Kinetics of Smelting Chromia–Bearing Vanadiferous Titanomagnetite Ore via High–Temperature CO2–Containing Gas Injection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
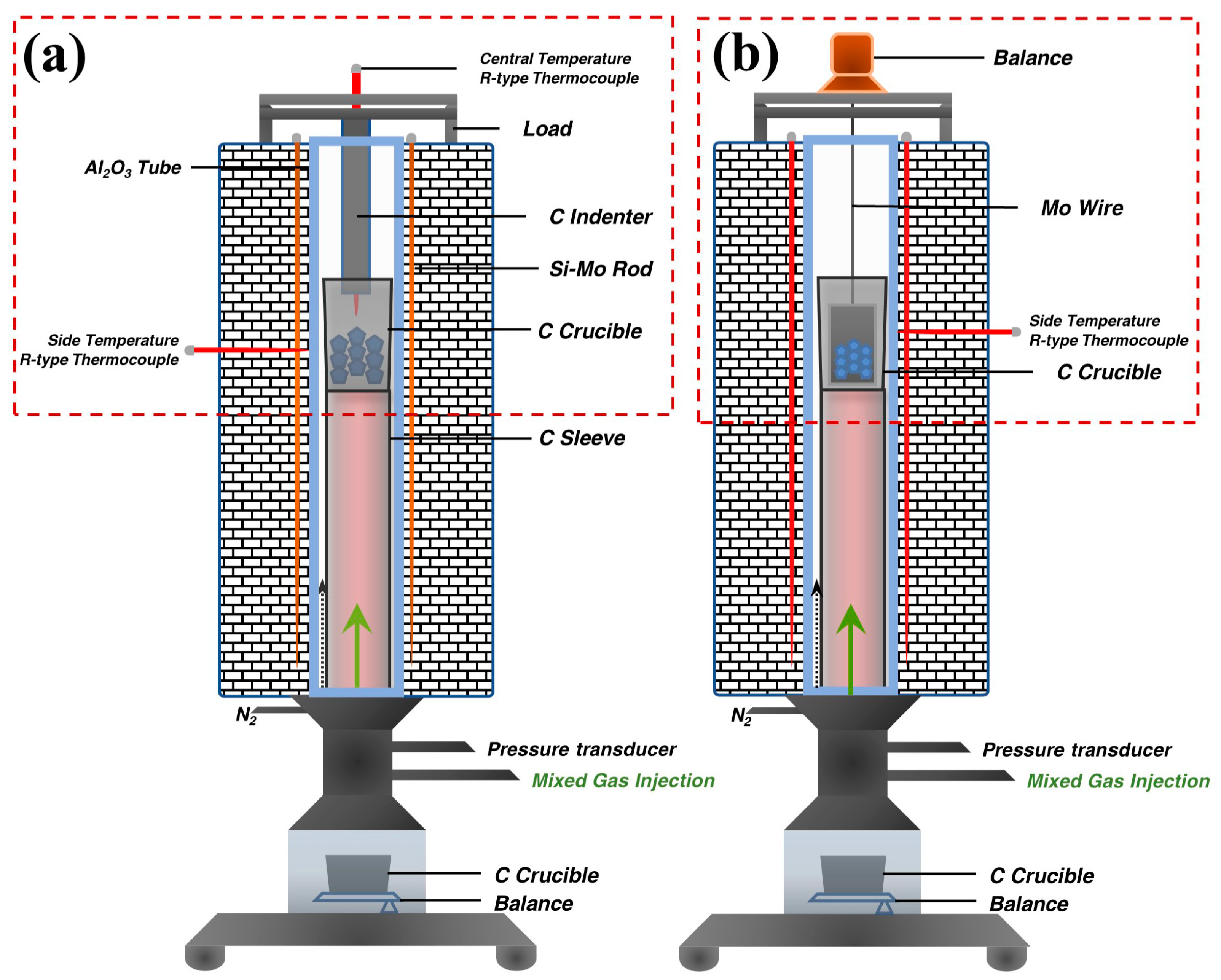
2.2. Apparatus and Methods
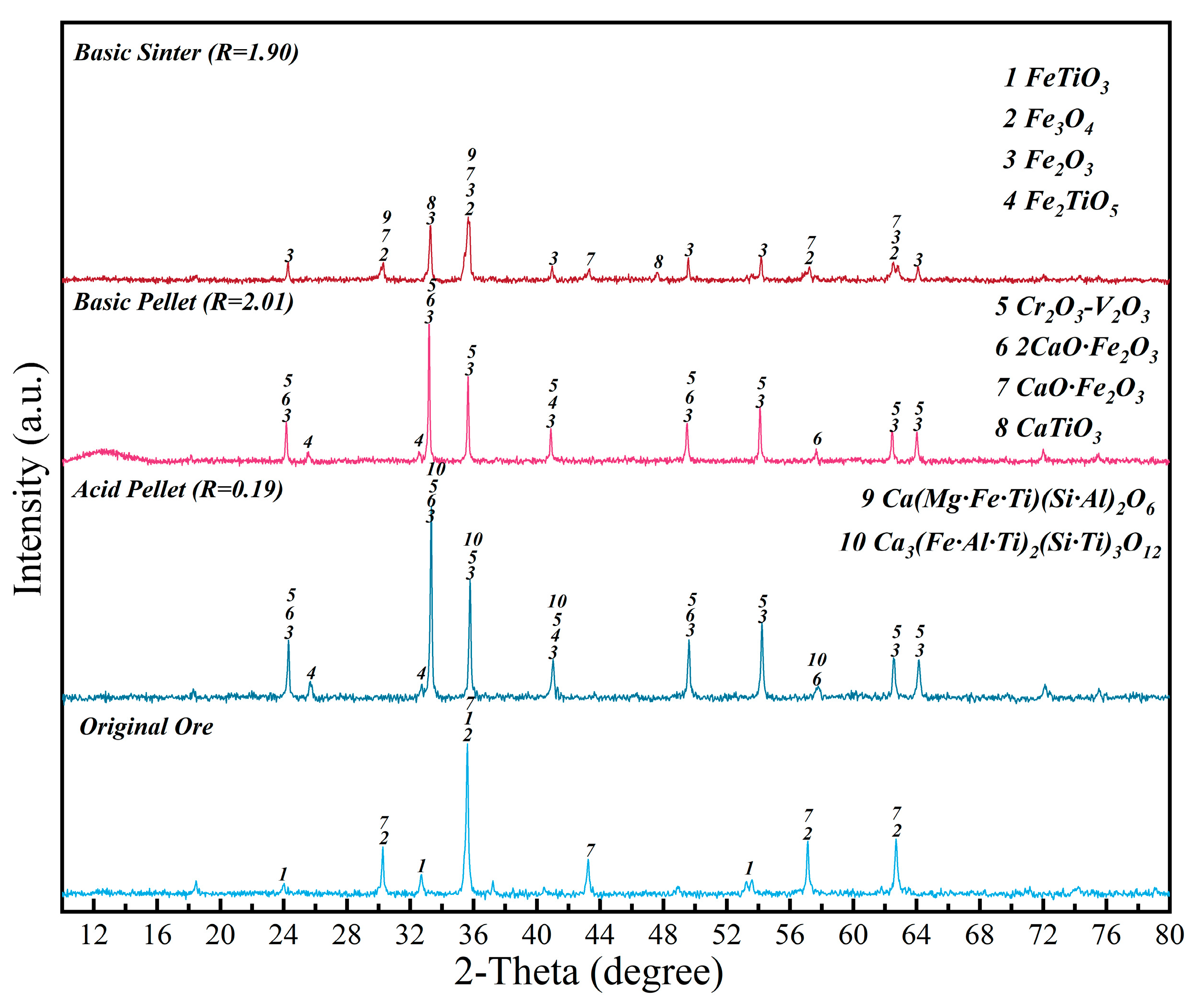
2.3. Characterizations
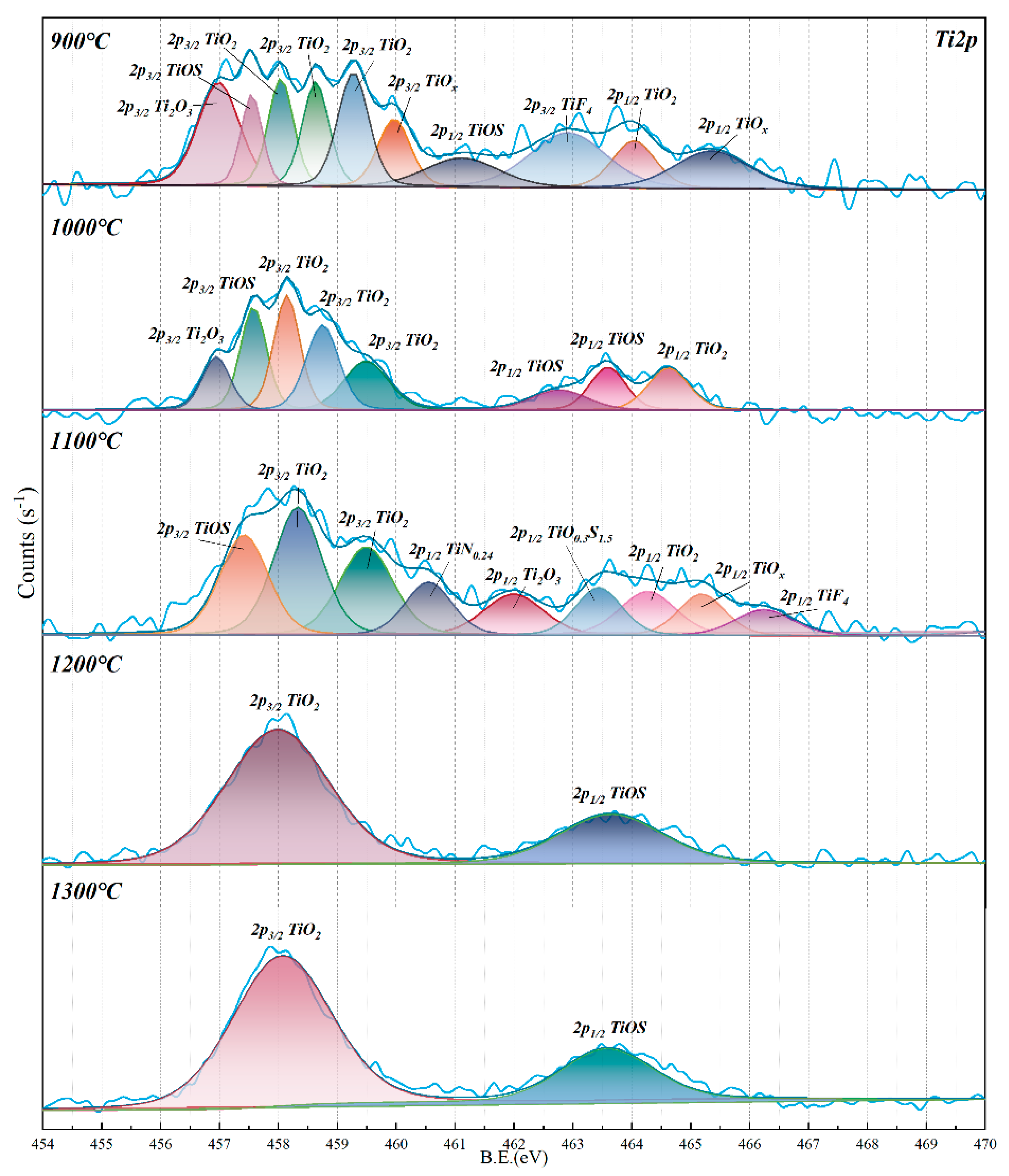
3. Results and Discussion
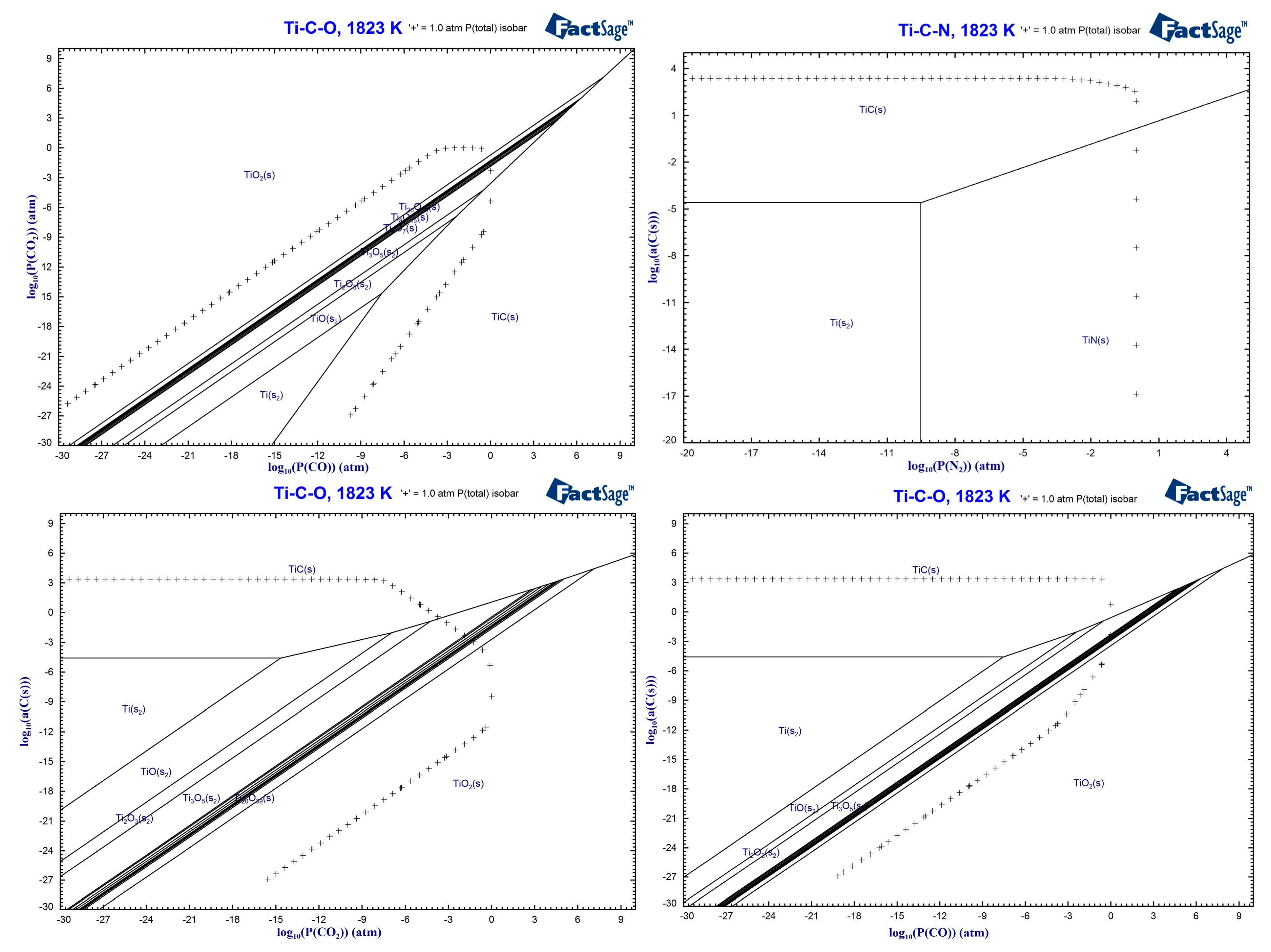
3.1. Effect of High–Temperature CO2–Containing Gas in the Thermodynamic Smelting Process
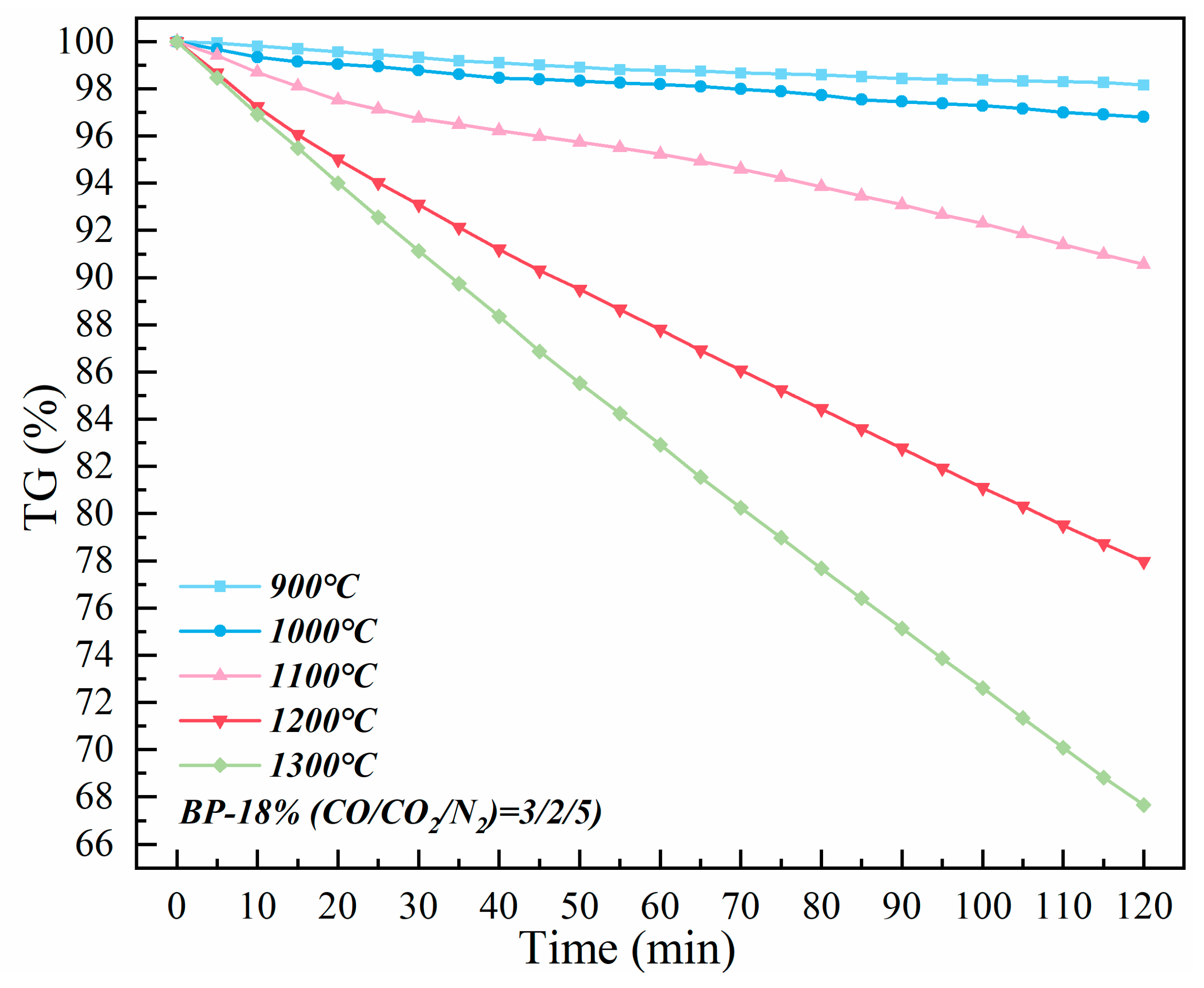
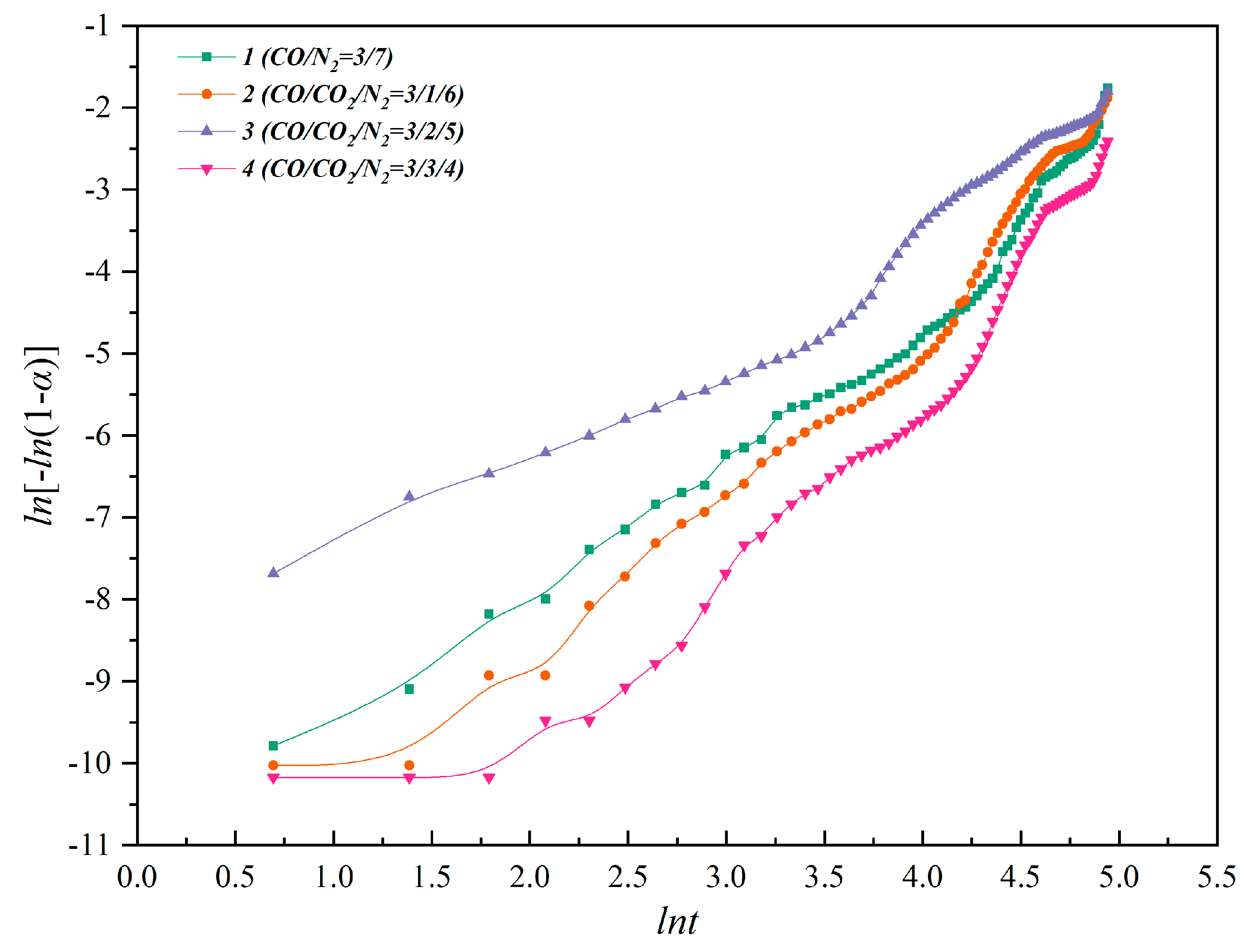
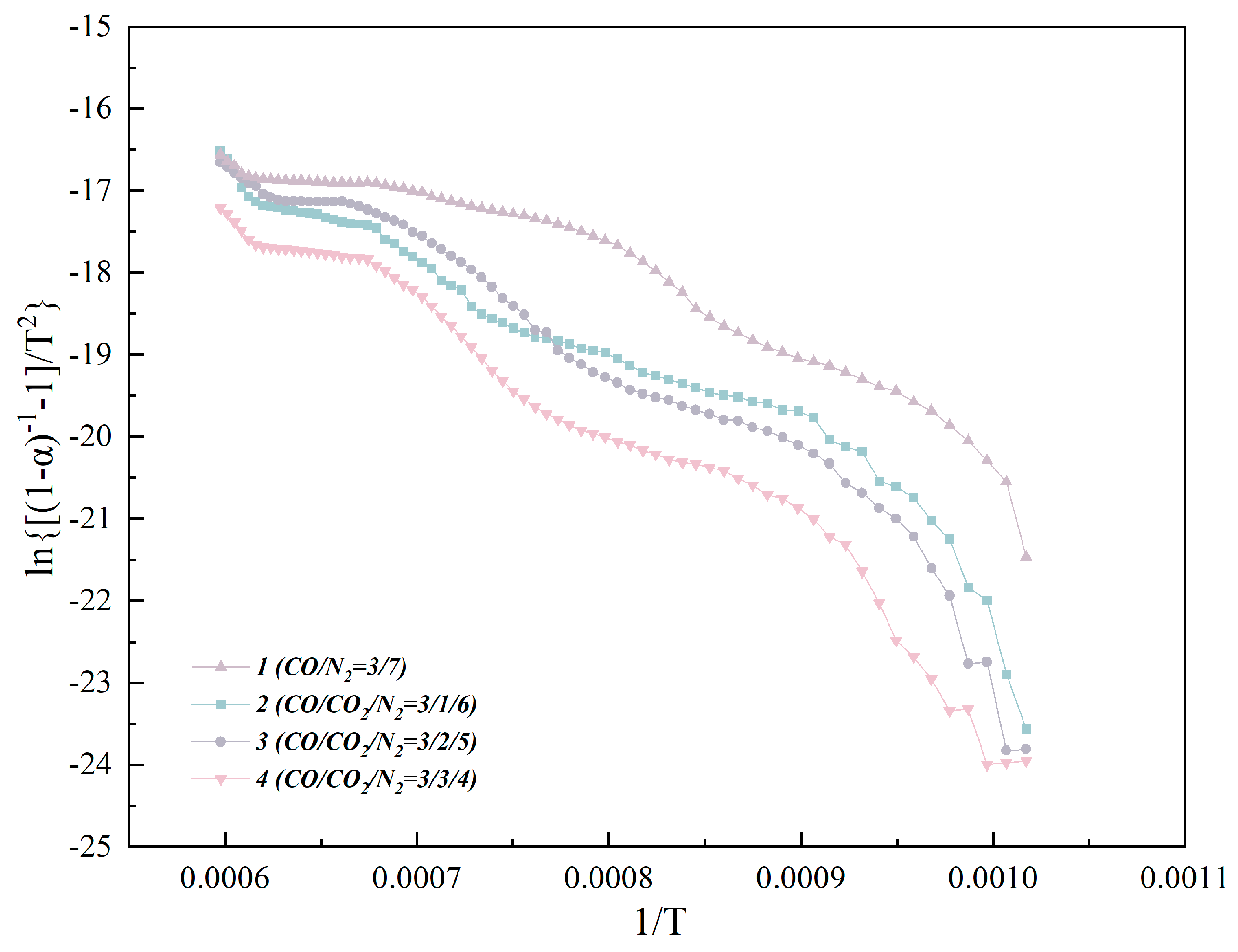
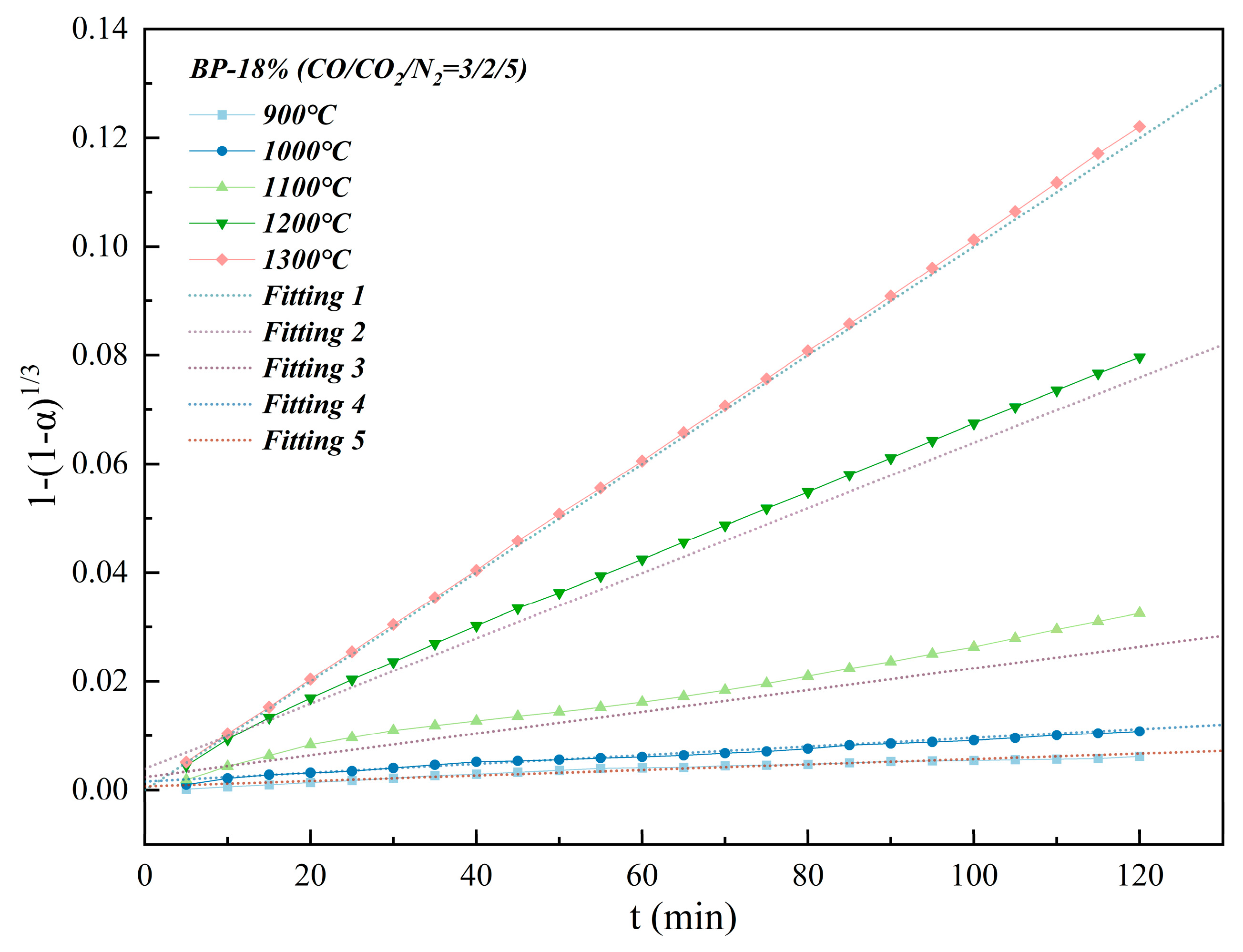
3.2. Isothermal and Nonisothermal Kinetics of High–Temperature CO2–Containing Gas in the Polymetallic Reduction Process
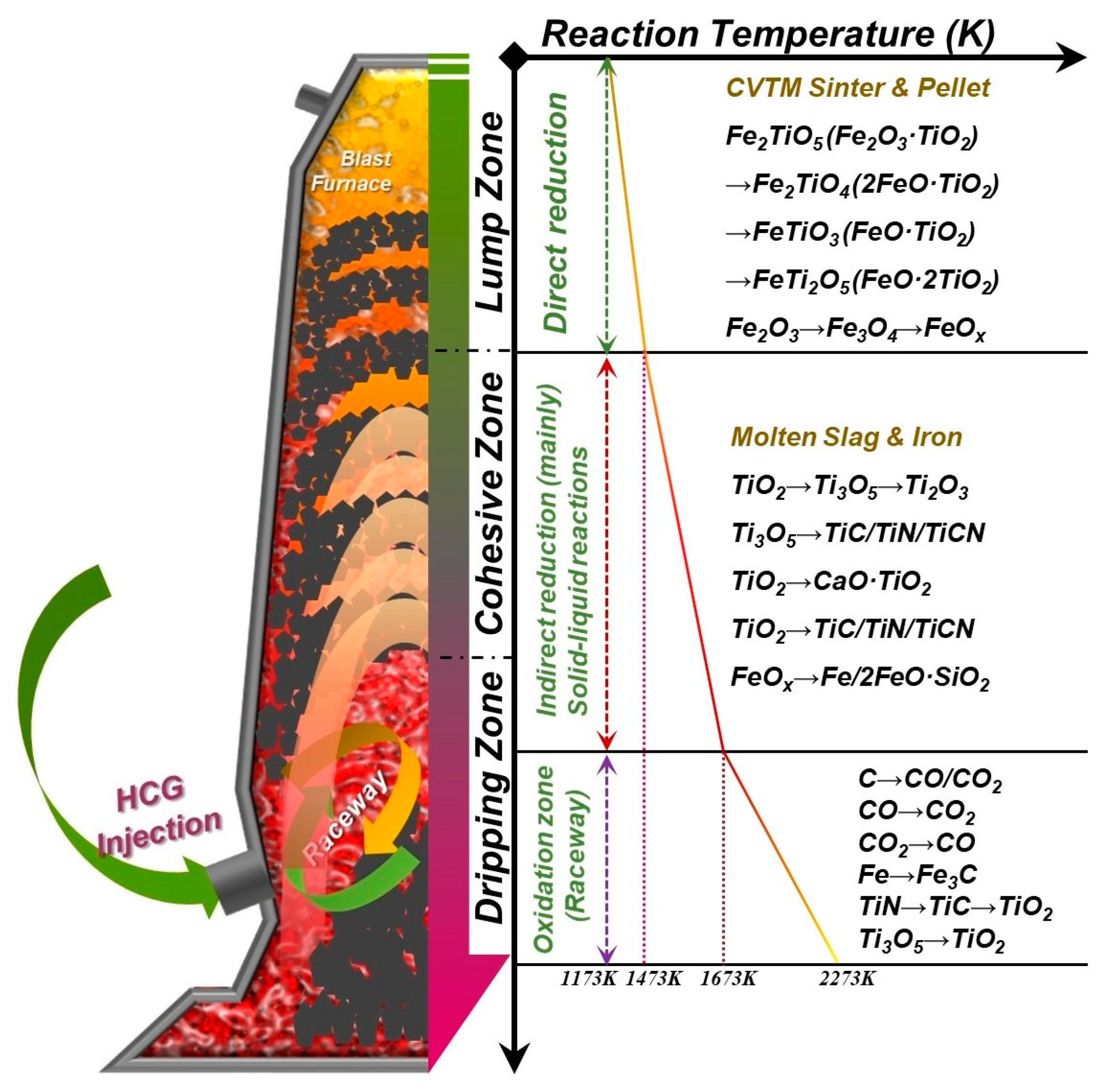
3.3. Reaction Mechanism of High–Temperature CO2–Containing Gas in the Polymetallic Smelting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.G. Principle of Smelting Vanadium-Titanium Magnetite, 1st ed.; Science Press: Beijing, China, 1996. [Google Scholar]
- Xue, X.Y.; Yang, S.T.; Zhang, Y. Blast Furnace Process Smelting Chromium-Containing Vanadia-Titania Magnetite: Theory and Practice, 1st ed.; Science Press: Beijing, China, 2020. [Google Scholar]
- Cheng, G.J.; Xue, X.X.; Jiang, T.; Duan, P.N. Effect of TiO2 on the Crushing Strength and Smelting Mechanism of High-Chromium Vanadium-Titanium Magnetite Pellets. Metall. Mater. Trans. B 2016, 47, 1713–1726. [Google Scholar] [CrossRef]
- Li, T.L.; Sun, C.Y.; Lan, D.; Song, J.; Song, S.; Wang, Q. Effect of Mineral Elements Migration on Softening-melting Properties of Ti-bearing High Basicity Sinter. ISIJ Int. 2019, 59, 245–252. [Google Scholar] [CrossRef]
- Liu, J.X.; Cheng, G.J.; Liu, Z.G.; Chu, M.S.; Xue, X.X. Softening and melting properties of different burden structures containing high chromic vanadium titano-magnetite. Int. J. Miner. Process. 2015, 142, 113–118. [Google Scholar] [CrossRef]
- Yue, H.R.; He, Z.W.; Jiang, T.; Duan, P.N.; Xue, X.X. Rheological Evolution of Ti-Bearing Slag with Different Volume Fractions of TiN. Metall. Mater. Trans. B 2018, 49, 2118–2127. [Google Scholar] [CrossRef]
- Chen, W.; Yin, X.; Ma, D. A bottom-up analysis of China’s iron and steel industrial energy consumption and CO2 emissions. Appl. Energy 2014, 136, 1174–1183. [Google Scholar] [CrossRef]
- Chung, W.; Roh, K.; Lee, J.H. Design and evaluation of CO2 capture plants for the steelmaking industry by means of amine scrubbing and membrane separation. Int. J. Greenh. Gas Control 2018, 74, 259–270. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, R.; Wang, X.; Li, Z. Utilization of CO2 in metallurgical processes in China. Miner. Process. Extr. Metall. 2017, 126, 47–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Xu, J.; Jia, G.Y. Carbon element flow analysis and CO2 emission reduction in iron and steel works. J. Clean. Prod. 2018, 172, 709–723. [Google Scholar] [CrossRef]
- Zhu, R. Theory and Practice of Carbon Dioxide Steelmaking, 1st ed.; Science Press: Beijing, China, 2019. [Google Scholar]
- Song, H.L.; Zhang, J.P.; Cheng, G.J.; Yang, S.T.; Xue, X.X. Study on the High-temperature Properties of High-titania Slags produced with Cr-bearing Vanadia-titania Magnetite Smelting in Blast Furnace. Surf. Interfaces 2020, 21, 100767. [Google Scholar] [CrossRef]
- Halli, P.; Taskinen, P.; Eric, R.H. Mechanisms and Kinetics of Solid State Reduction of Titano Magnetite Ore with Methane. J. Sustain. Metall. 2017, 3, 191–206. [Google Scholar] [CrossRef]
- Liu, S.S.; Guo, Y.F.; Qiu, G.Z.; Jiang, T.; Chen, F. Solid-state reduction kinetics and mechanism of pre-oxidized vanadium-titanium magnetite concentrate. Trans. Nonferrous Met. Soc. China 2014, 24, 3372–3377. [Google Scholar] [CrossRef]
- Lu, C.Y.; Zou, X.L.; Lu, X.G.; Xie, X.L.; Zheng, K.; Xiao, W.; Cheng, H.W.; Li, G.S. Reductive kinetics of Panzhihua ilmenite with hydrogen. Trans. Nonferrous Met. Soc. China 2016, 26, 3266–3273. [Google Scholar] [CrossRef]
- Lv, W.; Lv, X.W.; Zhang, Y.Y.; Li, S.P.; Tang, K.; Song, B. Isothermal oxidation kinetics of ilmenite concentrate powder from Panzhihua in air. Powder Technol. 2017, 320, 239–248. [Google Scholar] [CrossRef]
- Pan, F.; Zhu, Q.S.; Du, Z.; Sun, H.Y. Oxidation Kinetics, Structural Changes and Element Migration during Oxidation Process of Vanadium-titanium Magnetite Ore. J. Iron Steel Res. Int. 2016, 23, 1160–1167. [Google Scholar] [CrossRef]
- Sarkar, B.K.; Samanta, S.; Dey, R.; Das, G.C. A study on reduction kinetics of titaniferous magnetite ore using lean grade coal. Int. J. Miner. Process. 2016, 152, 36–45. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Qiu, G.Z. Reduction kinetics of oxidized vanadium titano-magnetite pellets using carbon monoxide and hydrogen. J. Alloys Compd. 2017, 706, 546–553. [Google Scholar] [CrossRef]
- Zhang, J.L.; Xing, X.D.; Cao, M.M.; Jiao, K.X.; Wang, C.L.; Ren, S. Reduction Kinetics of Vanadium Titano-Magnetite Carbon Composite Pellets Adding Catalysts Under High Temperature. J. Iron Steel Res. Int. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Nandy, B.; Chandra, S.; Bhattacharjee, D.; Ghosh, D. Assessment of blast furnace behaviour through softening-melting test. Ironmak. Steelmak. 2006, 33, 111–119. [Google Scholar] [CrossRef]
- Liu, X.L.; Honeyands, T.; Evans, G.; Zulli, P.; O’Dea, D. A review of high-temperature experimental techniques used to investigate the cohesive zone of the ironmaking blast furnace. Ironmak. Steelmak. 2019, 46, 953–967. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Wang, J.; Li, Q.J.; Shan, C.; Qiu, G.B.; Yu, W.Z.; Lv, X.W. Slag-foaming phenomenon originating from reaction of titanium-bearing blast furnace slag: Effects of TiO2 content and basicity. Can. Metall. Q. 2020, 59, 151–158. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Influence of TiO2 addition on the oxidation induration and reduction behavior of Hongge vanadium titanomagnetite pellets with simulated shaft furnace gases. Powder Technol. 2018, 326, 137–145. [Google Scholar] [CrossRef]
- Zhao, L.S.; Wang, L.N.; Chen, D.S.; Zhao, H.X.; Liu, Y.H.; Qi, T. Behaviors of vanadium and chromium in coal-based direct reduction of high-chromium vanadium-bearing titanomagnetite concentrates followed by magnetic separation. Trans. Nonferrous Met. Soc. China 2015, 25, 1325–1333. [Google Scholar] [CrossRef]






| Central Temperature | RT–500 °C | 500–1300 °C | 1300 °C–RT |
|---|---|---|---|
| Heating rate | 10 °C/min | 5 °C/min | 5 °C/min |
| Operating time | 50 min | 160 min | >300 min |
| Reaction gas | N2 100% | CO/CO2/N2 (Total 5 L/min) | Ar 100% |
| Conditions | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Slope | 2.5804 | 2.2243 | 1.5415 | 2.287 |
| Intercept | −13.871 | −13.326 | −9.635 | −14.34 |
| R2 | 0.893 | 0.96 | 0.9637 | 0.9393 |
| Conditions | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Slope | 0.9985 | 0.6783 | 0.808 | 0.8905 | 1.0151 |
| Intercept | −8.5879 | −6.7163 | −6.2483 | −5.6679 | −5.8198 |
| R2 | 0.9579 | 0.9891 | 0.9866 | 0.9991 | 0.9998 |
| Type | G(α) | n |
|---|---|---|
| D1 | α2 = kt | 0.62 |
| D2 | (1 − α) ln(1 − α) + α = kt | 0.57 |
| D3 | [1 − (1 − α)1/3]2 = kt | 0.54 |
| D4 | (1 − 2/3α) − (1 − α)2/3 = kt | 0.57 |
| CG2 | 1 − (1 − α)1/2 = kt | 1.11 |
| CG3 | 1 − (1 − α)1/3 = kt | 1.07 |
| R1 | −ln(1 − α) = kt | 1.00 |
| A2 | [−ln(1 − α)]1/2 = kt | 2.00 |
| A3 | [−ln(1 − α)]1/3 = kt | 3.00 |
| Conditions | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Slope | −10,217 | −14,273 | −9090 | −14,952 |
| Intercept | −9.46 | −7.80 | −10.81 | −8.05 |
| R2 | 0.84 | 0.95 | 0.92 | 0.96 |
| Ea | 84.94 | 118.67 | 75.58 | 124.31 |
| Conditions | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Slope (×10−5) | 5 | 8 | 20 | 60 | 100 |
| Intercept (×10−4) | 7 | 16 | 24 | 39 | 0.02 |
| R2 | 0.9549 | 0.9902 | 0.9904 | 0.999 | 0.9999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Cheng, G.; Liu, J.; Zhang, J.; Xue, X. Kinetics of Smelting Chromia–Bearing Vanadiferous Titanomagnetite Ore via High–Temperature CO2–Containing Gas Injection. Metals 2021, 11, 1008. https://doi.org/10.3390/met11071008
Song H, Cheng G, Liu J, Zhang J, Xue X. Kinetics of Smelting Chromia–Bearing Vanadiferous Titanomagnetite Ore via High–Temperature CO2–Containing Gas Injection. Metals. 2021; 11(7):1008. https://doi.org/10.3390/met11071008
Chicago/Turabian StyleSong, Hanlin, Gongjin Cheng, Jianxing Liu, Jinpeng Zhang, and Xiangxin Xue. 2021. "Kinetics of Smelting Chromia–Bearing Vanadiferous Titanomagnetite Ore via High–Temperature CO2–Containing Gas Injection" Metals 11, no. 7: 1008. https://doi.org/10.3390/met11071008
APA StyleSong, H., Cheng, G., Liu, J., Zhang, J., & Xue, X. (2021). Kinetics of Smelting Chromia–Bearing Vanadiferous Titanomagnetite Ore via High–Temperature CO2–Containing Gas Injection. Metals, 11(7), 1008. https://doi.org/10.3390/met11071008





