Synthesis and Electrochemical Properties of TiNb2O7 and Ti2Nb10O29 Anodes under Various Annealing Atmospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. TiNb2O7 and Ti2Nb10O29 Synthesis
2.2. Material Characterization
2.3. Electrochemical Characteristics
3. Results and Discussion
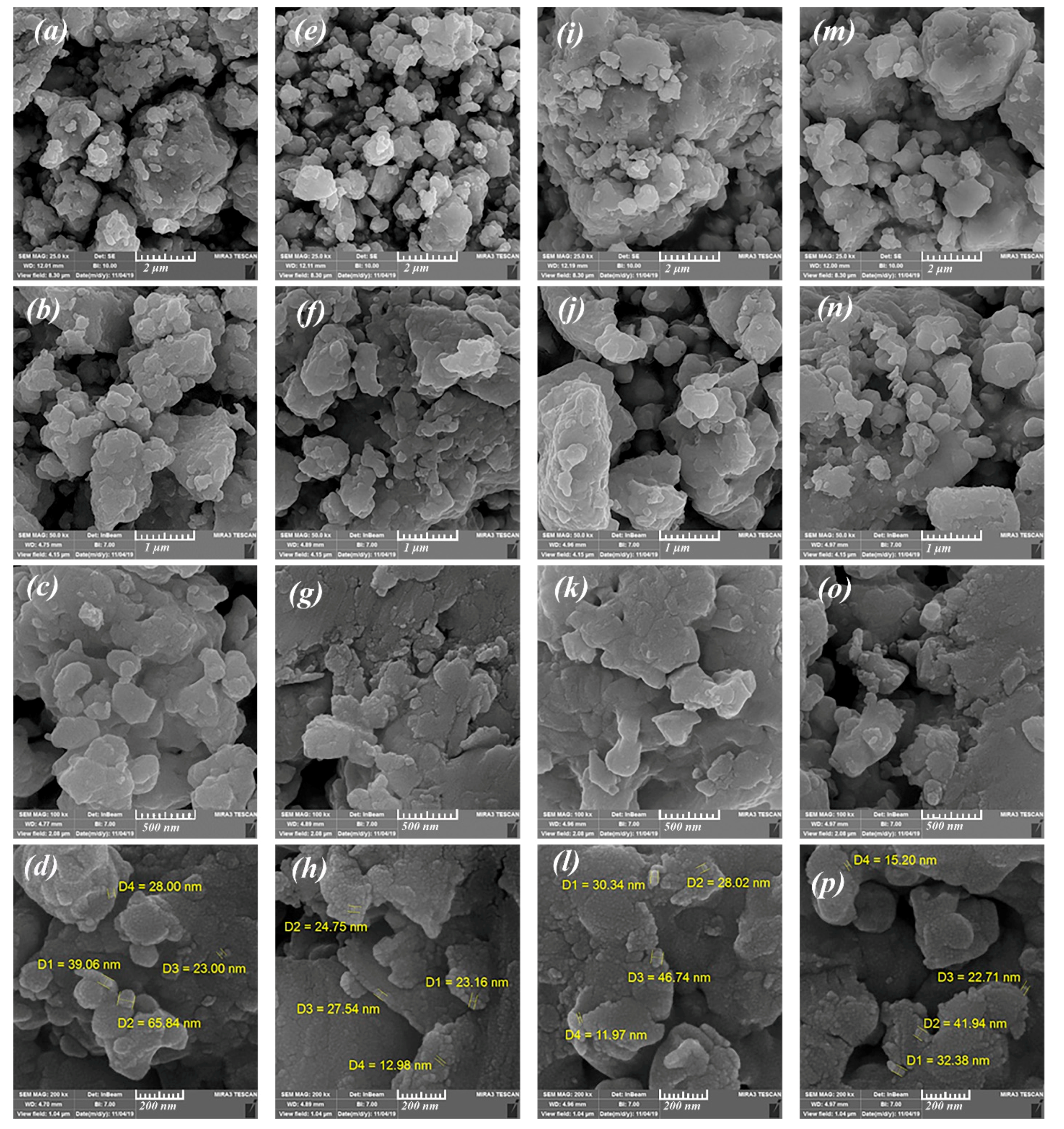
3.1. Structure and Morphology
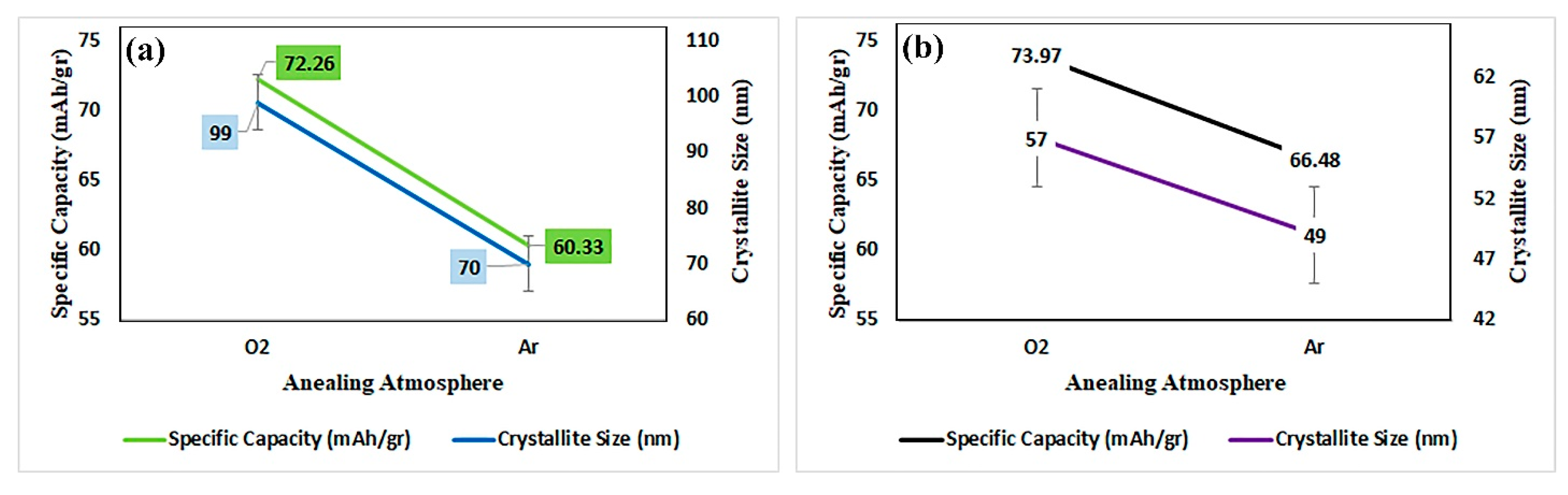
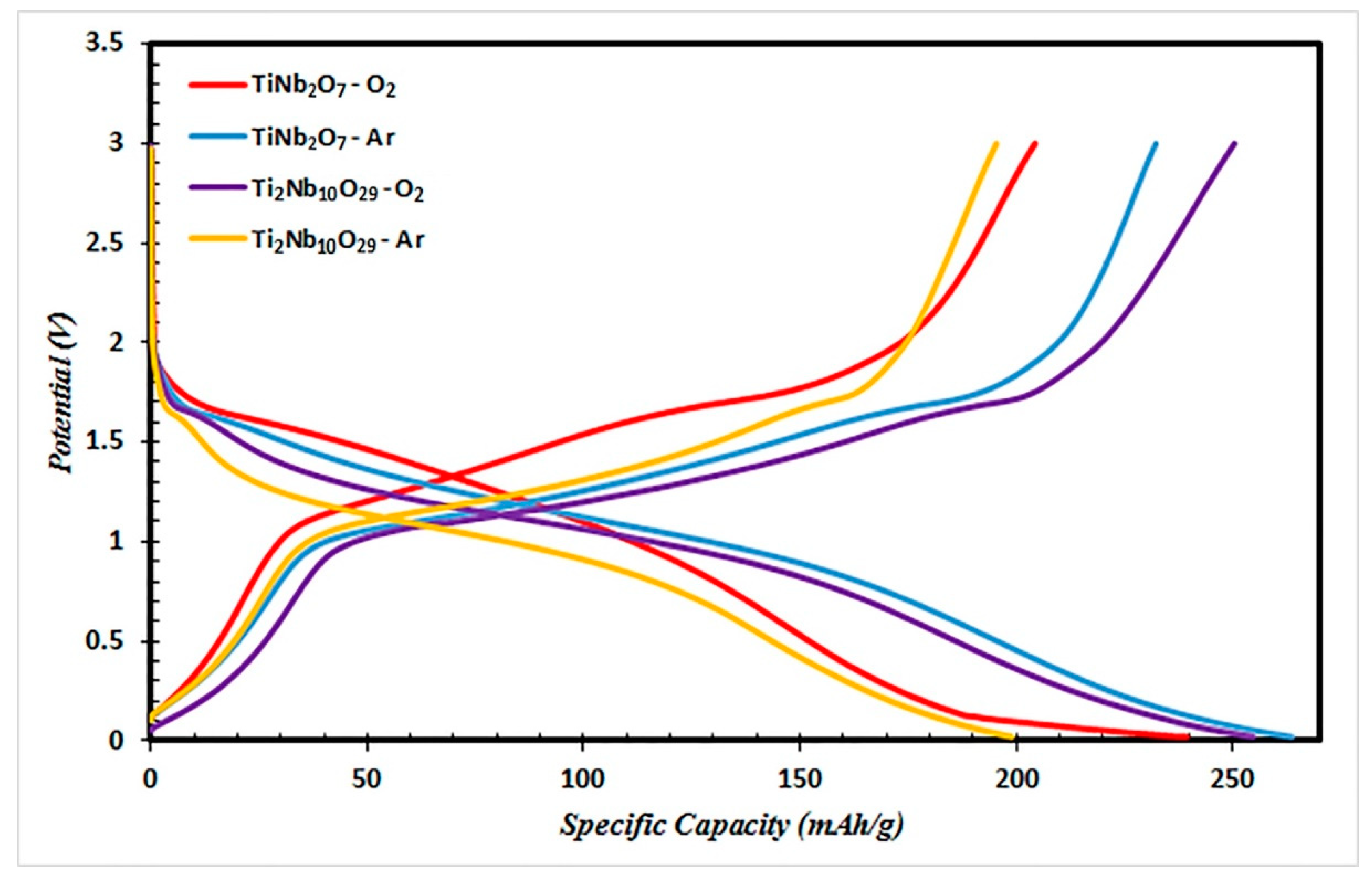
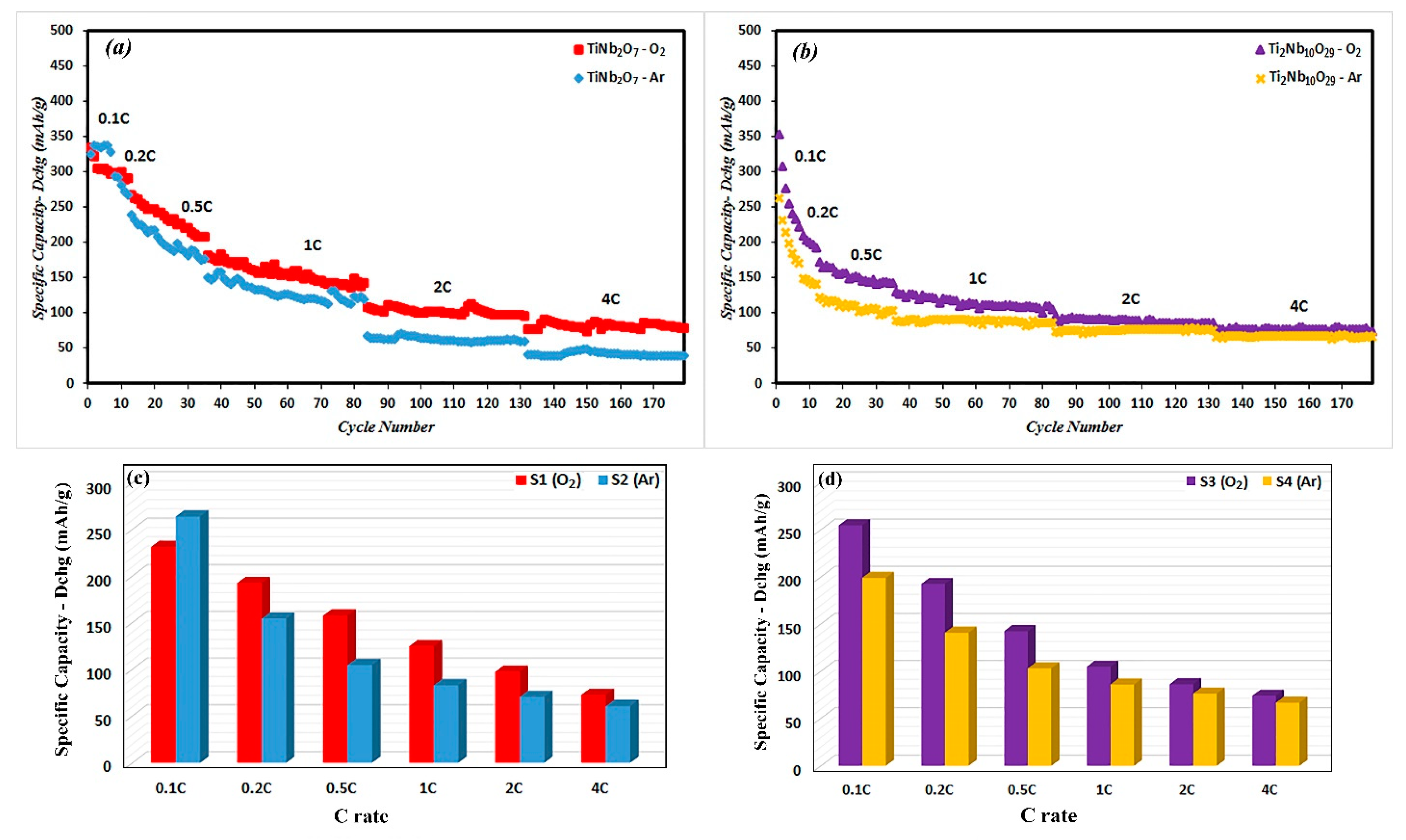
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Sun, R.; Liu, G.; Cao, S.; Dong, B.; Liu, X.; Hu, M.; Liu, M.; Duan, X. High rate capability performance of ordered mesoporous TiNb6O17 microsphere anodes for lithium ion batteries. Dalton Trans. 2017, 46, 17061–17066. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Deng, S.; Kautz, D.J.; Xu, Z.; Liu, T.; Li, J.; Wang, N.; Lin, F. Intercalating Ti2Nb14O39 Anode Materials for Fast-Charging, High-Capacity and Safe Lithium-Ion Batteries. Small 2017, 13, 1702903. [Google Scholar] [CrossRef]
- Yao, F.; Pham, D.; Lee, Y. Carbon-based materials for lithium-ion batteries, electrochemical capacitors, and their hybrid devices. ChemSusChem 2015, 8, 2284–2311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y. Recent progress of TiO2-based anodes for Li ion batteries. J. Nanomater. 2016, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Croy, J.R.; Balasubramanian, M.; Gallagher, K.G.; Burrell, A.K. Review of the US Department of Energy’s “deep dive” effort to understand voltage fade in Li-and Mn-rich cathodes. Acc. Chem. Res. 2015, 48, 2813–2821. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Pang, G.; Fang, S.; Luo, H.; Yang, K.; Zhang, X. TiNb2O7 nanoparticles assembled into hierarchical microspheres as high-rate capability and longcycle-life anode materials for lithium ion batteries. Nanoscale 2014, 7, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Catti, M.; Pinus, I.; Knight, K. Lithium insertion properties of LixTiNb2O7 investigated by neutron diffraction and first-principles modelling. J. Solid State Chem. 2015, 229, 19–25. [Google Scholar] [CrossRef]
- Park, H.; Wu, H.B.; Song, T.; Lou, X.W.; Paik, U. Porosity controlled TiNb2O7 microspheres with partial nitridation as a practical negative electrode for high-power lithium-ion batteries. Adv. Energy Mater. 2015, 5, 1401945. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Zhao, Y.; Lushington, A.; Gao, J.; Li, Q.; Zuo, P.; Wang, B.; Gao, Y.; Ma, Y.; et al. Superior performance of ordered macroporous TiNb2O7 anodes for lithium ion batteries: Understanding from the structural and pseudocapacitive insights on achieving high rate capability. Nano Energy 2017, 34, 15–25. [Google Scholar] [CrossRef]
- Wang, G.; Wen, Z.; Du, L.; Yang, Y.; Li, S.; Sun, J.; Ji, S. Hierarchical Ti-Nb oxide microspheres with synergic multiphase structure as ultra-long-life anode materials for lithium-ion batteries. J. Power Sour. 2017, 367, 106–115. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Xia, Y. Ti-based compounds as anode materials for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6652–6667. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Kundu, M.; Balakrishnan, B.; Kim, H.; Sevensson, A.M.; Jayasayee, K. Hematite microdisks as an alternative anode material for lithium-ion batteries. Mater. Lett. 2019, 247, 163–166. [Google Scholar] [CrossRef]
- Li, S.; Cao, X.; Schmidt, C.N.; Xu, Q.; Uchaker, E.; Pei, Y.; Cao, G. TiNb2O7/graphene composites as high-rate anode materials for lithium/sodium ion batteries. J. Mater. Chem. A 2016, 4, 4242–4251. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Deng, S.; Feng, S.; Wu, J.; Tu, J. Hierarchical porous Ti2Nb10O29 Nanospheres as superior anode materials for lithiumion storage. J. Mater. Chem. A 2017, 5, 21134–21139. [Google Scholar] [CrossRef]
- Tang, K.; Mu, X.; Aken, P.A.; Yu, Y.; Maier, J. Nano-Pearl-String TiNb2O7 as Anodes for Rechargeable Lithium Batteries. Adv. Energy Mater. 2012, 3, 49–53. [Google Scholar] [CrossRef]
- Jo, C.; Kim, Y.; Hwang, J.; Shim, J.; Chun, J.; Lee, J. Block copolymer directed ordered mesostructured TiNb2O7 multimetallic oxide constructed of nanocrystals as high power Li-ion battery anodes. Chem. Mater. 2014, 26, 3508–3514. [Google Scholar] [CrossRef]
- Lin, C.; Wang, G.; Lin, S.; Li, J.; Lu, L. TiNb6O17: A new electrode material for lithium-ion batteries. Communication 2015, 51, 8970–8973. [Google Scholar] [CrossRef]
- Perfler, L.; Kahlenberg, V.; Wikete, C.; Schmidmair, D.; Tribus, M.; Kaindl, R. Nanoindentation, High-temperature behavior, and crystallographic/spectroscopic characterization of the high-refractive-index materials TiTa2O7 and TiNb2O7. Inorg. Chem. 2015, 54, 6836–6848. [Google Scholar] [CrossRef]
- Pham-Cong, D.; Kim, J.; Tran, V.T.; Kim, S.J.; Jeong, S.; Choi, J.; Cho, C. Electrochemical behavior of interconnected Ti2Nb10O29 nanoparticles for high-power Li-ion battery anodes. Electrochim. Acta 2017, 236, 451–459. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Hu, S.; Li, F.; Fan, Z. Preparation of dihydroxy naphthalene/TiO2 complex via surface modification and their photocatalytic H2 production performances under visible light. Bull. Korean Chem. Soc. 2013, 34, 2056–2062. [Google Scholar] [CrossRef] [Green Version]
- Hansen, P.L.; Wagner, J.B.; Helveg, S.; Rostrup-Nielsen, J.R.; Clausen, B.S.; Topsøe, H. Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science 2002, 295, 2053–20555. [Google Scholar] [CrossRef]
- Lin, J.; Lin, Y.; Liu, P.; Meziani, M.J.; Allard, L.F.; Sun, Y.P. Hot-fluid annealing for crystalline titanium dioxide nanoparticles in stable suspension. J. Am. Chem. Soc. 2002, 38, 11514–11518. [Google Scholar] [CrossRef]
- Rai, A.K.; Anh, L.T.; Gim, J.; Mathew, V.; Kang, J.; Paul, B.J.; Song, J.; Kim, J. Simple synthesis and particle size effects of TiO2 nanoparticle anodes for rechargeable lithium ion batteries. Electrochim. Acta 2013, 90, 112–118. [Google Scholar] [CrossRef]
- Liu, N.; Schneider, C.; Freitag, D.; Hartmann, M.; Venkatesan, U.; Müller, J.; Spiecker, E.; Schmuki, P. Black TiO2 nanotubes: Cocatalyst-free open-circuit hydrogen generation. Nano Lett. 2014, 14, 3309–3313. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xiao, P.; Zhang, Y.; Garcia, B.B.; Zhang, Q.; Guo, Q.; Champion, R.; Cao, G. TiO2 nanotube arrays annealed in N2 for efficient lithium-ion intercalation. J. Phys. Chem. C 2008, 112, 11175–11180. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, Y.; Xiao, P.; García, B.B.; Zhang, Q.; Zhou, X.; Jeong, Y.-H.; Cao, G. TiO2 nanotube arrays annealed in CO exhibiting high performance for lithium-ion intercalation. Electrochim. Acta 2009, 54, 6816–6820. [Google Scholar] [CrossRef]
- Williason, G.K.; Hall, W.H. X-ray Line Broadening from Filed Aluminum and Wolfram. Acta Metall. 1953, 1, 122–131. [Google Scholar]
- Madram, A.R.; Daneshtalab, R.; Sovizi, M.R. Effect of Na+ and K+ co-doping on the structure and electrochemical behaviors of LiFePO4/C cathode material for lithium-ion batteries. R. Soc. Chem. 2016, 6, 101477–101484. [Google Scholar] [CrossRef]
- Jiang, W.T.; Peacor, D.R.; Rkaip, A.; Toth, M.; Kim, J.W. TEM and XRD Determination of Crystallite Size and Lattice Strain as a Function of Illite Crystallinity in Pelitic Rocks. J. Metamorph. Geol. 2004, 15, 267–281. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.; Pralong, V.; Varadaraju, U.V.; Raveau, B. Crystallite Size Constraints on Lithium Insertion into Brookite TiO2. Electrochem. Solid State Lett. 2008, 11, A132–A134. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Zhang, Q.; Cao, G. Engineering nanostructured electrodes away from equilibrium for lithium-ion batteries. J. Mater. Chem. 2011, 21, 9969–9983. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Garcia, B.B.; Zhang, Q.; Pan, A.; Jeong, Y.-H.; Cao, G. V2O5 xerogel electrodes with much enhanced lithium-ion intercalation properties with N2 annealing. J. Mater. Chem. 2009, 19, 8789–8795. [Google Scholar] [CrossRef]
- Griffith, K.J.; Seymour, I.D.; Hope, M.A.; Butala, M.M.; Lamontagne, L.K.; Preefer, M.B.; Koçer, C.P.; Henkelman, G.; Morris, A.J.; Cliffe, M.J.; et al. Ionic and Electronic Conduction in TiNb2O7. J. Am. Chem. Soc. 2019, 141, 16706–16725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerold, E.; Luidold, S.; Antrekowitsch, H. Selective Precipitation of Metal Oxalates from Lithium Ion Battery Leach Solutions. Metals 2020, 10, 1435. [Google Scholar] [CrossRef]
- Jenei, P.; Kádár, C.; Han, G.; Hung, P.T.; Choe, H.; Gubicza, J. Annealing-Induced Changes in the Microstructure and Mechanical Response of a Cu Nanofoam Processed by Dealloying. Metals 2020, 10, 1128. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, K.; Ahn, J.; Lee, J. Application of Multistage Concentration (MSC) Electrodialysis to Concentrate Lithium from Lithium-Containing Waste Solution. Metals 2020, 10, 851. [Google Scholar] [CrossRef]
- Klimko, J.; Oráč, D.; Miškufová, A.; Vonderstein, C.; Dertmann, C.; Sommerfeld, M.; Friedrich, B.; Havlík, T. A Combined Pyro- and Hydrometallurgical Approach to Recycle Pyrolyzed Lithium-Ion Battery Black Mass Part 2: Lithium Recovery from Li Enriched Slag—Thermodynamic Study, Kinetic Study, and Dry Digestion. Metals 2020, 10, 1558. [Google Scholar] [CrossRef]
- Schwich, L.; Küpers, M.; Finsterbusch, M.; Schreiber, A.; Fattakhova-Rohlfing, D.; Guillon, O.; Friedrich, B. Recycling Strategies for Ceramic All-Solid-State Batteries—Part I: Study on Possible Treatments in Contrast to Li-Ion Battery Recycling. Metals 2020, 10, 1523. [Google Scholar] [CrossRef]
- Schwich, L.; Sabarny, P.; Friedrich, B. Recycling Potential of Lithium–Sulfur Batteries—A First Concept Using Thermal and Hydrometallurgical Methods. Metals 2020, 10, 1513. [Google Scholar] [CrossRef]
- Sinn, T.; Flegler, A.; Wolf, A.; Stübinger, T.; Witt, W.; Nirschl, H.; Gleiß, M. Investigation of Centrifugal Fractionation with Time-Dependent Process Parameters as a New Approach Contributing to the Direct Recycling of Lithium-Ion Battery Components. Metals 2020, 10, 1617. [Google Scholar] [CrossRef]
- Lin, C.; Hu, L.; Cheng, C.; Sun, K.; Guo, X.; Shao, Q.; Li, J.; Wang, N.; Guo, Z. Nano-TiNb2O7/carbon nanotubes composite anode for enhanced lithium-ion storage. Electrochim. Acta 2018, 260, 65–72. [Google Scholar] [CrossRef]
- Babu, B.; Shaijumon, M.M. Studies on kinetics and diffusion characteristics of lithium ions in TiNb2O7. Electrochim. Acta 2020, 345, 136208. [Google Scholar] [CrossRef]
- Ise, K.; Morimoto, S.; Harada, Y.; Takami, N. Large lithium storage in highly crystalline TiNb2O7 nanoparticles synthesized by a hydrothermal method as anodes for lithium-ion batteries. Solid State Ion. 2018, 320, 7–15. [Google Scholar] [CrossRef]


| Sample No. | Stoichiometric Reaction | Milling Time (h) | Furnace/Annealing Atmosphere | Annealing Time (h) | Annealing Temperature (°C) |
|---|---|---|---|---|---|
| S1 | TiNb2O7 | 5 | Tube/oxygen | 2 | 900 |
| S2 | TiNb2O7 | 5 | Tube/argon | 2 | 900 |
| S3 | Ti2Nb10O29 | 5 | Tube/oxygen | 2 | 900 |
| S4 | Ti2Nb10O29 | 5 | Tube/argon | 2 | 900 |
| Sample No. | Cycle No. | No. of Cycles per Each C Rate | Capacity (mAh/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1C | 0.2C | 0.5C | 1C | 2C | 4C | 0.1C | 0.2C | 0.5C | 1C | 2C | 4C | ||
| S1 | 179 | 4 | 8 | 23 | 48 | 48 | 48 | 231 | 192 | 157 | 124 | 97 | 72 |
| S2 | 179 | 4 | 8 | 23 | 48 | 48 | 48 | 264 | 154 | 104 | 83 | 70 | 60 |
| S3 | 179 | 4 | 8 | 23 | 48 | 48 | 48 | 255 | 193 | 142 | 105 | 86 | 74 |
| S4 | 179 | 4 | 8 | 23 | 48 | 48 | 48 | 199 | 141 | 103 | 86 | 76 | 66 |
| Sample | Re (Ω cm2) | Rct (Ω cm2) | i0 (mA cm2) | σw (Ω cm2 s1/2) | DC (cm2/s) |
|---|---|---|---|---|---|
| TiNb2O7 (O2) | 16.19 | 29 | 8.85 × 10−4 | 3.74 | 1.24 × 10−11 |
| TiNb2O7 (Ar) | 9.76 | 41 | 6.26 × 10−4 | 3.92 | 1.13 × 10−11 |
| Ti2Nb10O29 (O2) | 20.81 | 54 | 4.76 × 10−4 | 5.27 | 6.22 × 10−12 |
| Ti2Nb10O29 (Ar) | - | - | - | 107.74 | 1.49 × 10−14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhami, T.; Ebrahimi-Kahrizsangi, R.; Bakhsheshi-Rad, H.R.; Majidi, S.; Ghorbanzadeh, M.; Berto, F. Synthesis and Electrochemical Properties of TiNb2O7 and Ti2Nb10O29 Anodes under Various Annealing Atmospheres. Metals 2021, 11, 983. https://doi.org/10.3390/met11060983
Adhami T, Ebrahimi-Kahrizsangi R, Bakhsheshi-Rad HR, Majidi S, Ghorbanzadeh M, Berto F. Synthesis and Electrochemical Properties of TiNb2O7 and Ti2Nb10O29 Anodes under Various Annealing Atmospheres. Metals. 2021; 11(6):983. https://doi.org/10.3390/met11060983
Chicago/Turabian StyleAdhami, Touraj, Reza Ebrahimi-Kahrizsangi, Hamid Reza Bakhsheshi-Rad, Somayeh Majidi, Milad Ghorbanzadeh, and Filippo Berto. 2021. "Synthesis and Electrochemical Properties of TiNb2O7 and Ti2Nb10O29 Anodes under Various Annealing Atmospheres" Metals 11, no. 6: 983. https://doi.org/10.3390/met11060983
APA StyleAdhami, T., Ebrahimi-Kahrizsangi, R., Bakhsheshi-Rad, H. R., Majidi, S., Ghorbanzadeh, M., & Berto, F. (2021). Synthesis and Electrochemical Properties of TiNb2O7 and Ti2Nb10O29 Anodes under Various Annealing Atmospheres. Metals, 11(6), 983. https://doi.org/10.3390/met11060983







