Effect of Co/Ni Substituting Fe on Magnetocaloric Properties of Fe-Based Bulk Metallic Glasses
Abstract
:1. Introduction
2. Experiment
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franco, V.; Blázquez, J.S.; Ingale, A.; Conde, B. The magnetocaloric effect and magnetic refrigeration near room temperature: Materials and models. Annu. Rev. Mater. Res. 2012, 42, 305–342. [Google Scholar] [CrossRef] [Green Version]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, lighter, and more energy efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.-H.; Yu, S.-C. Review of the magnetocaloric effect in manganite materials. J. Magn. Magn. Mater. 2007, 308, 325–340. [Google Scholar] [CrossRef]
- Tishin, A.M.; Spichkin, Y.I. The Magnetocaloric Effect and Its Applications, 1st ed.; Institute of Physics: London, UK, 2003. [Google Scholar]
- Alves, C.S.; Gama, S.; Coelho, A.D.A.; Plaza, E.J.R.; Carvalho, A.M.G.; Cardoso, L.P.; Persiano, A.C. Giant magnetocaloric effect in Gd5 (Si2 Ge2) alloy with low purity Gd. Mater. Res. Mater. Res. 2004, 7, 535–538. [Google Scholar] [CrossRef]
- Kimel, A.V.; Kirilyuk, A.; Tsvetkov, A.; Pisarev, R.V.; Rasing, T. Laser-induced ultrafast spin reorientation in the antiferromagnet TmFeO3. Nature 2004, 429, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Moore, J.D.; Skokov, K.P.; Krautz, M.; Löwe, K.; Barcza, A.; Katter, M.; Gutfleisch, O. Exploring La (Fe,Si)13-based magnetic refrigerants towards application. Scr. Mater. 2012, 67, 584–589. [Google Scholar] [CrossRef]
- Tegus, O.; Brück, E.; Buschow, K.H.J.; De Boer, F.R. Transition-metal-based magnetic refrigerants for room-temperature applications. Nat. Cell Biol. 2002, 415, 150–152. [Google Scholar] [CrossRef]
- Li, X.; Pan, Y. Magnetocaloric effect in Fe-Zr-B-M (M = Ni, Co, Al, and Ti) amorphous alloys. J. Appl. Phys. 2014, 116, 093910. [Google Scholar] [CrossRef]
- Wang, Y.F.; Qin, F.X.; Wang, Y.H.; Wang, H.; Das, R.; Phan, M.H.; Peng, H.X. Magnetocaloric effect of Gd-based microwires from binary to quaternary system. AIP Adv. 2017, 7, 056422. [Google Scholar] [CrossRef]
- Wang, W. The nature and properties of amorphous materials. Prog. Phys. 2013, 33, 177–350. [Google Scholar]
- Wang, F.; Inoue, A.; Han, Y. Excellent soft magnetic Fe-Co-B-based amorphous alloys with extremely high saturation magnetization above 1.85 T and low coercivity below 3 A/m. J. Alloys Compd. 2017, 711, 132–142. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Xu, T.; Liu, F.; Zhang, T. Near room-temperature magnetocaloric effect in FeMnPBC metallic glasses with tunable Curie temperature. J. Magn. Magn. Mater. 2013, 347, 131–135. [Google Scholar] [CrossRef]
- Zhao, L.; Tian, H.; Zhong, X.; Liu, Z.; Greneche, J.M.; Ramanujan, R.V. Influence of gadolinium and dysprosium substitution on magnetic properties and magnetocaloric effect of Fe78-xRExSi4Nb5B12Cu1 amorphous alloys. J. Rare Earths 2020, 38, 1317–1321. [Google Scholar] [CrossRef]
- Tian, H.; Zhong, X.; Liu, Z.; Zheng, Z.; Min, J.X. Achieving table-like magnetocaloric effect and large refrigerant capacity around room temperature in Fe78-xCexSi4Nb5B12Cu1 (x = 0−10) composite materials. Mater. Lett. 2015, 138, 64–66. [Google Scholar] [CrossRef]
- Yang, W.; Li, W.; Wan, C.; Huo, J.; Mo, J.; Liu, H.; Shen, B. Low-temperature magnetic properties and magnetocaloric effect of Fe-Zr-Cu amorphous alloys. J. Low Temp. Phys. 2020, 200, 51–61. [Google Scholar] [CrossRef]
- Caballero-Flores, R.; Franco, V.; Conde, A.; Knipling, K.E.; Willard, M.A. Influence of Co and Ni addition on the magnetocaloric effect in Fe88−2xCoxNixZr7B4Cu1 soft magnetic amorphous alloys. Appl. Phys. Lett. 2010, 96, 182506. [Google Scholar] [CrossRef]
- Yang, W.; Huo, J.; Liu, H.; Li, J.; Song, L.; Li, Q.; Xue, L.; Shen, B.; Inoue, A. Extraordinary magnetocaloric effect of Fe-based bulk glassy rods by combining fluxing treatment and J-quenching technique. J. Alloys Compd. 2016, 684, 29–33. [Google Scholar] [CrossRef]
- Xu, K.; Ling, H.; Li, Q.; Li, J.; Yao, K.; Guo, S. Effects of Co substitution for Fe on the glass forming ability and properties of Fe80P13C7 bulk metallic glasses. Intermetallics 2014, 51, 53–58. [Google Scholar] [CrossRef]
- Ma, X.H.; Zhang, L.; Yang, X.H.; Li, Q.; Huang, Y.D. Effect of Ni addition on corrosion resistance of FePC bulk glassy alloy. Corros. Eng. Sci. Technol. 2014, 50, 433–437. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Kucuk, I.; Şarlar, K.; Adam, A.; Civan, E. Magnetocaloric and magnetoresistance properties in Co based (Co0.402Fe0.201Ni0.067B0.227Si0.053Nb0.05) 100-xCux (x = 0−1) glassy alloys. Philos. Mag. 2016, 96, 3120–3130. [Google Scholar] [CrossRef]
- Gschneidner Jr, K.A.; Pecharsky, V.K. Magnetocaloric materials. Annu. Rev. Mater. Sci. 2000, 30, 387–429. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhao, Z.; Zhang, X.; Li, H.; Zhao, G. Peculiar effect of rare earth doping on magnetic and magnetocaloric properties in Fe-rich amorphous ribbons. J. Alloys Compd. 2018, 735, 104–108. [Google Scholar] [CrossRef]
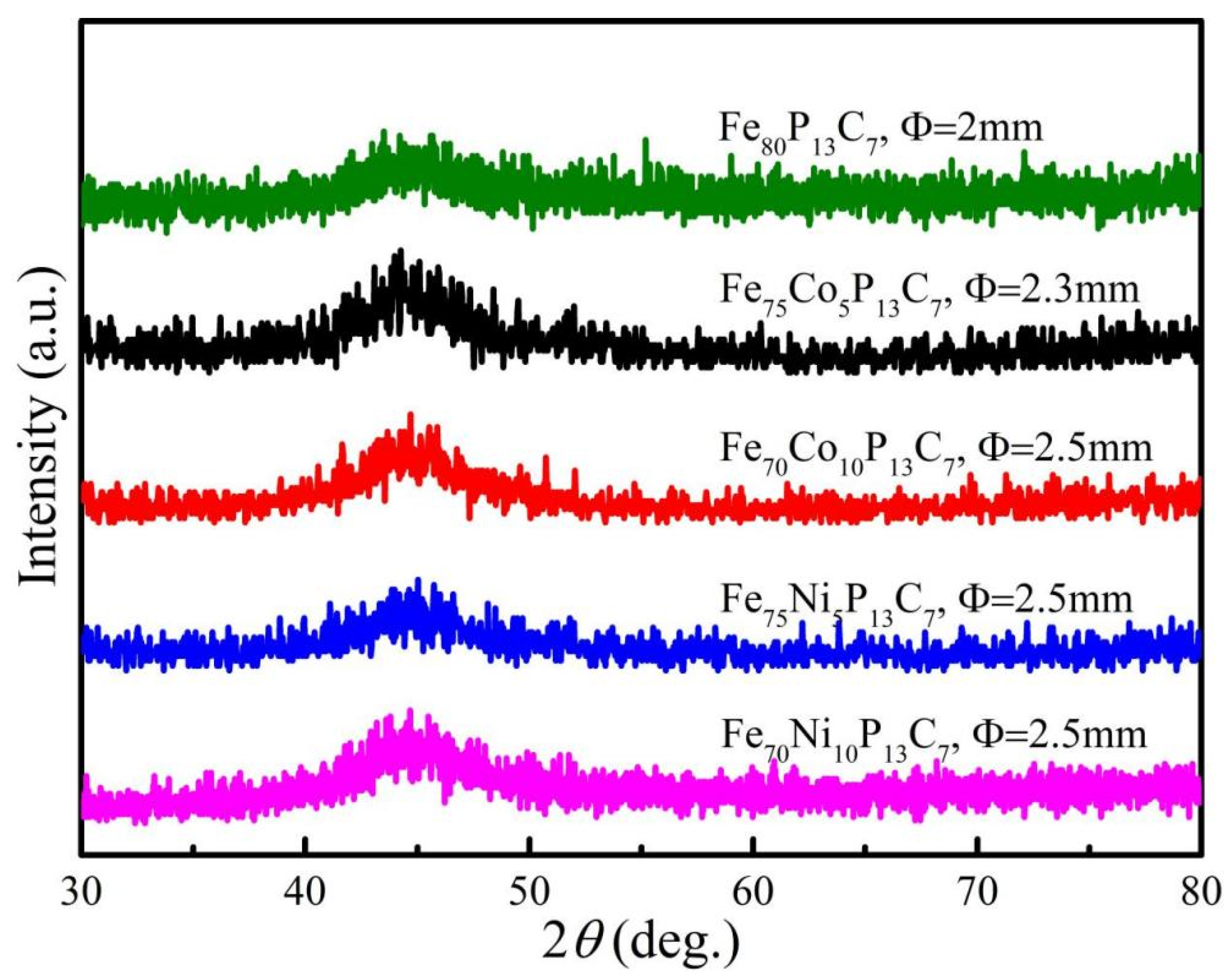

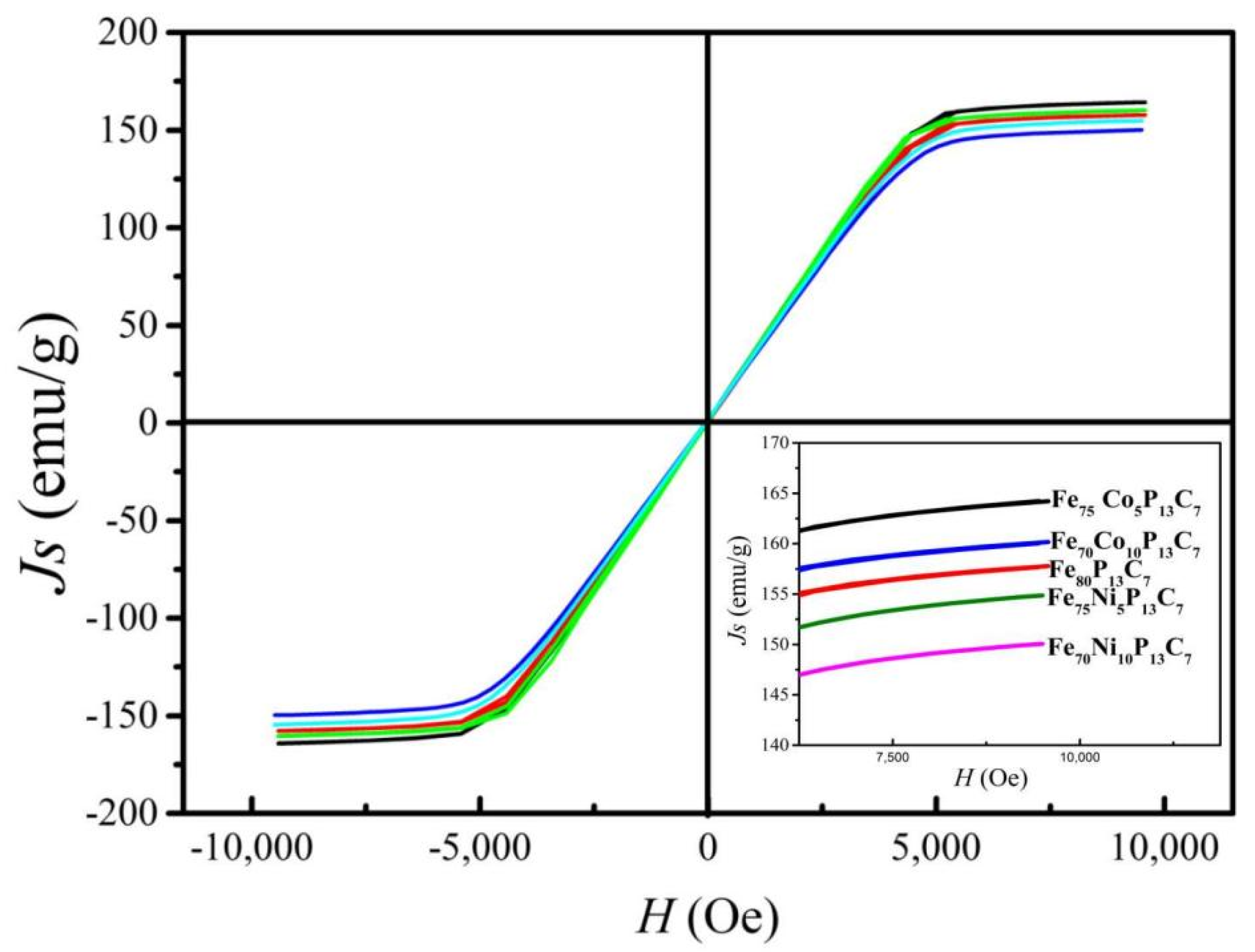
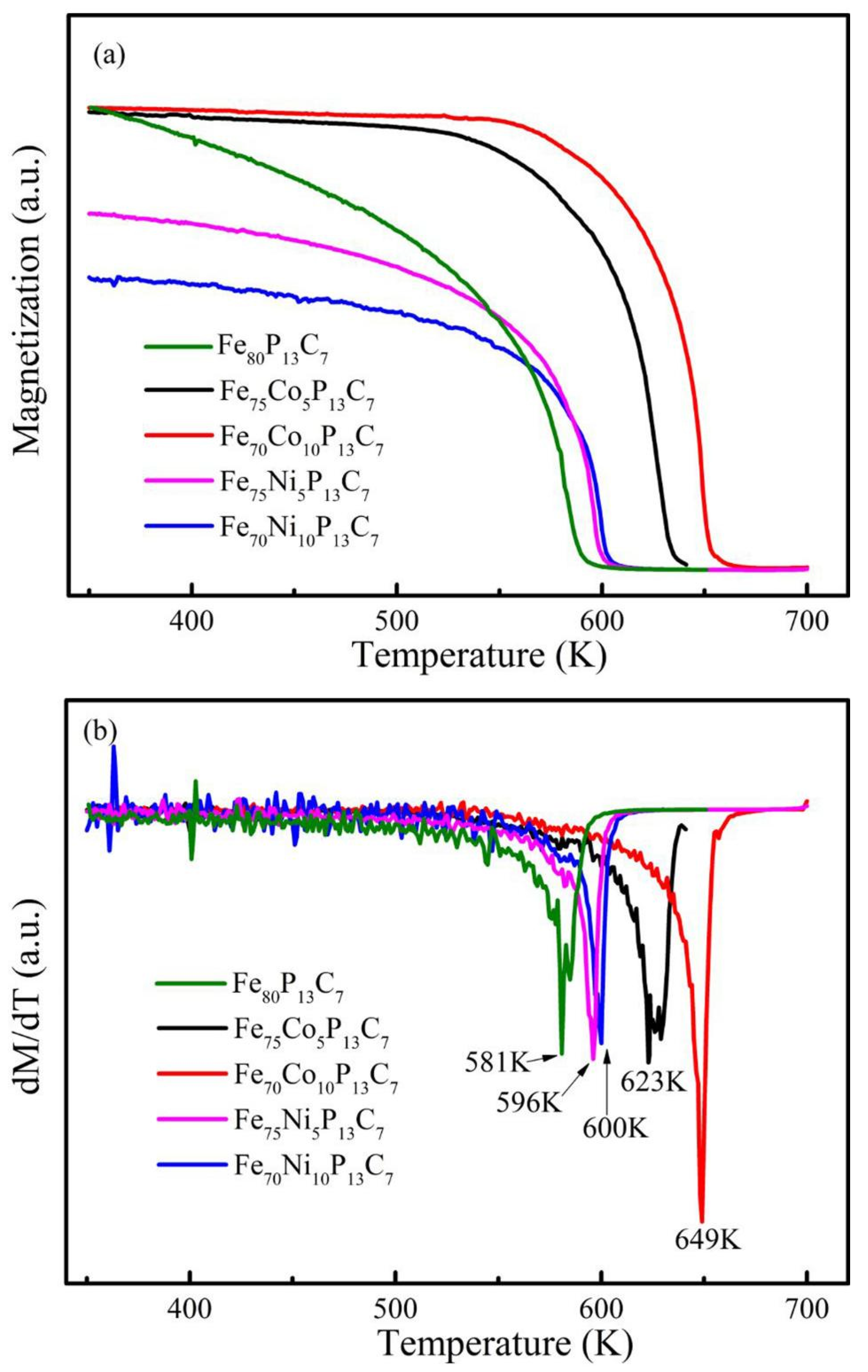
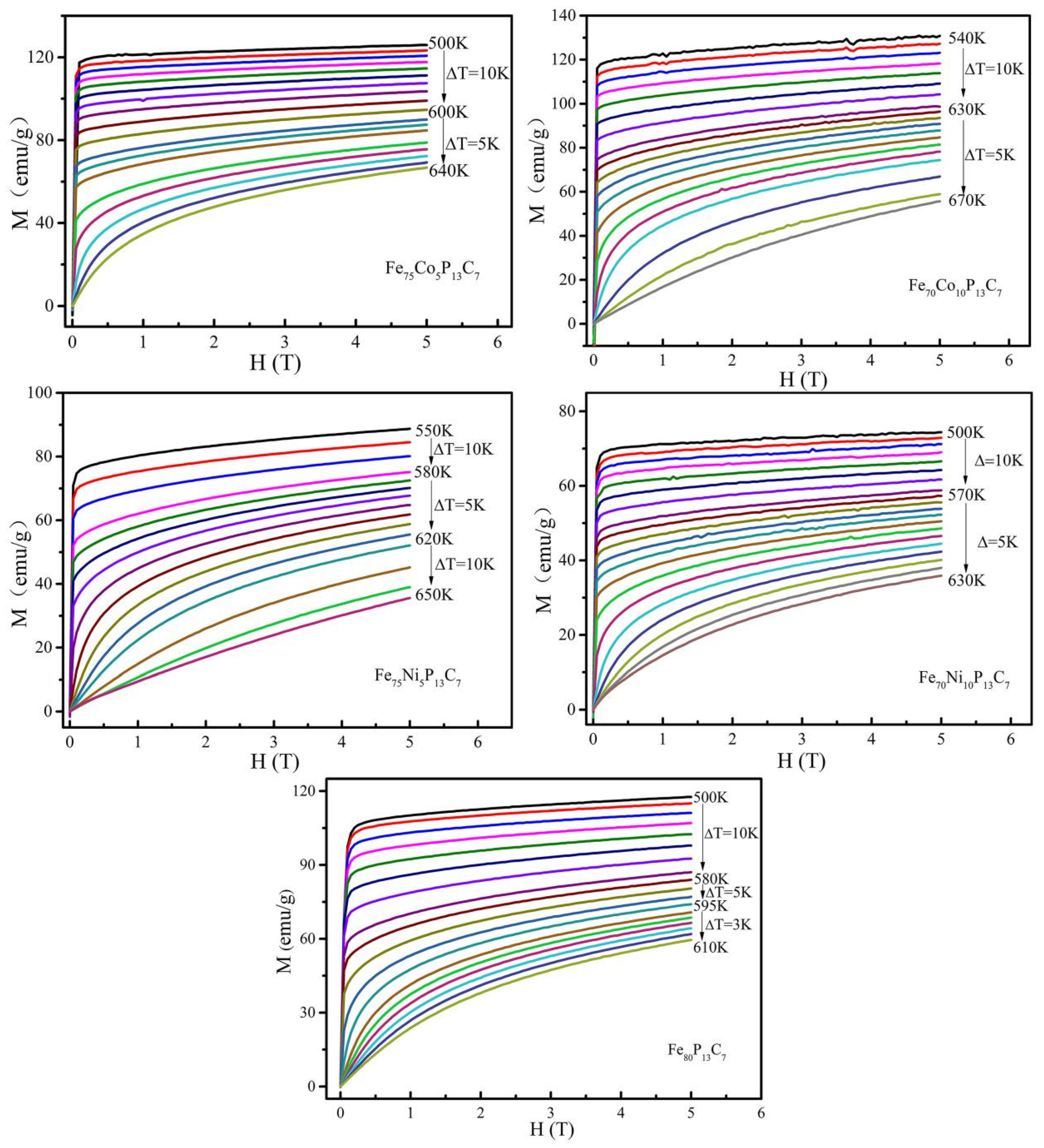


| Composition (at.%) | Dmax (mm) | TC (K) | Js (emu/g) | −ΔSM (J kg−1 K−1) | ΔTFWHM (K) | RCFWHM (J kg−1) | ||
|---|---|---|---|---|---|---|---|---|
| 1.5 T | 5 T | 1.5 T | 1.5 T | 5 T | ||||
| Fe80P13C7 | 2.0 | 581 | 160 | 2.20 | 5.05 | 57.1 | 125.6 | 479.8 |
| Fe75Co5P13C7 | 2.3 | 623 | 165 | 2.34 | 5.21 | 39.1 | 91.6 | 331.6 |
| Fe70Co10P13C7 | 2.5 | 649 | 157 | 2.08 | 4.98 | 55.4 | 115.2 | 462.1 |
| Fe75Ni5P13C7 | 2.5 | 596 | 155 | 2.04 | 4.60 | 52.2 | 106.5 | 381.8 |
| Fe70Ni10P13C7 | 2.5 | 600 | 150 | 1.46 | 3.26 | 48.7 | 71.1 | 211.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Xie, L.; Liu, C.; Li, Q.; Huo, J.; Chang, C.; Li, H.; Ma, X. Effect of Co/Ni Substituting Fe on Magnetocaloric Properties of Fe-Based Bulk Metallic Glasses. Metals 2021, 11, 950. https://doi.org/10.3390/met11060950
Guo J, Xie L, Liu C, Li Q, Huo J, Chang C, Li H, Ma X. Effect of Co/Ni Substituting Fe on Magnetocaloric Properties of Fe-Based Bulk Metallic Glasses. Metals. 2021; 11(6):950. https://doi.org/10.3390/met11060950
Chicago/Turabian StyleGuo, Jia, Lei Xie, Cong Liu, Qiang Li, Juntao Huo, Chuntao Chang, Hongxiang Li, and Xu Ma. 2021. "Effect of Co/Ni Substituting Fe on Magnetocaloric Properties of Fe-Based Bulk Metallic Glasses" Metals 11, no. 6: 950. https://doi.org/10.3390/met11060950






