Evaluation of Hardness, Sliding Wear and Strength of a Hypoeutectic White Iron with 25%Cr after Heat Treatments
Abstract
:1. Introduction
2. Materials and Methods
- Volume fraction of eutectic carbides by quantitative metallography by manual point counting on a total of 30 micrographs at a magnification of 400×.
- Vickers hardness with a load of 30 kgf (~294 N), averaging the results of 12 indentations per experiment.
- Sliding wear resistance evaluated through a reciprocating pin on disk wear test performed according to the ASTM G133-05 standard, with the following characteristics: ball characteristics: WC 4/7%Co, 4 mm diameter, 1550–1780 HV; applied load: 30 N; stroke length: 40 mm; rotating speed: 250 rev⋅min−1 (equivalent to a linear average speed of 20 m⋅min−1); total distance tested: 10 km.
- Where the mass loss at the end of testing was weighted and registered.
- Three-point flexural bending test with the following characteristics: support span, L = 30 mm; width (w) and thickness (t) of tested beams, 14 mm and 3.5 mm, respectively; center roll diameter for load application and support roll diameters: 10 mm; center roll linear speed: 5 mm⋅min−1.
3. Results
4. Conclusions
- Vickers hardness increases when destabilization of austenite takes place at 1050 °C and tempering is conducted at 400 °C. High destabilization temperatures yield a high-C austenite and, on further quenching, provide hard martensite and a high fraction of retained austenite. Low tempering temperature is insufficient to decompose the hard martensite but allows structural strengthening by alloy carbide precipitation from residual austenite.
- For improved adhesive wear resistance, tempering treatment parameters are critical, the best results being obtained at 400 °C and 2 h. The technical literature underlines the relevance of a high fraction and fineness of secondary carbides from destabilization. Low temperature and short tempering times impede secondary carbide coarsening. Such tempering conditions are also favorable for increased wear resistance of the matrix due to minimal conversion of martensite (α’→α) and possible conditioning of retained austenite giving alloy carbides from tempering.
- Maximum flexural strength could be obtained with high destabilization temperatures (1050 °C) and slow quenching rates (samples introduced in an air convection furnace set at 150 °C). These conditions are favorable for maximizing the volume fraction of ductile retained austenite which allows the deflection required for the high strengths registered.
- The energy absorbed to fracture in bending, measured as the total area below the force–displacement curve in a bend test curve, is a valuable source of information of combined strength and ductility. It has been seen that this energy could be enhanced by slow cooling quenching (an air convection furnace set at 150 °C) followed by a tempering treatment at 550 °C. The novelty here is that high-temperature tempering favors: (a) total conversion of martensite (α’→α + alloy carbides), (b) conditioning of austenite (γ rich in C and alloy elements) → alloy carbides + depleted-γ); and (c) the possibility of transformation of depleted austenite (depleted-γ → lower bainite). In summary, this provides a ductile matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeytin, H.K.; Yildirim, H.; Berme, B.; Duduoĝlu, S.; Kazdal, G.; Deniz, A. Effect of boron and heat treatment on mechanical properties of white cast iron for mining application. J. Iron Steel Res. Int. 2011, 18, 31–39. [Google Scholar] [CrossRef]
- Fu, H.; Xiao, Q.; Fu, H. Heat treatment of multi-element low alloy wear-resistant steel. Mater. Sci. Eng. A 2005, 396, 206–212. [Google Scholar] [CrossRef]
- Gasan, H.; Erturk, F. Effects of a Destabilization Heat Treatment on the Microstructure and Abrasive Wear Behavior of High-Chromium White Cast Iron Investigated Using Different Characterization Techniques. Metall. Mater. Trans. A 2013, 44, 4993–5005. [Google Scholar] [CrossRef]
- Wieczerzak, K.; BaŁa, P.; Stępień, M.; Cios, G.; KozieŁ, T. The characterization of cast Fe-Cr-C alloy. Arch Metall. Mater. 2015, 60, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Huang, Y.; Zuo, X.; Liu, Y.; Chen, N.; Rong, Y. High hardness-toughness and wear resistance of white cast iron treated by a multicycle quenching-partitioning-tempering process. Heat Treat Surf. Eng. 2019, 1, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Lindroos, M.; Apostol, M.; Heino, V.; Valtonen, K.; Laukkanen, A.; Holmberg, K.; Kuokkala, V.T. The deformation, strain hardening, and wear behavior of chromium-alloyed hadfield steel in abrasive and impact conditions. Tribol. Lett. 2015, 57, 1–11. [Google Scholar] [CrossRef]
- Lindroos, M.; Ratia, V.; Apostol, M.; Valtonen, K.; Laukkanen, A.; Molnar, W.; Holmberg, K.; Kuokkala, V.T. The effect of impact conditions on the wear and deformation behavior of wear resistant steels. Wear 2015, 328–329, 197–205. [Google Scholar] [CrossRef]
- Uygura, I.; Gerengib, H.; Arslanc, Y.; Kurtayb, M. The Effects of Cryogenic Treatment on the Corrosion of AISI D3 Steel. Mater. Res. 2015, 18, 569–574. [Google Scholar] [CrossRef] [Green Version]
- Amini, K.; Akhbarizadeh, A.; Javadpour, S. Effect of Carbide Distribution on Corrosion Behavior of the Deep Cryogenically Treated 1.2080 Steel. J. Mater. Eng. Perform. 2016, 25, 365–373. [Google Scholar] [CrossRef]
- Hill, H.; Huth, S.; Weber, S.; Theisen, W. Corrosion properties of a plastic mould steel with special focus on the processing route. Werkst. Korros. 2011, 62, 436–443. [Google Scholar] [CrossRef]
- Hudakova, M.; Bartkowska, A.; Jurci, P. The effect of conventional heat treatment and sub-zero treatment in liquid nitrogen on corrosion resistance of vanadis 6 steel. In Proceedings of the 28th International Conference on Metallurgy and Materials 2019, Brno, Czech Republic, 22 May 2019; pp. 867–873. [Google Scholar]
- Qu, Y.; Xing, J.; Zhi, X.; Peng, J.; Fu, H. Effect of cerium on the as-cast microstructure of a hypereutectic high chromium cast iron. Mater. Lett. 2008, 62, 3024–3027. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, J.; Fan, H.; Yang, H.S.; Liu, H.H.; Shen, B.L. Effects of high temperature and cryogenic treatment on the microstructure and abrasion resistance of a high chromium cast iron. J. Mater. Process Technol. 2009, 209, 3236–3240. [Google Scholar] [CrossRef]
- Ding, H.; Liu, S.; Zhang, H.; Guo, J. Improving impact toughness of a high chromium cast iron regarding joint additive of nitrogen and titanium. Mater. Des. 2016, 90, 958–968. [Google Scholar] [CrossRef]
- Guo, Z.H.; Xiao, F.R.; Lu, S.L.; Liu, R.L.; Liao, B. Effects of heat treatment and counterface on the wear behaviour of Cr21 cast iron. Ironmak. Steelmak. 2018, 45, 257–263. [Google Scholar] [CrossRef]
- Zheng, B.C.; Xing, J.D.; Liu, Y.Z.; Li, W. Two-Body Abrasion Behaviors Characterization of White Cast Iron with Various Chromium Concentrations. Tribol. Trans. 2020, 63, 519–527. [Google Scholar] [CrossRef]
- Groche, P.; Moeller, N.; Hoffmann, H.; Suh, J. Influence of gliding speed and contact pressure on the wear of forming tools. Wear 2011, 271, 2570–2578. [Google Scholar] [CrossRef]
- Jurci, P. Cr-V Ledeburitic cold-work tool steels. Mater. Tehnol. 2011, 45, 383–394. [Google Scholar]
- Gao, X.J.; Jiang, Z.Y.; Wei, D.B.; Kosasih, B. Effect of thermomechanical treatment on sliding wear of high-Cr cast iron with large plastic deformation. Tribol. Int. 2015, 92, 117–125. [Google Scholar] [CrossRef]
- Efremenko, V.; Shimizu, K.; Chabak, Y. Effect of Destabilizing Heat Treatment on Solid-State Phase Transformation in High-Chromium Cast Irons. Metall. Mater. Trans. 2013, 44, 5434–5446. [Google Scholar] [CrossRef]
- Kishore, K.; Kumar, U.; Dinesh, N.; Adhikary, M. Effect of Soaking Temperature on Carbide Precipitation, Hardness, and Wear Resistance of High-Chromium Cast Iron. J. Fail. Anal. Prev. 2020, 20, 249–260. [Google Scholar] [CrossRef]
- Pero-Sanz, J.A.; Plaza, D.; Verdeja, J.I.; Asensio, J. Metallographic Characterization of Hypoeutectic Martensitic White Cast Irons: Fe-C-Cr System Experimental procedure and results composition and microstructural development. Mater. Charact. 1999, 43, 33–39. [Google Scholar] [CrossRef]
- Mandal, S.S.; Ghosh, K.S.; Mondal, D.K. Correlation between microstructure, hardness, wear and electrochemical behaviour in 8.0%, 16.0% and 20.0% (by wt) chromium white irons. Mater. Chem. Phys. 2017, 193, 401–412. [Google Scholar] [CrossRef]
- Wiengmoon, A.; Pearce, J.T.H.; Chairuangsri, T. Relationship between microstructure, hardness and corrosion resistance in 20 wt.%Cr, 27 wt.%Cr and 36 wt.%Cr high chromium cast irons. Mater. Chem. Phys. 2011, 125, 739–748. [Google Scholar] [CrossRef]
- Zumelzu, E.; Goyos, I.; Cabezas, C.; Opitz, O.; Parada, A. Wear and corrosion behaviour of high-chromium (14–30% Cr) cast iron alloys. J. Mater. Process Technol. 2002, 128, 250–255. [Google Scholar] [CrossRef]
- Tang, X.H.; Chung, R.; Li, D.Y.; Hinckley, B.; Dolman, K. Variations in microstructure of high chromium cast irons and resultant changes in resistance to wear, corrosion and corrosive wear. Wear 2009, 267, 116–121. [Google Scholar] [CrossRef]
- Pawlak, K.; Białobrzeska, B.; Konat, Ł. The influence of austenitizing temperature on prior austenite grain size and resistance to abrasion wear of selected low-alloy boron steel. Arch. Civ. Mech. Eng. 2016, 16, 913–926. [Google Scholar] [CrossRef]
- Wang, C.C.; Shen, C.G.; Zhang, Z.; Xu, W. Simulation and verification of core–shell MC carbide design in Fe–C–Ni–V–Ti steel. J. Iron Steel Res. Int. 2021, 28, 58–65. [Google Scholar] [CrossRef]
- Karantzalis, A.E.; Lekatou, A.; Mavros, H. Microstructural Modifications of As-Cast High-Chromium White Iron by Heat Treatment. J. Mater. Eng. Perform 2009, 18, 174–181. [Google Scholar] [CrossRef]
- Bedolla-Jacuinde, A.; Arias, L.; Hernández, B. Kinetics of secondary carbides precipitation in a hiqh-chromium white iron. J. Mater. Eng. Perform 2003, 12, 371–382. [Google Scholar] [CrossRef]
- Çöl, M.; Koç, F.G.; Öktem, H.; Kir, D. The role of boron content in high alloy white cast iron (Ni-Hard 4) on microstructure, mechanical properties and wear resistance. Wear 2016, 348–349, 158–165. [Google Scholar] [CrossRef]
- Gelfi, M.; Pola, A.; Girelli, L.; Zacco, A.; Masotti, M.; La Vecchia, G.M. Effect of heat treatment on microstructure and erosion resistance of white cast irons for slurry pumping applications. Wear 2019, 428–429, 438–448. [Google Scholar] [CrossRef]
- Guitar, M.A.; Nayak, U.P.; Britz, D.; Mücklich, F. The effect of thermal processing and chemical composition on secondary carbide precipitation and hardness in high-chromium cast irons. Int. J. Metalcast. 2020, 14, 465–755. [Google Scholar] [CrossRef] [Green Version]
- Karantzalis, A.E.; Lekatou, A.; Diavati, E. Effect of Destabilization Heat Treatments on the Microstructure of High-Chromium Cast Iron: A Microscopy Examination Approach. J. Mater. Eng. Perform. 2009, 18, 1078–1085. [Google Scholar] [CrossRef]
- Powell, G.L.F.; Laird, G. Structure, nucleation, growth and morphology of secondary carbides in high chromium and Cr-Ni white cast irons. J. Mater. Sci. 1992, 27, 29–35. [Google Scholar] [CrossRef]
- Yang, J.R.; Tsai, M.C.; Du, J.S.; Chiou, C.S. Phase transformations in AISI 410 stainless steel. Jpn. Inst. Met. Proc. 1999, 332, 1605–1608. [Google Scholar]
- Kusumoto, K.; Shimizu, K.; Yaer, X.; Zhang, Y.; Ota, Y.; Ito, J. Abrasive wear characteristics of Fe-2C-5Cr-5Mo-5W-5Nb multi-component white cast iron. Wear 2017, 376–377, 22–29. [Google Scholar] [CrossRef]
- Wang, M.Q.; Dong, H.; Hui, W.J.; Shi, J. Effect of heat treatment on microstructure and mechanical properties of Cr-Ni-Mo-Nb steel. Mater. Sci. Technol. 2007, 23, 963–969. [Google Scholar] [CrossRef]
- Morito, S.; Saito, H.; Maki, T.; Furuhara, T. Effect of PAGS on crystallography and morphology of lath martensite in low carbon steels. ISIJ Int. 2004, 45, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Shaeri, M.H.; Saghafian, H.; Shabestari, S.G. Effect of heat treatment on microstructure and mechanical properties of Cr-Mo steels (FMU-226) used in mills liner. Mater. Des. 2012, 34, 192–200. [Google Scholar] [CrossRef]
- Coronado, J.J. Effect of load and carbide orientation on abrasive wear resistance of white cast iron. Wear 2011, 270, 823–827. [Google Scholar] [CrossRef]
- Tabrett, C.P.; Sare, I.R.; Ghomashchi, M.R. Microstructure-property relationships in high chromium white iron alloys. Int. Mater. Rev. 2014, 41, 59–82. [Google Scholar] [CrossRef]
- Imurai, S.; Thanachayanont, C.; Pearce, J.T.H.; Tsuda, K.; Chairuangsri, T. Effects of Mo on microstructure of as-cast 28 wt.% Cr-2.6 wt.% C-(0-10) wt.% Mo irons. Mater. Charact. 2014, 90, 99–112. [Google Scholar] [CrossRef]
- Wieczerzak, K.; Bala, P.; Dziurka, R.; Tokarski, T.; Cios, G.; Koziel, T.; Gondek, L. The effect of temperature on the evolution of eutectic carbides and M7C3 → M23C6carbides reaction in the rapidly solidified Fe-Cr-C alloy. J. Alloys. Compd. 2017, 698, 673–684. [Google Scholar] [CrossRef]
- Karantzalis, E.; Lekatou, A.; Mavros, H. Microstructure and properties of high chromium cast irons: Effect of heat treatments and alloying additions. Int. J. Cast. Met. Res. 2009, 22, 448–456. [Google Scholar] [CrossRef]
- Sabet, H.; Khierandish, S.; Mirdamadi, S.; Goodarzi, M. The microstructure and abrasive wear resistance of Fe-Cr-C hardfacing alloys with the composition of hypoeutectic, eutectic, and hypereutectic at Cr/C 6. Tribol. Lett. 2011, 44, 237–245. [Google Scholar] [CrossRef]
- Taşgin, Y.; Kaplan, M.; Yaz, M. Investigation of effects of boron additives and heat treatment on carbides and phase transition of highly alloyed duplex cast iron. Mater. Des. 2009, 30, 3174–3179. [Google Scholar] [CrossRef]
- Filipovic, M.; Kamberovic, Z.; Korac, M.; Gavrilovski, M. Microstructure and mechanical properties of Fe-Cr-C-Nb white cast irons. Mater. Des. 2013, 47, 41–48. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Zhang, Y.; Ye, C.; Ren, X.; Yang, Y.; Yang, Q. Phase diagram calculation of high chromium cast irons and influence of its chemical composition. Mater. Des. 2009, 30, 340–345. [Google Scholar] [CrossRef]
- Kim, C.K.; Lee, S.; Jung, J.Y. Effects of heat treatment on wear resistance and fracture toughness of duo-cast materials composed of high-chromium white cast iron and low-chromium steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2006, 37, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Doǧan, Ö.N.; Hawk, J.A.; Laird, G. Solidification structure and abrasion resistance of high chromium white irons. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1997, 28, 1315–1328. [Google Scholar] [CrossRef]
- Carpenter, S.D.; Carpenter, D.; Pearce, J.T.H. XRD and electron microscope study of a heat treated 26.6% chromium white iron microstructure. Mater. Chem. Phys. 2007, 101, 49–55. [Google Scholar] [CrossRef]
- Lai, J.P.; Pan, Q.L.; Wang, Z.B.; Cui, H.R.; Wang, X.D.; Gao, Z.Z. Effects of Destabilization Temperature on the Microstructure and Mechanical Properties of High Chromium Cast Iron. J. Mater. Eng. Perform. 2017, 26, 4667–4675. [Google Scholar] [CrossRef]
- González-Pociño, A.; Asensio-Lozano, J.; Álvarez-Antolín, F.; García-Diez, A. Improvement of impact toughness and abrasion resistance of a 3C-25Cr-0.5Mo alloy using a design of experiment statistical technique: Microstructural correlations after heat treatments. Metals 2021, 11, 595. [Google Scholar] [CrossRef]
- Prat-Bartés, A.; Tort-Martorell, X.; Grima-Cintas, P.; Pozueta-Fernández, L.; Solé-Vidal, I. Métodos Estadísticos, 2nd ed.; UPC: Barcelona, Spain, 2004; p. 376. [Google Scholar]
- Maratray, F.; Usseglio-Nanot, R. Transformation Characteristics of Cr and Cr-Mo White Irons; Climax Molybdenum S.A.: Paris, France, 1969; p. 150. [Google Scholar]

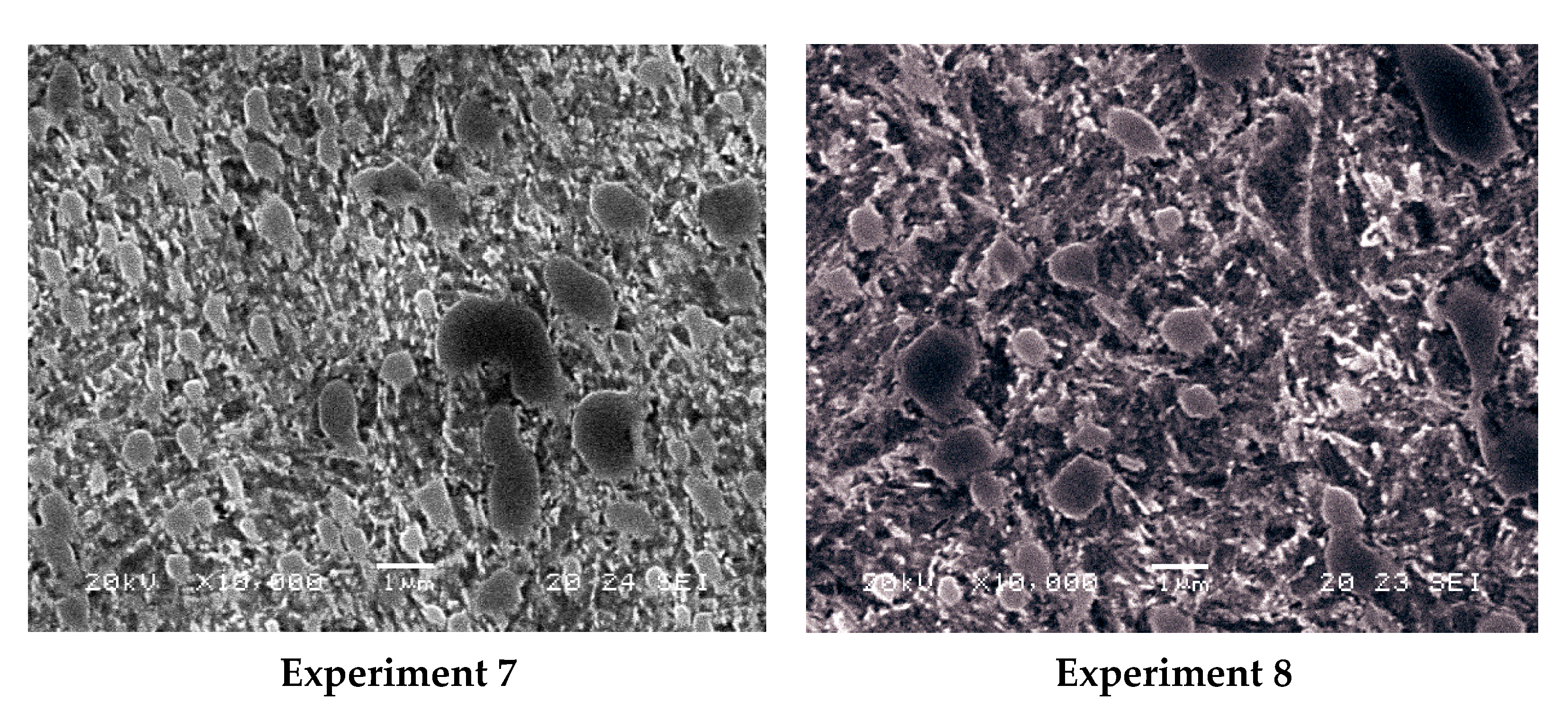
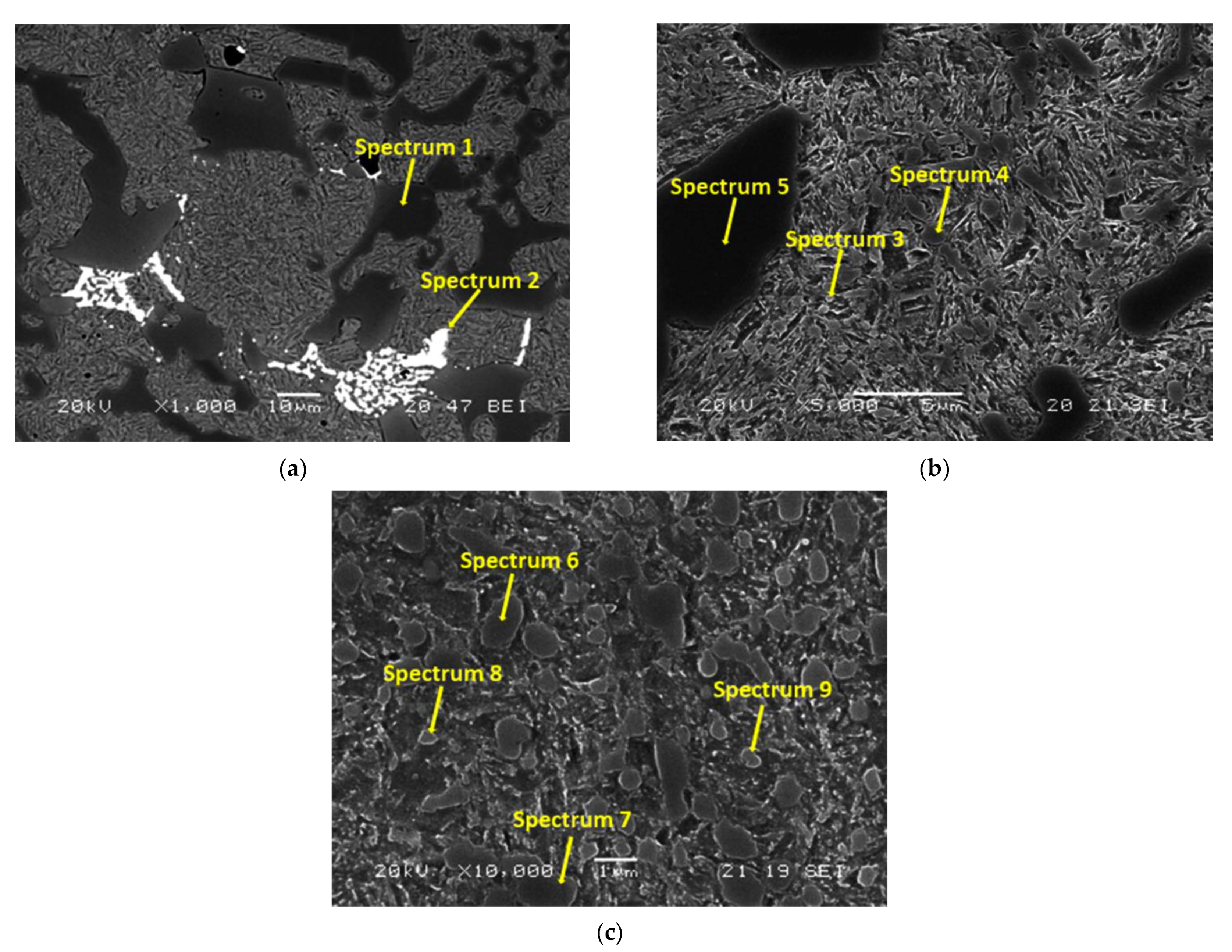


| C | Si | Mn | Cr | Mo | Cr:C |
|---|---|---|---|---|---|
| 2.7 | 1.2 | 0.8 | 25.1 | 0.5 | 9.3 |
| Factors | Levels | ||
|---|---|---|---|
| Code | Heat Treatment Parameters | Level −1 | Level +1 |
| A | Destabilization temperature (°C) for 5 h | 950 | 1050 |
| B | Cooling media after gamma destabilizing | Air convection within a furnace set @150 °C | Gently stirred in oil at RT |
| C | Tempering temperature (°C) | 400 | 550 |
| D | Dwell time at tempering temperature (h) | 2 | 6 |
| No. | A | B | C | D | Restricted Confounding Patterns |
|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | A B C D AB+CD AC+BD AD+BC |
| 2 | +1 | −1 | −1 | +1 | |
| 3 | −1 | +1 | −1 | +1 | |
| 4 | +1 | +1 | −1 | −1 | |
| 5 | −1 | −1 | +1 | +1 | |
| 6 | +1 | −1 | +1 | −1 | |
| 7 | −1 | +1 | +1 | −1 | |
| 8 | +1 | +1 | +1 | +1 |
| Carbide Spectrum | C | Fe | Cr | Si | Mo | Most Likely Stoichiometry |
|---|---|---|---|---|---|---|
| 1 | 34.27 | 26.61 | 39.12 | - | - | M7C3 |
| 2 | 45.24 | 23.70 | 4.88 | 5.34 | 20.84 | M2C |
| 3 | 52.89 | 38.81 | 7.01 | 1.29 | - | M23C6 |
| 4 | 62.08 | 19.71 | 18.21 | - | - | M7C3 |
| 5 | 64.56 | 14.59 | 20.85 | - | - | M7C3 |
| 6 | 51.57 | 32.31 | 16.12 | - | - | M7C3 |
| 7 | 52.72 | 32.11 | 15.17 | - | - | M7C3 |
| 8 | 16.12 | 44.73 | 5.00 | 1.29 | - | M23C6 |
| 9 | 15.17 | 35.91 | 6.29 | 1.14 | - | M23C6 |
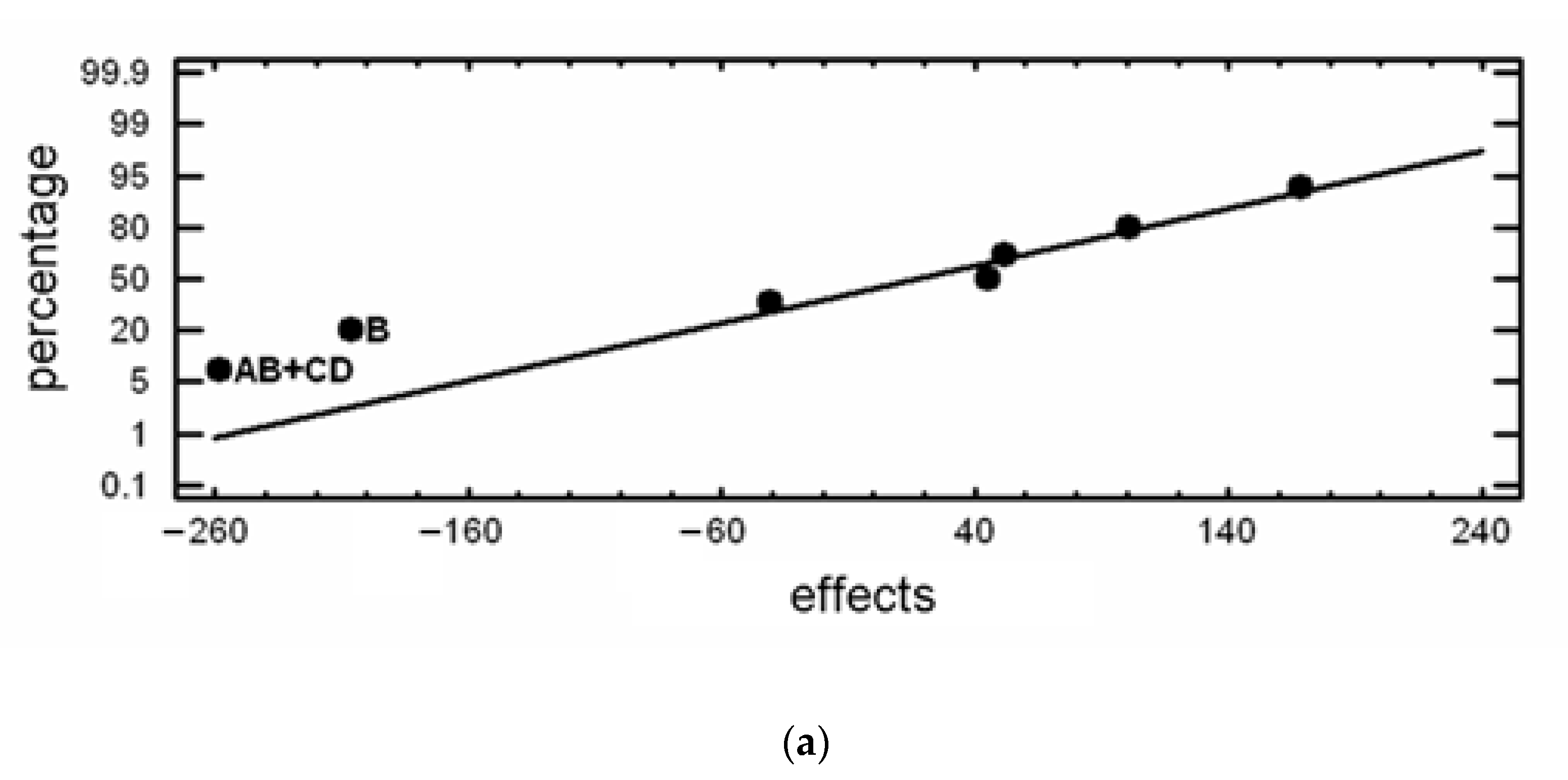
| Experiment No. | Hardness | Mass Loss | Confounding Pattern | ||
|---|---|---|---|---|---|
| HV30 | Effect | mg | Effect | ||
| 1 | 741 | 668.25 | 103.1 | 102.288 | Average |
| 2 | 707 | 75.5 | 41.3 | −21.025 | A |
| 3 | 746 | −42.0 | 88.9 | 4.675 | B |
| 4 | 726 | −123.5 | 58.3 | 58.775 | C |
| 5 | 567 | −2.0 | 195.5 | 62.075 | D |
| 6 | 742 | 5.0 | 59.9 | 77.675 | AB+CD |
| 7 | 468 | 102.5 | 63.7 | 25.175 | AC+BD |
| 8 | 649 | −54.0 | 207.6 | 3.275 | AD+BC |
| A(↓)×C(→) | −1 | +1 | B(↓)×D(→) | −1 | +1 |
|---|---|---|---|---|---|
| −1 | 743.5 | 517.5 | −1 | 741.5 | 637 |
| +1 | 716.5 | 695 | +1 | 597 | 697.5 |
| A(↓)×B(→) | −1 | +1 | C(↓)×D(→) | −1 | +1 |
|---|---|---|---|---|---|
| −1 | 149.3 | 76.3 | −1 | 80.7 | 65.1 |
| +1 | 50.6 | 133 | +1 | 61.8 | 201.6 |
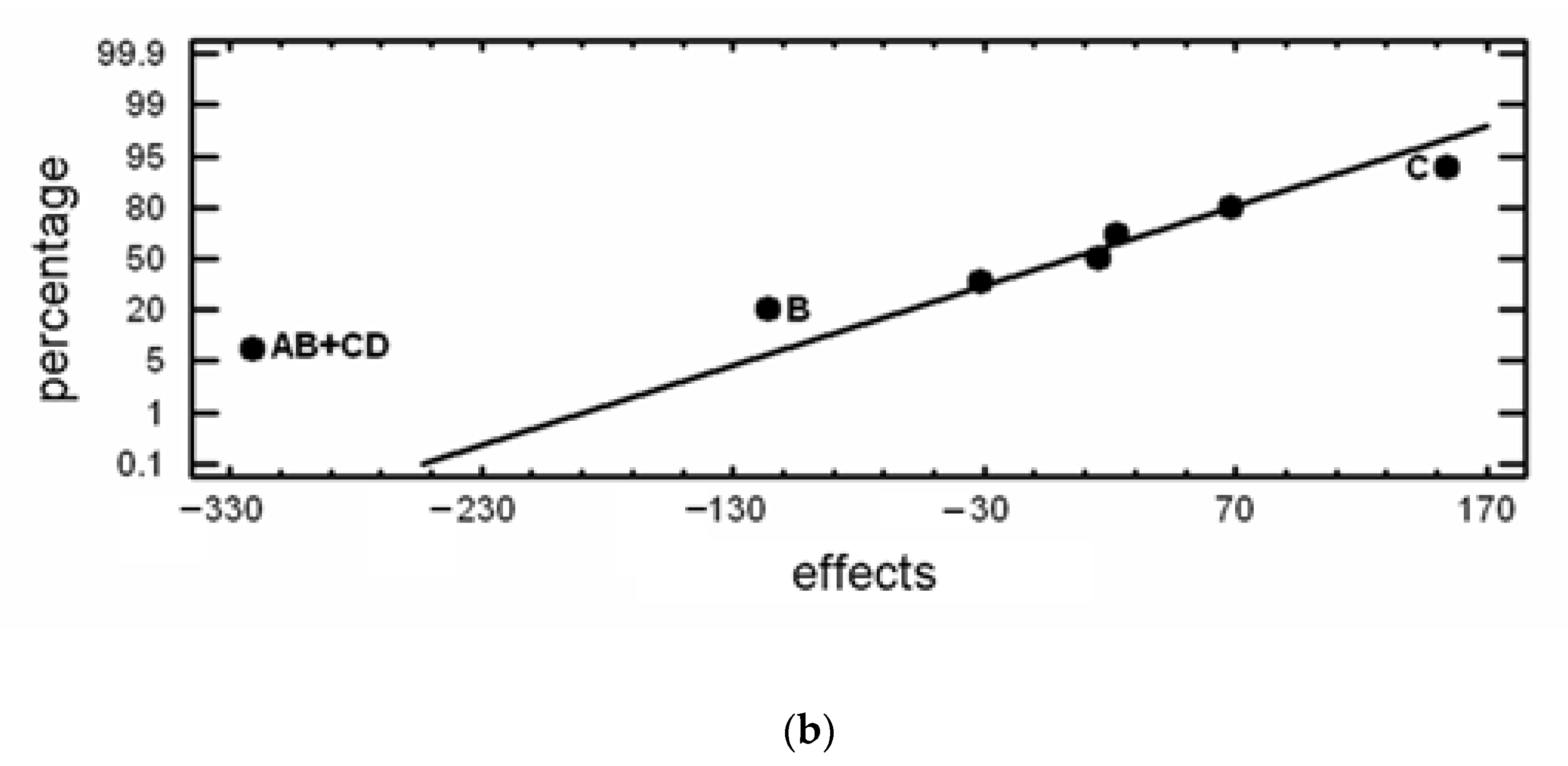
| No. | Maximum Flexural Strength (σmax) | Absorbed Energy to Fracture (W) | Confounding Pattern | ||
|---|---|---|---|---|---|
| MPa | Effect | mJ | Effect | ||
| 1 | 945.9 | 1054.05 | 489.85 | 670.433 | Average |
| 2 | 1286.1 | −7.4 | 780.86 | 15.99 | A |
| 3 | 1114.4 | −238.9 | 749.11 | −115.465 | B |
| 4 | 602.6 | 133.6 | 352.92 | 154.495 | C |
| 5 | 1117.7 | 135.2 | 629.85 | 22.96 | D |
| 6 | 1344.3 | −290.8 | 1012.1 | −320.64 | AB+CD |
| 7 | 1053 | 78.4 | 780.94 | 68.58 | AC+BD |
| 8 | 968.4 | 18.6 | 567.83 | −31.125 | AD+BC |
| A(↓)×B(→) | −1 | +1 | C(↓)×D(→) | −1 | +1 |
|---|---|---|---|---|---|
| −1 | 1031.8 | 1083.7 | −1 | 774.3 | 1200.3 |
| +1 | 1315.2 | 785.5 | +1 | 1198.7 | 1043.1 |
| A(↓)×B(→) | −1 | +1 | C(↓)×D(→) | −1 | +1 |
|---|---|---|---|---|---|
| −1 | 559.9 | 765 | −1 | 421.4 | 764.9 |
| +1 | 896.5 | 460.4 | +1 | 895.5 | 598.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Pociño, A.; Asensio-Lozano, J.; Álvarez-Antolín, F.; García-Diez, A. Evaluation of Hardness, Sliding Wear and Strength of a Hypoeutectic White Iron with 25%Cr after Heat Treatments. Metals 2021, 11, 947. https://doi.org/10.3390/met11060947
González-Pociño A, Asensio-Lozano J, Álvarez-Antolín F, García-Diez A. Evaluation of Hardness, Sliding Wear and Strength of a Hypoeutectic White Iron with 25%Cr after Heat Treatments. Metals. 2021; 11(6):947. https://doi.org/10.3390/met11060947
Chicago/Turabian StyleGonzález-Pociño, Alejandro, Juan Asensio-Lozano, Florentino Álvarez-Antolín, and Ana García-Diez. 2021. "Evaluation of Hardness, Sliding Wear and Strength of a Hypoeutectic White Iron with 25%Cr after Heat Treatments" Metals 11, no. 6: 947. https://doi.org/10.3390/met11060947






