Application of Commercial Surface Pretreatments on the Formation of Cerium Conversion Coating (CeCC) over High-Strength Aluminum Alloys 2024-T3 and 7075-T6
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
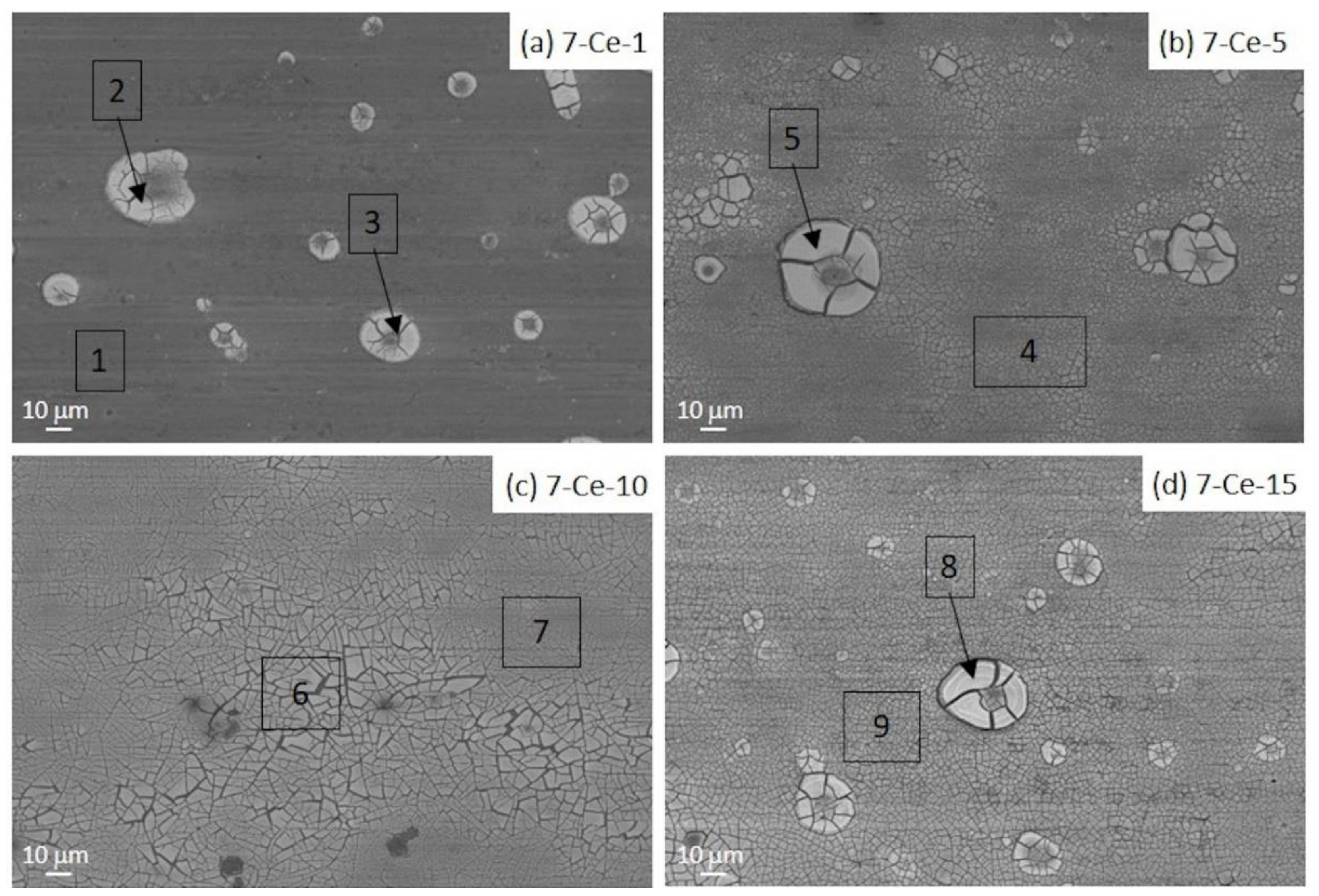
3.1. Scanning Electron Microscopy (SEM-EDX)
3.2. Glow Discharge Optical Emission Spectroscopy (GDOES)
3.3. Potentiodynamic Linear Polarization (LP)
3.4. Electrochemical Impedance Spectroscopy (EIS)
3.5. Neutral Salt Spray (NSS) Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parvizi, R.; Tan, M.Y.; Hughes, A.E. Chapter 14. Recent Insights in to Corrosion Initiation at the Nanoscale. In Fundamentals of Aluminium Metallurgy; Lumley, R.N., Ed.; Woodhead Publishing, Elsevier: Cambridge, UK, 2018; pp. 525–551. [Google Scholar]
- Zhu, Y.; Sun, K.; Frankel, G.S. Intermetallic Phases in Aluminum Alloys and Their Roles in Localized Corrosion. J. Electrochem. Soc. 2018, 165, C807–C820. [Google Scholar] [CrossRef]
- Hughes, A.E.; Parvizi, R.; Forsyth, M. Microstructure and corrosion of AA2024. Corros. Rev. 2015, 33, 1–30. [Google Scholar] [CrossRef]
- Kendig, M.; Jeanjaquet, S.; Addison, R.; Waldrop, J. Role of hexavalent chromium in the inhibition of corrosion of aluminum alloys. Surf. Coat. Technol. 2001, 140, 58–66. [Google Scholar] [CrossRef]
- Hughes, E. Conversion coatings. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 108–114. [Google Scholar] [CrossRef]
- Ferreira, G.S.; Zheludkevich, M.L.; Tedim, J.; Yasakau, K.A. 9-Self-healing nanocoatings for corrosion control. In Corrosion Protection and Control Using Nanomaterials; Saji, V.S., Cook, R., Eds.; Woodhead Publishing Series in Metals and Surface Engineering: Cambridge, UK, 2012; pp. 213–263. [Google Scholar] [CrossRef]
- Zarras, P.; Stenger-Smith, J.D. Chapter 3. Smart inorganic and organic pretreatment coatings for the inhibition of corrosion on metals/alloys. In Intelligent Coatings for Corrosion Control; Tiwari, A., Rawlins, J., Hihara, L.H., Eds.; Butterworth-Heinemannpp: London, UK, 2014; pp. 59–91. [Google Scholar] [CrossRef]
- Kwakernaak, A.; Hofstede, J.; Poulis, J.; Benedictus, R. Improvements in bonding metals for aerospace and other application. In Welding and Joining of Aerospace Materials; Chaturvedi, M.C., Ed.; Woodhead Publishing Series in Welding and Other Joining Technologies: Cambridge, UK, 2012; pp. 235–287. [Google Scholar] [CrossRef]
- Eichinger, E.; Osborne, J.; Van Cleave, T. Hexavalent chromium elimination: An aerospace industry progress report. Met. Finish. 1997, 95, 36–41. [Google Scholar] [CrossRef]
- Qi, J.; Gao, L.; Li, Y.; Wang, Z.; Thompson, G.E.; Skeldon, P. An Optimized Trivalent Chromium Conversion Coating Process for AA2024-T351 Alloy. J. Electrochem. Soc. 2017, 164, C390–C395. [Google Scholar] [CrossRef]
- Kendig, M.W.; Buchheit, R.G. Corrosion Inhibition of Aluminum and Aluminum Alloys by Soluble Chromates, Chromate Coatings, and Chromate-Free Coatings. Corrosion 2003, 59, 379–400. [Google Scholar] [CrossRef]
- Becker, M. Chromate-free chemical conversion coatings for aluminum alloys. Corros. Rev. 2019, 37, 321–342. [Google Scholar] [CrossRef]
- Stoica, A.-I.; Światowska, J.; Romaine, A.; Di Franco, F.; Qi, J.; Mercier, D.; Seyeux, A.; Zanna, S.; Marcus, P. Influence of post-treatment time of trivalent chromium protection coating on aluminium alloy 2024-T3 on improved corrosion resistance. Surf. Coat. Technol. 2019, 369, 186–197. [Google Scholar] [CrossRef]
- Harvey, T.G. Cerium-based conversion coatings on aluminium alloys: A process review. Corros. Eng. Sci. Technol. 2013, 48, 248–269. [Google Scholar] [CrossRef]
- Volarič, B.; Mazare, A.; Virtanen, S.; Milošev, I. The Effect of Deposition Parameters on the Properties of CeCl3 and LaCl3 Conversion Coatings Deposited on Three Al-Based Substrates. Corrosion 2019, 76, 18–38. [Google Scholar] [CrossRef]
- Milošev, I.; Frankel, G.S. Review—Conversion Coatings Based on Zirconium and/or Titanium. J. Electrochem. Soc. 2018, 165, C127–C144. [Google Scholar] [CrossRef]
- Klumpp, R.E.; Donatus, U.; Da Silva, R.M.P.; Antunes, R.A.; Machado, C.D.S.C.; Milagre, M.X.; Araujo, J.V.D.S.; De Viveiros, B.V.G.; Costa, I. Corrosion protection of the AA2198-T8 alloy by environmentally friendly organic-inorganic sol-gel coating based on bis-1,2-(triethoxysilyl) ethane. Surf. Interface Anal. 2021, 53, 314–329. [Google Scholar] [CrossRef]
- Bouali, A.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.; Zheludkevich, M. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Milošev, I. Contemporary Modes of Corrosion Protection and Functionalization of Materials. Acta Chim. Slov. 2019, 66, 511–533. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.; Calvino, J.J.; Marcos, M.; Rodríguez-Chacón, M. Lanthanide compounds as environmentally-friendly corrosion inhibitors of aluminium alloys: A review. Corros. Sci. 1998, 40, 1803–1819. [Google Scholar] [CrossRef]
- Sainis, S.; Roșoiu, S.; Ghassemali, E.; Zanella, C. The role of microstructure and cathodic intermetallics in localised deposition mechanism of conversion compounds on Al (Si, Fe, Cu) alloy. Surf. Coat. Technol. 2020, 402, 126502. [Google Scholar] [CrossRef]
- Czerwinski, F. Cerium in aluminum alloys. J. Mater. Sci. 2020, 55, 24–72. [Google Scholar] [CrossRef]
- Decroly, A.; Petitjean, J.-P. Study of the deposition of cerium oxide by conversion on to aluminium alloys. Surf. Coat. Technol. 2005, 194, 1–9. [Google Scholar] [CrossRef]
- Scholes, F.; Soste, C.; Hughes, A.; Hardin, S.; Curtis, P. The role of hydrogen peroxide in the deposition of cerium-based conversion coatings. Appl. Surf. Sci. 2006, 253, 1770–1780. [Google Scholar] [CrossRef]
- Amaya, J.M.S.; Blanco, G.; Garcia-Garcia, F.J.; Bethencourt, M.; Botana, F. XPS and AES analyses of cerium conversion coatings generated on AA5083 by thermal activation. Surf. Coat. Technol. 2012, 213, 105–116. [Google Scholar] [CrossRef]
- Hughes, A.; Harvey, T.; Birbilis, N.; Kumar, A.; Buchheit, R. Coatings for corrosion prevention based on rare earths. In Rare Earth-Based Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2014; pp. 186–232. [Google Scholar]
- Klumpp, R.E.; Donatus, U.; Araujo, J.V.S.; Redigolo, M.; Machado, C.D.S.C.; Costa, I. The Effect of Acid Pickling on the Corrosion Behavior of a Cerium Conversion-Coated AA2198-T851 Al-Cu-Li Alloy. J. Mater. Eng. Perform. 2020, 29, 167–174. [Google Scholar] [CrossRef]
- Andreeva, R. On the Role of pre-treatment of Aluminum Substrate on Deposition of Cerium Based Conversion Layers and Their Corrosion-Protective Ability. Int. J. Electrochem. Sci. 2018, 13, 5333–5351. [Google Scholar] [CrossRef]
- Alba-Galvín, J.J.; González-Rovira, L.; Bethencourt, M.; Botana, F.J.; Sánchez-Amaya, J.M. Influence of Aerospace Standard Surface Pretreatment on the Intermetallic Phases and CeCC of 2024-T3 Al-Cu Alloy. Metals 2019, 9, 320. [Google Scholar] [CrossRef]
- ISO 9227:2017. Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. Available online: https://www.iso.org/standard/63543.html (accessed on 29 April 2019).
- ASTM B117-18. Standards Practice for Operating Salt Spray (Fog) Apparatus. Available online: https://www.astm.org/Standards/B117 (accessed on 29 April 2019).
- Lau, D.; Glenn, A.; Hughes, A.; Scholes, F.; Muster, T.; Hardin, S. Factors influencing the deposition of Ce-based conversion coatings, Part II: The role of localised reactions. Surf. Coat. Technol. 2009, 203, 2937–2945. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I. Corrosion resistance of cerium-conversion coatings formed from cerium(III) salts on aluminium alloy 7075-T6. Studia Univ. Babeș-Bolyai Chem. 2020, 65, 227–244. [Google Scholar] [CrossRef]
- Shimizu, K.; Brown, G.; Habazaki, H.; Kobayashi, K.; Skeldon, P.; Thompson, G.; Wood, G. Impurity distributions in barrier anodic films on aluminium: A GDOES depth profiling study. Electrochim. Acta 1999, 44, 2297–2306. [Google Scholar] [CrossRef]
- Kuypers, S.; Buytaert, G.; Terryn, H. Depth profiling of rolled aluminium alloys by means of GDOES. Surf. Interface Anal. 2004, 36, 833–836. [Google Scholar] [CrossRef]
- De Nicolò, A.; Paussa, L.; Gobessi, A.; Lanzutti, A.; Cepek, C.; Andreatta, F.; Fedrizzi, L. Cerium conversion coating and sol-gel multilayer system for corrosion protection of AA6060. Surf. Coat. Technol. 2016, 287, 33–43. [Google Scholar] [CrossRef]
- Andreatta, F.; Lanzutti, A.; Paussa, L.; Fedrizzi, L. Addition of phosphates or copper nitrate in a fluotitanate conversion coating containing a silane coupling agent for aluminium alloy AA6014. Prog. Org. Coat. 2014, 77, 2107–2115. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.J.; Cano, M.J.; González-Rovira, L.; Marcos, M.; Amaya, J.M.S. Protection by Thermal and Chemical Activation with Cerium Salts of the Alloy AA2017 in Aqueous Solutions of NaCl. Met. Mater. Trans. A 2011, 43, 182–194. [Google Scholar] [CrossRef]
- De Frutos, A.; Arenas, M.A.; Liu, Y.; Skeldon, P.; Thompson, G.; De Damborenea, J.; Conde, A. Influence of pre-treatments in cerium conversion treatment of AA2024-T3 and 7075-T6 alloys. Surf. Coat. Technol. 2008, 202, 3797–3807. [Google Scholar] [CrossRef]
- García-Rubio, M.; Ocón, P.; Climent-Font, A.; Smith, R.; Curioni, M.; Thompson, G.; Skeldon, P.; Lavía, A.; Garcia, I. Influence of molybdate species on the tartaric acid/sulphuric acid anodic films grown on AA2024 T3 aerospace alloy. Corros. Sci. 2009, 51, 2034–2042. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Tang, S.; Ren, W.; Zhang, Z. Corrosion resistance of cerium-based conversion coatings on alumina borate whisker reinforced AA6061 composite. Appl. Surf. Sci. 2007, 253, 8879–8884. [Google Scholar] [CrossRef]
- Feliu, J.S. Electrochemical Impedance Spectroscopy for the Measurement of the Corrosion Rate of Magnesium Alloys: Brief Review and Challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Yasakau, K.; Bastos, A.; Karavai, O.; Ferreira, M. On the application of electrochemical impedance spectroscopy to study the self-healing properties of protective coatings. Electrochem. Commun. 2007, 9, 2622–2628. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.; Cano, M.; Marcos, M.; Amaya, J.M.S.; Rovira, L.G. Behaviour of the alloy AA2017 in aqueous solutions of NaCl. Part I: Corrosion mechanisms. Corros. Sci. 2009, 51, 518–524. [Google Scholar] [CrossRef]
- Ilevbare, G.O.; Scully, J.R.; Yuan, J.; Kelly, R.G. Inhibition of Pitting Corrosion on Aluminum Alloy 2024-T3: Effect of Soluble Chromate Additions vs Chromate Conversion Coating. Corrosion 2000, 56, 227–242. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Marcos, M.; Rodríguez-Chacón, M.A. Seguimiento de la corrosión de la aleación AA2024 en disoluciones de NaCl mediante la medida del ruido electroquímico. Rev. Met. 1998, 34, 42–46. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; O’Keefe, M.J.; Zhou, H.; Grant, J. Characterization of cerium-based conversion coatings for corrosion protection of aluminum alloys. Surf. Coat. Technol. 2002, 155, 208–213. [Google Scholar] [CrossRef]
- Rivera, B.F.; Johnson, B.Y.; O’Keefe, M.J.; Fahrenholtz, W. Deposition and characterization of cerium oxide conversion coatings on aluminum alloy 7075-T6. Surf. Coat. Technol. 2004, 176, 349–356. [Google Scholar] [CrossRef]








| Alloy | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Ti + Zr | V | Al | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2024-T3 | 0.07 | 0.19 | 4.30 | 0.58 | 1.30 | 0.01 | 0.10 | 0.02 | - | 0.01 | 93.42 | - |
| 7075-T6 | 0.08 | 0.13 | 1.7 | 0.02 | 2.5 | 0.19 | 5.7 | 0.04 | 0.04 | - | 89.61 | 0.03 |
| Area. | Al | Cu | Mg | O | Mn | Fe | C | Ce |
|---|---|---|---|---|---|---|---|---|
| 1 | 52.33 | 20.81 | 1.28 | 2.81 | 3.59 | 6.36 | 12.64 | - |
| 2 | 88.65 | 4.85 | 2.01 | 4.48 | – | – | – | – |
| 3 | 81.19 | 3.64 | 1.62 | 2.95 | 0.49 | – | 10.11 | – |
| 4 | 68.57 | 3.90 | 1.18 | 14.21 | – | – | 5.50 | 6.64 |
| 5 | 38.48 | 3.73 | – | 27.22 | – | – | 5.75 | 24.81 |
| 6 | 44.38 | 15.04 | 1.05 | 24.70 | 1.28 | 1.26 | – | 9.41 |
| 7 | 0.89 | – | – | 26.71 | – | – | – | 72.40 |
| 8 | 55.05 | 3.31 | 1.16 | 22.58 | – | – | – | 17.90 |
| 9 | 74.22 | 3.50 | 1.39 | 14.01 | – | – | – | 6.78 |
| 10 | 41.95 | 3.32 | 0.60 | 26.87 | – | – | 6.53 | 20.73 |
| 11 | 31.80 | 3.18 | – | 29.78 | – | – | 5.60 | 29.68 |
| Area | Al | Cu | Mg | O | Zn | C | Ce |
|---|---|---|---|---|---|---|---|
| 1 | 70.52 | 1.59 | 2.76 | 16.02 | 5.35 | - | 3.76 |
| 2 | 36.82 | – | 2.12 | 27.24 | 3.26 | 7.01 | 23.55 |
| 3 | 27.39 | 0.82 | 0.97 | 27.68 | 2.92 | 6.42 | 33.80 |
| 4 | 40.41 | 0.87 | 1.27 | 27.16 | 4.78 | 6.27 | 19.19 |
| 5 | 20.37 | – | 0.66 | 29.02 | 2.57 | 4.77 | 42.61 |
| 6 | 30.54 | – | 1.26 | 28.33 | 3.28 | – | 36.59 |
| 7 | 4.07 | 1.19 | – | 25.60 | 4.12 | 6.99 | 22.04 |
| 8 | 0.62 | – | – | 12.45 | – | – | 86.93 |
| 9 | 43.80 | – | 1.05 | 28.26 | 4.38 | – | 22.51 |
| Treatment Time (Minutes) | CeCC (nm) | Al Oxide/Hydroxide (nm) | ||
|---|---|---|---|---|
| 2024 | 7075 | 2024 | 7075 | |
| 1 | 120 | 260 | – | 60 |
| 5 | 200 | 470 | 20 | 110 |
| 10 | 590 | 590 | – | 90 |
| 15 | 850 | 950 | – | 210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alba-Galvín, J.J.; González-Rovira, L.; Botana, F.J.; Lekka, M.; Andreatta, F.; Fedrizzi, L.; Bethencourt, M. Application of Commercial Surface Pretreatments on the Formation of Cerium Conversion Coating (CeCC) over High-Strength Aluminum Alloys 2024-T3 and 7075-T6. Metals 2021, 11, 930. https://doi.org/10.3390/met11060930
Alba-Galvín JJ, González-Rovira L, Botana FJ, Lekka M, Andreatta F, Fedrizzi L, Bethencourt M. Application of Commercial Surface Pretreatments on the Formation of Cerium Conversion Coating (CeCC) over High-Strength Aluminum Alloys 2024-T3 and 7075-T6. Metals. 2021; 11(6):930. https://doi.org/10.3390/met11060930
Chicago/Turabian StyleAlba-Galvín, Juan Jesús, Leandro González-Rovira, Francisco Javier Botana, Maria Lekka, Francesco Andreatta, Lorenzo Fedrizzi, and Manuel Bethencourt. 2021. "Application of Commercial Surface Pretreatments on the Formation of Cerium Conversion Coating (CeCC) over High-Strength Aluminum Alloys 2024-T3 and 7075-T6" Metals 11, no. 6: 930. https://doi.org/10.3390/met11060930
APA StyleAlba-Galvín, J. J., González-Rovira, L., Botana, F. J., Lekka, M., Andreatta, F., Fedrizzi, L., & Bethencourt, M. (2021). Application of Commercial Surface Pretreatments on the Formation of Cerium Conversion Coating (CeCC) over High-Strength Aluminum Alloys 2024-T3 and 7075-T6. Metals, 11(6), 930. https://doi.org/10.3390/met11060930







