Research Progress of First Principles Studies on Oxidation Behaviors of Ti-Al Alloys and Alloying Influence
Abstract
:1. Introduction
2. Alloying Methods
3. Theoretical Study
3.1. Surface Oxidation Properties
3.2. Stability and Adhesion Properties of the Interfaces
3.3. Bulk Properties
4. Summary
- (1)
- The surface oxidation properties of Ti-Al alloys such as the surface stability, oxygen adsorption properties, and doping effects have received the most attention. Although there is a strong tendency to form TiO2 on the original alloy surfaces, the interactions between oxygen and titanium can be inhibited to a certain extent by alloying. The alloying elements like B, Si, Cl show an obvious segregation tendency on the surfaces of the alloys and weaken the binding between oxygens and metal atoms. The high cation valence elements like Ta and W are effective in hinder the reaction between Ti and O, agreeing with the valence control mechanism.
- (2)
- In order to explore the bonding properties between the oxides and the alloy matrix, the interface systems are greatly studied, mainly focusing on the representative oxides (TiO2 and Al2O3) and Ti-Al matrix. Not surprisingly, the alloy elements have different influences on the stability and adhesion properties of the interfaces, such as Nb can enhance the strength of the TiO2/γ-TiAl interface while Mo may play a negative role in this system, generally, the elements that own obvious segregation tendency may have more effects on the adhesion strength.
- (3)
- The diffusion properties and the stability of the oxides have also been studied. Some typical alloying elements such as Nb can not only segregate in the oxides and optimize the structure of the oxides. They also reduce the diffusion rate of oxygen atoms, and thus inhibit the rapid growth of the oxide.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Pollock, T.M. Alloy design for aircraft engines. Nat. Mater. 2016, 15, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L.; Li, Y. The Electronic Theory Study on High-temperature Oxidation Mechanism of TiAl alloy. Acta Phys. Sin. 2012, 61, 177101. [Google Scholar]
- Swadzba, R.; Marugi, K.; Pyclik, L. STEM Investigations of γ-TiAl Produced by Additive Manufacturing after Isothermal Oxidation. Corros. Sci. 2020, 169, 108617. [Google Scholar] [CrossRef]
- Mogale, N.F.; Matizamhuka, W.R. Spark Plasma Sintering of Titanium Aluminides: A Progress Review on Processing, Structure-Property Relations, Alloy Development and Challenges. Metals 2020, 10, 1080. [Google Scholar] [CrossRef]
- Garip, Y. Investigation of isothermal oxidation performance of TiAl alloys sintered by different processing methods. Intermetallics 2020, 127, 106985. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, H.; Zhang, G.; Zhang, Y.; Lin, H.; Yang, Y. Investigation on the initial oxidation behavior of TiAl alloy. Mater. Res. Express 2019, 6, 106595. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.; Chen, C.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Lim, H.; Liew, W.; Melvin, G.; Jiang, Z.-T. A Short Review on the Phase Structures, Oxidation Kinetics, and Mechanical Properties of Complex Ti-Al Alloys. Materials 2021, 14, 1677. [Google Scholar] [CrossRef]
- Cobbinah, P.V.; Matizamhuka, W.; Machaka, R.; Shongwe, M.B.; Yamabe-Mitarai, Y. The effect of Ta additions on the oxidation resistance of SPS-produced TiAl alloys. Int. J. Adv. Manuf. Technol. 2020, 106, 3203–3215. [Google Scholar] [CrossRef]
- Bacos, M.P.; Ceccacci, S.; Monchoux, J.P.; Davoine, C.; Gheno, T.; Rio, C.; Morel, A.; Merot, J.-S.; Fossard, F.; Thomas, M. Oxidation Behavior of a Spark Plasma Sintered Ti–48Al–2W–0.1B Alloy at 800 °C. Oxid. Met. 2020, 93, 587–600. [Google Scholar] [CrossRef]
- Galetz, M.C.; Ulrich, A.S.; Oskay, C.; Fahsing, D.; Laska, N.; Schulz, U.; Schutze, M. Oxidation-induced Microstructural Changes of the TiAl TNM-B1 Alloy after Expo-sure at 900 °C in Air. Intermetallics 2020, 123, 106830. [Google Scholar] [CrossRef]
- Knaislová, A.; Novák, P.; Cabibbo, M.; Jaworska, L.; Vojtěch, D. Development of TiAl–Si Alloys—A Review. Materials 2021, 14, 1030. [Google Scholar] [CrossRef] [PubMed]
- Ralison, A.; Dettenwanger, F.; Schutze, M. Oxidation of Orthorhombic Ti2AlNb Alloys in the Temperature Range 550–1000 Degrees C in Air. Mater. High Temp. 2003, 20, 607–629. [Google Scholar] [CrossRef]
- Chou, K.; Chu, P.-W.; Marquis, E.A. Early oxidation behavior of Si-coated titanium. Corros. Sci. 2018, 140, 297–306. [Google Scholar] [CrossRef]
- Garip, Y.; Ozdemir, O. Investigation of Cyclic Oxidation Behavior of (Cr, Mo, Si)-Containing (α2 + γ) TiAl-Based Alloys Synthesized by ECAS Sintering. Phys. Met. Metallogr. 2020, 121, 322–329. [Google Scholar] [CrossRef]
- Mengis, L.; Ulrich, A.S.; Watermeyer, P.; Liebscher, C.H.; Galetz, M.C. Oxidation Behaviour and Related Microstructural Changes of two β0–phase containing TiAl Alloys between 600 °C and 900 °C. Corros. Sci. 2021, 178, 109085. [Google Scholar] [CrossRef]
- Neelam, N.S.; Banumathy, S.; Bhattacharjee, A.; Rao, N.; Zafir, A. Comparison of the Isothermal and Cyclic Oxidation Behavior of Cr and Mo Containing γ-TiAlNb alloys. Corros. Sci. 2019, 163, 108300. [Google Scholar] [CrossRef]
- Garip, Y.; Ozdemir, O. A study of the cycle oxidation behavior of the Cr/Mn/Mo alloyed Ti–48Al–based intermetallics prepared by ECAS. J. Alloys Compd. 2020, 818, 152818. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, X.; Hayat, M.D.; Yang, F.; Liu, C.; Li, Y.; Li, X.; Xu, W.; Qu, X.; Cao, P. Effect of Sn addition on the high-temperature oxidation behavior of high Nb-containing TiAl alloys. Corros. Sci. 2020, 166, 108449. [Google Scholar] [CrossRef]
- Dadé, M.; Esin, V.; Nazé, L.; Sallot, P. Short- and long-term oxidation behaviour of an advanced Ti2AlNb alloy. Corros. Sci. 2019, 148, 379–387. [Google Scholar] [CrossRef]
- Liu, C.C. Investigation of Properties and Metal Injection Moulding of Sn Micro-alloyed High Nb Containing TiAl Alloy. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2018. [Google Scholar]
- Zeng, S.W. Research on Hot Deformation and Oxidation Behavior of TiAl Containing Nb, Mo. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]
- Zhao, P.; Li, X.; Tang, H.; Ma, Y.; Chen, B.; Xing, W.; Liu, K.; Yu, J. Improved High-Temperature Oxidation Properties for Mn-Containing Beta-Gamma TiAl with W Addition. Oxid. Met. 2020, 93, 433–448. [Google Scholar] [CrossRef]
- Liu, J. Research on the Effect of Microstructure on the Oxidation Behavior of γ-TiAl Alloys. Ph.D. Thesis, Northwestern Polytechnical University, Xi’an, China, 2017. [Google Scholar]
- Li, D.; Zhang, G.; Lu, G.; Wang, J.; Liu, C. Optimizing high-temperature oxidation behaviors of high-Nb-containing TiAl alloys by addition of boron. Corros. Sci. 2020, 177, 108971. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, X.; Hui, T.; Liu, C.; Liu, B.; Xu, W.; Zhang, C.; Sun, J.; Qu, X.; Zhang, J. High-temperature oxidation behaviour of TiAl alloys with Co addition. J. Mater. Sci. 2020, 56, 815–827. [Google Scholar] [CrossRef]
- Song, Y.; Dai, J.H.; Yang, R. Mechanism of Oxygen Adsorption on Surfaces of Gamma-TiAl. Surf. Sci. 2012, 606, 852–857. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.M.; Wang, S.Q.; Ye, H. First-Principles Study of Oxygen Atom Adsorption on γ-TiAl(111) Surface. Acta Metall. Sin. 2006, 42, 897–902. [Google Scholar]
- Wang, L.; Shang, J.X.; Wang, F.H.; Chen, Y.; Zhang, Y. Oxygen Adsorption on Gamma-TiAl Surfaces and the Related Surface Phase Diagrams: A Density-functional Theory Study. Acta. Mater. 2013, 61, 1726–1738. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkova, S.E.; Hu, Q.-M.; Yang, R. Initial oxidation of TiAl: An ab-initio investigation. AIP Conf. Proc. 2014, 1623, 39–42. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.Q.; Ye, H.Q. Initial Oxidation of γ-TiAl(111) Surface: Density-functional Theory Study. J. Mater. Sci. Technol. 2009, 25, 569–576. [Google Scholar]
- Zhou, Y.; Xiong, H.; Yin, Y.; Zhong, S. First principles study of surface properties and oxygen adsorption on the surface of Al3Ti intermetallic alloys. RSC Adv. 2019, 9, 1752–1758. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Dai, J.H.; Song, Y. Adsorption Properties of Oxygen Atom on the Surface of Ti2AlNb by First Principles Calculations. Comput. Mater. Sci. 2017, 139, 412–418. [Google Scholar] [CrossRef]
- Duan, X.; Warschkow, O.; Soon, A.; Delley, B.; Stampfl, C. Density functional study of oxygen on Cu(100) and Cu(110) surfaces. Phys. Rev. B 2010, 81, 075430. [Google Scholar] [CrossRef] [Green Version]
- Das, N.K.; Shoji, T. Early Stage Oxidation of Ni-Cr Binary Alloy (111), (110) and (100) Surfaces: A Combined Density Func-tional and Quantum Chemical Molecular Dynamics Study. Corros. Sci. 2013, 73, 18–31. [Google Scholar] [CrossRef]
- Liu, S.Y.; Shang, J.X.; Wang, F.H.; Liu, S.Y.; Zhang, Y.; Xu, H.B. Ab Initio Atomistic Thermodynamics Study on the Selective Oxidation Mechanism of the Surfaces of Intermetallic Compounds. Phys. Rev. B 2009, 80, 5. [Google Scholar] [CrossRef]
- Liu, S.Y.; Shang, J.X.; Wang, F.H.; Zhang, Y. Surface Segregation of Si and Its Effect on Oxygen Adsorption on a Gamma-TiAl(111) Surface from First Principles. J. Phys. Condes. Matter. 2009, 21, 7. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Liu, G.L.; Bao, J.S.; Liu, C.M.; Zhang, H. Energetic Studies on the B Effect on the Oxidation of γ-TiAl Alloys. Adv. Mater. Res. 2014, 1052, 28–33. [Google Scholar] [CrossRef]
- Wu, X.X.; Wang, Q.E.; Wang, F.H.; Zhou, Y.S. First-principles Study on Chemisorption of Cl on γ-TiAl(111) Surface. Acta Phys. Sin. Chin. Ed. 2010, 59, 7278–7284. [Google Scholar]
- Kulkova, S.E.; Bakulin, A.V.; Kulkov, S.S. Oxygen adsorption on the doped TiAl(1 0 0) surface. Comput. Mater. Sci. 2019, 170, 109136. [Google Scholar] [CrossRef]
- Wei, D.B.; Li, F.K.; Li, S.Q.; Wang, S.; Ding, F.; Liang, H.; Yan, Y.; Zhang, P. A Combined Experimental and First-principle Study on the Effect of Plasma Surface Ta–W Co-alloying on the Oxidation Behavior of γ-TiAl at 900 °C. J. Mater. Res. 2020, 35, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Pu, D.L.; Li, Y.Q.; Zheng, Q.H. Origin of the Antioxidation Mechanism of RuAl(1 1 0) Surface from First Principles Calculations. Meter. Sci. Eng. B Adv. 2020, 259, 114580. [Google Scholar] [CrossRef]
- Tian, X.F.; Wang, Y.; Li, L.S.; Wu, M.; Yu, Y. First Principles Studies of Oxygen Adsorption on the g-U (1 1 0) Surface and Influences of Mo Doping. Comp. Mater. Sci. 2020, 179, 109633. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Huang, J.; Wang, W.; Ye, Z.; Chen, S.; Zhao, Y. First-principles calculations on interface structure and fracture characteristic of TiC/TiZrC nano-multilayer film based on virtual crystal approximation. J. Alloys Compd. 2018, 755, 211–223. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Huang, J.; Ye, Z.; Sun, X.; Chen, S.; Zhao, Y. First-principles calculations on Ni/W interfaces in Steel/Ni/W hot isostatic pressure diffusion bonding layer. Appl. Surf. Sci. 2019, 475, 906–916. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, S.; Zhou, Y.; Xing, X.; Ren, J.; Yang, Q. Structure and properties of YAlO3/NbC heterogeneous nucleation interface: First principles calculation and experimental research. J. Alloys Compd. 2019, 773, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.Z.; Shao, W.; Hu, T.S.; Zhao, C.; Xing, X.; Zhou, Y.; Yang, Q. Adhesive Sliding and Interfacial Property of YAlO3/TiC Interface: A First Principles Investigation. J. Alloys Compd. 2019, 805, 1052–1059. [Google Scholar] [CrossRef]
- Wang, J.; Enomoto, M.; Shang, C. First-principles study on the interfacial segregation at coherent Cu precipitate/Fe matrix interface. Scr. Mater. 2020, 185, 42–46. [Google Scholar] [CrossRef]
- Ren, Z.; Qin, J.; Liu, R.; Zhang, X. Vacancy mediated alloying strengthening effects on Al/Al3Zr interface and stabilization of L12-Al3Zr: A first-principles study. J. Alloys Compd. 2020, 825, 153825. [Google Scholar] [CrossRef]
- Wang, B.; Dai, J.; Wu, X.; Song, Y.; Yang, R. First-principles study of the bonding characteristics of TiAl(111)/Al2O3(0001) interface. Intermetallics 2015, 60, 58–65. [Google Scholar] [CrossRef]
- Song, Y.; Xing, F.; Dai, J.; Yang, R. First-principles study of influence of Ti vacancy and Nb dopant on the bonding of TiAl/TiO2 interface. Intermetallics 2014, 49, 1–6. [Google Scholar] [CrossRef]
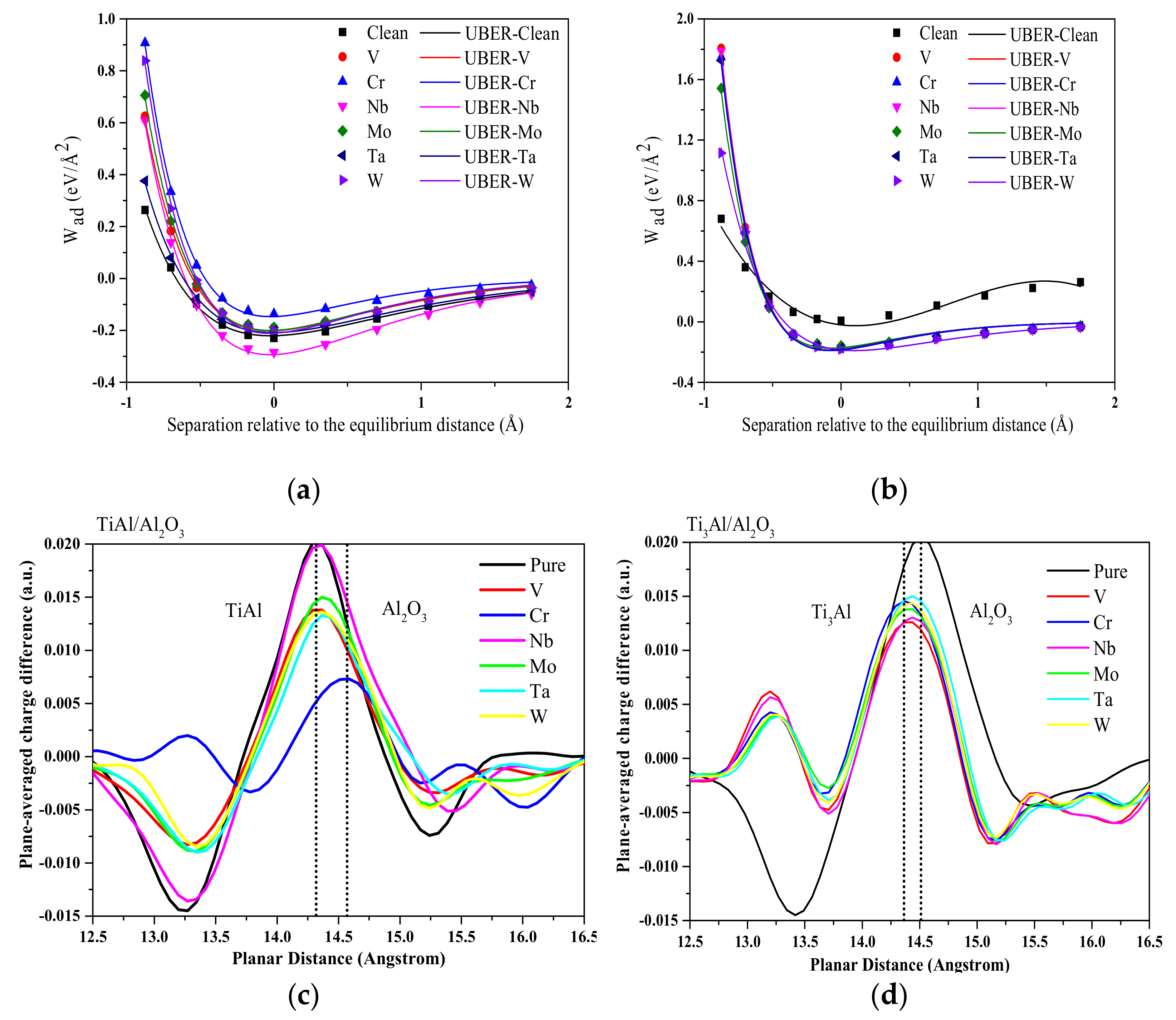
- Dai, J.; Song, Y.; Yang, R. Influence of alloying elements on stability and adhesion ability of TiAl/TiO2 interface by first-principles calculations. Intermetallics 2017, 85, 80–89. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Song, Y. Enhancing adhesion of Al2O3 scale on Ti-Al intermetallics by alloying: A first principles study. Comput. Mater. Sci. 2020, 181, 109756. [Google Scholar] [CrossRef]
- Rose, J.H.; Smith, J.R.; Ferrante, J. Universal features of bonding in metals. Phys. Rev. B 1983, 28, 1835–1845. [Google Scholar] [CrossRef]
- Rose, J.H.; Smith, J.R.; Guinea, F.; Ferrante, J. Universal features of the equation of state of metals. Phys. Rev. B 1984, 29, 2963–2969. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkov, S.S.; Fuks, A.A.; Kulkova, S.E. The impurity influence on the formation of oxide layers on TiAL surface. AIP Conf. Proc. 2018, 2051, 020023. [Google Scholar] [CrossRef]
- Hao, H.N.; Wang, X.; Wang, F.H. First-principles Study on the Clean and Nb Doped TiO2(110)/γ-TiAl(111) Interfaces. Adv. Mater. Res. 2013, 602–604, 555–558. [Google Scholar]
- Wang, A.; Liu, P.; Xie, J.; Ma, D.; Mao, Z. First-principles investigation on the atomic structure, stability and electronic property of O(001)/B2(110) interface in Ti2AlNb alloys. J. Alloys Compd. 2020, 817, 152734. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Song, Y. First-principles investigation on stability and oxygen adsorption behavior of a O/B2 interface in Ti2AlNb alloys. J. Alloys Compd. 2020, 818, 152926. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Zhou, H.-B.; Lu, G.-H.; Xu, H. First-principles investigation on shear deformation of a TiAl/Ti3Al interface and effects of oxygen. Intermetallics 2012, 22, 41–46. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, H.B.; Zhang, Y.; Lu, G.H.; Xu, H. Effects of O in a Binary-phase TiAl-Ti3Al Alloy: From Site Occupancy to Interfacial Energetics. J. Phys. Condensed Matter. 2011, 23, 7. [Google Scholar] [CrossRef]
- Li, Z.Z.; Wei, Y.; Zhou, H.B.; Lu, G.H. Investigating the Effects of Phosphorus in a Binary-phase TiAl-Ti3Al Alloy by First-principles: From Site Preference, Interfacial Energetics to Mechanical Properties. Eur. Phys. J. B 2016, 89, 280. [Google Scholar] [CrossRef]
- Ping, F.P.; Hu, Q.M.; Bakulin, A.V.; Kulkova, S.E.; Yang, R. Alloying Effects on Properties of Al2O3 and TiO2 in Connection with Oxidation Resistance of TiAl. Intermetallics 2016, 68, 57–62. [Google Scholar] [CrossRef]
- Ping, F.P.; Hu, Q.M.; Yang, R. Investigation on Effects of Alloying on Oxidation Resistance of γ-TiAl by Using First Principle. Acta Metall. Sin. 2013, 49, 385–390. [Google Scholar] [CrossRef]
- Shang, S.L.; Wang, Y.; Brian, G.; Liu, Z. Understanding Slow-growing Alumina Scale Mediated by Reactive Elements: Perspec-tive via Local Metal-oxygen Bonding Strength. Scr. Mater. 2018, 150, 139–142. [Google Scholar] [CrossRef]
- Wu, G.; Cui, G.; Qu, S.; Feng, A.; Cao, G.; Ge, B.; Xiang, H.; Shen, J.; Chen, D. High-temperature oxidation mechanisms of nano-/submicro-scale lamellar structures in an intermetallic alloy. Scr. Mater. 2019, 171, 102–107. [Google Scholar] [CrossRef]
- Cheng, F. First-Principles Calculations of the Effect of Nb on Oxidation Resistance and Friction and Wear of TiAl Alloys. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2019. [Google Scholar]
- Epifano, E.; Hug, G. First-principle Study of the Solubility and Diffusion of Oxygen and Boron in γ-TiAl. Comp. Mater. Sci. 2020, 174, 109475. [Google Scholar] [CrossRef]
- Bakulin, A.V.; Kulkov, S.S.; Kulkova, S.E. Diffusion Properties of Oxygen in the γ-TiAl Alloy. J. Exp. Theor. Phys. 2020, 130, 579–590. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Wang, X.; Wang, F.H. First-principles Study of Nb Doping Effect on the Diffusion of Oxygen Atom in γ-TiAl. Adv. Mater. Res. 2011, 304, 148–153. [Google Scholar] [CrossRef]
- Song, Q.G.; Wang, L.G.; Zhu, Y.X.; Kang, J.H.; Gu, W.F.; Wang, M.C.; Liu, Z.F. Effects of Si and Y Co-doping on Stability and Oxidation Resistance of γ-TiAl Based Alloys. Acta Phys. Sin. 2019, 68, 196101. [Google Scholar]
- Dai, J.; Wang, L.; Wu, X.; Song, Y.; Yang, R. Effects of alloying elements on the stability of TiO2 and their diffusion properties studied by first principles calculations. Mater. Today Commun. 2018, 17, 40–45. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, K.O. Band Theory and Mott Insulators: Hubbard U Instead of Stoner I. Phys. Rev. B 1991, 44, 943. [Google Scholar] [CrossRef] [Green Version]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
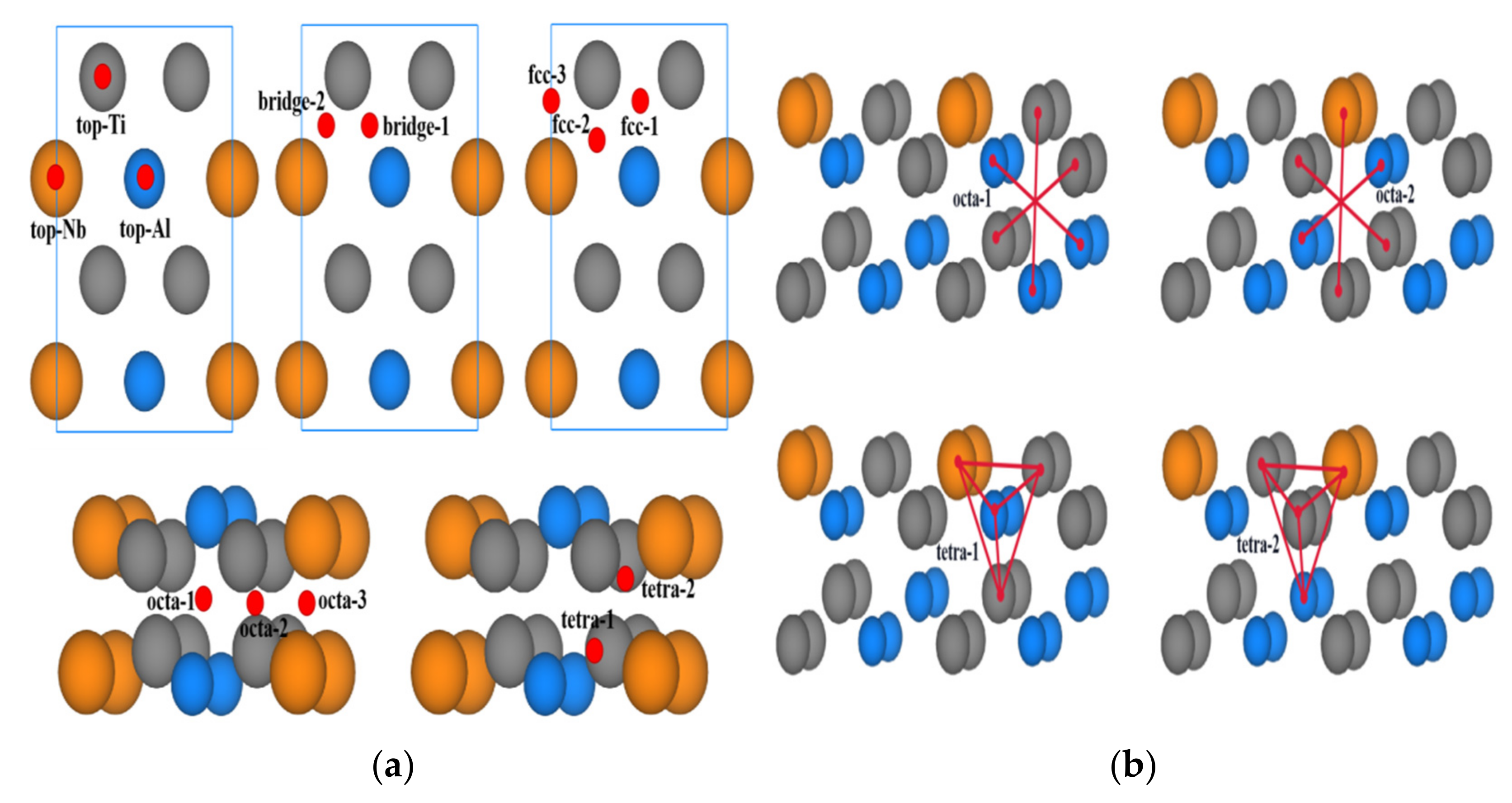
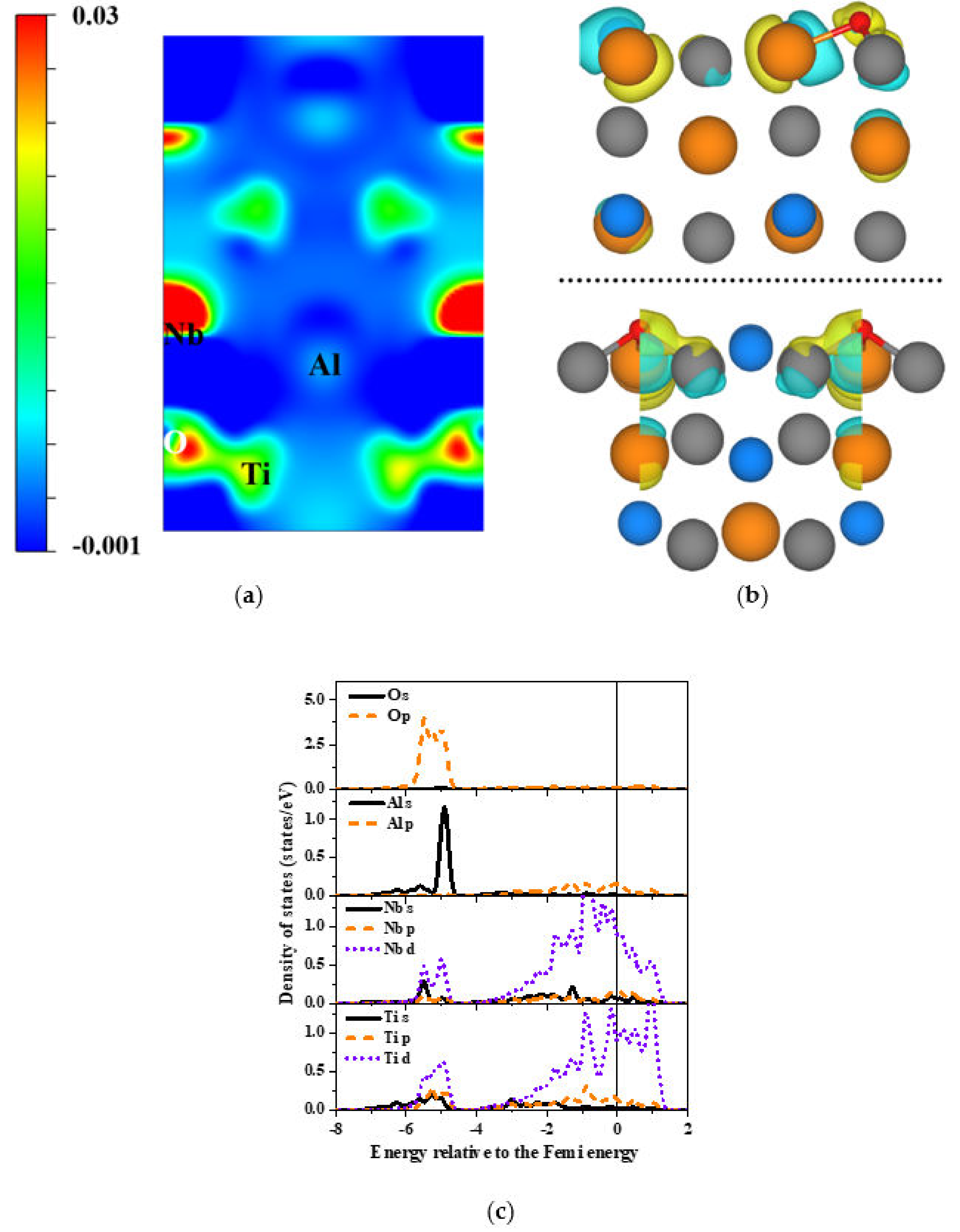

| Surface | Site | E(O-TiAl) (eV) | Eads (eV) |
|---|---|---|---|
| Al-(001) | B | −285.454 | −3.94 |
| T1 | −283.557 | −2.04 | |
| T2 | −285.133 | −3.62 | |
| Ti-(001) | B | −286.506 | −5.08 |
| T1 | −287.196 | −5.78 | |
| T2 | −284.938 | −3.51 | |
| Al-(110) | T1 | −140.811 | −0.92 |
| T2 | −142.212 | −2.32 | |
| C1 | −144.114 | −4.22 | |
| C2 | −143.654 | −3.76 | |
| Ti-(110) | T1 | −143.911 | −3.86 |
| T2 | −145.203 | −5.15 | |
| C1 | −145.015 | −4.97 | |
| C2 | −145.401 | −5.36 | |
| (100) | A1 | −287.049 | −2.00 |
| A2 | −289.560 | −4.51 | |
| T1 | −288.023 | −2.97 | |
| T2 | −289.548 | −4.39 | |
| (111) | fcc-Al | −150.912 | −5.18 |
| hcp-Al | −150.815 | −5.08 | |
| fcc-Ti | −149.918 | −4.18 | |
| hcp-Ti | −149.871 | −4.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Dai, J.; Song, Y. Research Progress of First Principles Studies on Oxidation Behaviors of Ti-Al Alloys and Alloying Influence. Metals 2021, 11, 985. https://doi.org/10.3390/met11060985
Li Y, Dai J, Song Y. Research Progress of First Principles Studies on Oxidation Behaviors of Ti-Al Alloys and Alloying Influence. Metals. 2021; 11(6):985. https://doi.org/10.3390/met11060985
Chicago/Turabian StyleLi, Yue, Jianhong Dai, and Yan Song. 2021. "Research Progress of First Principles Studies on Oxidation Behaviors of Ti-Al Alloys and Alloying Influence" Metals 11, no. 6: 985. https://doi.org/10.3390/met11060985
APA StyleLi, Y., Dai, J., & Song, Y. (2021). Research Progress of First Principles Studies on Oxidation Behaviors of Ti-Al Alloys and Alloying Influence. Metals, 11(6), 985. https://doi.org/10.3390/met11060985






