2A12-T4 Aluminum Alloy under Thermal Strain. Metals 2021, 11, 924. https://doi.org/10.3390/met11060924
Qian S, Zhang T, Chen Y, Li H, Xie J, He P. Study on the Correlation between the Microstructure Characteristics and Corrosion Behaviors of 2A12-T4 Aluminum Alloy under Thermal Strain. Metals. 2021; 11(6):924. https://doi.org/10.3390/met11060924
Chicago/Turabian StyleQian, Shuaihao, Timing Zhang, Yuhua Chen, Hongxiang Li, Jilin Xie, and Peng He. 2021. "Study on the Correlation between the Microstructure Characteristics and Corrosion Behaviors of 2A12-T4 Aluminum Alloy under Thermal Strain" Metals 11, no. 6: 924. https://doi.org/10.3390/met11060924





