Dephosphorization in Double Slag Converter Steelmaking Process at Different Temperatures by Industrial Experiments
Abstract
1. Introduction
2. Experimental Procedure
2.1. Double Slag Converter Steelmaking Experiments
2.2. Compositions of the Initial Hot Metal, the Hot Metal at the Endpoint of Dephosphorization, and Dephosphorization Slag
3. Results
3.1. Effect of Dephosphorization Endpoint Temperature on Liquidus Region of Dephosphorization Slag
3.2. Effect of Dephosphorization Endpoint Temperature on Dephosphorization
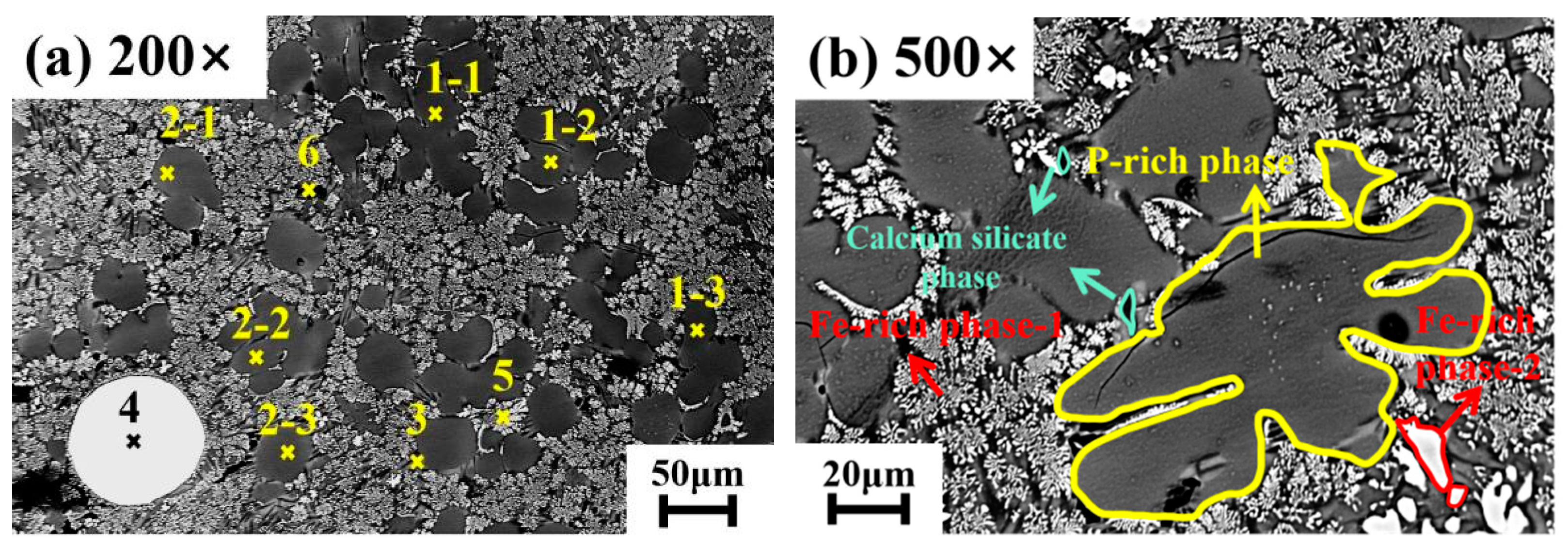
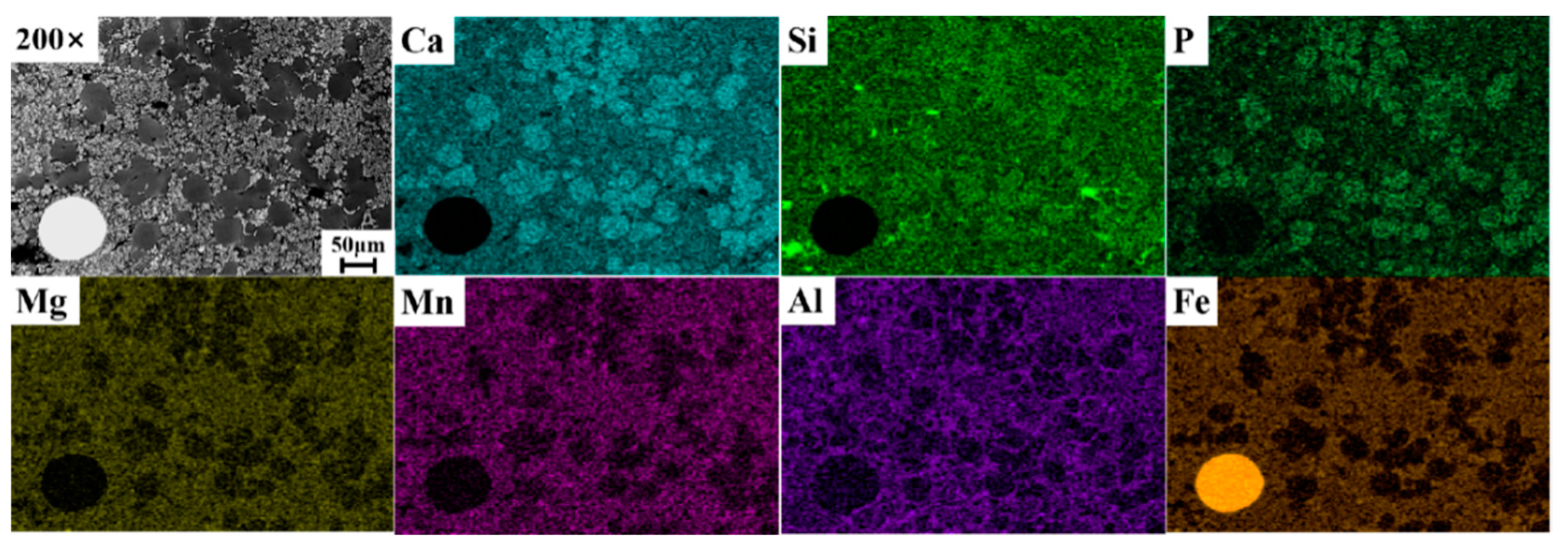
3.3. Analysis of Dephosphorization Slag at the Optimum Dephosphorization Temperature of 1405 °C
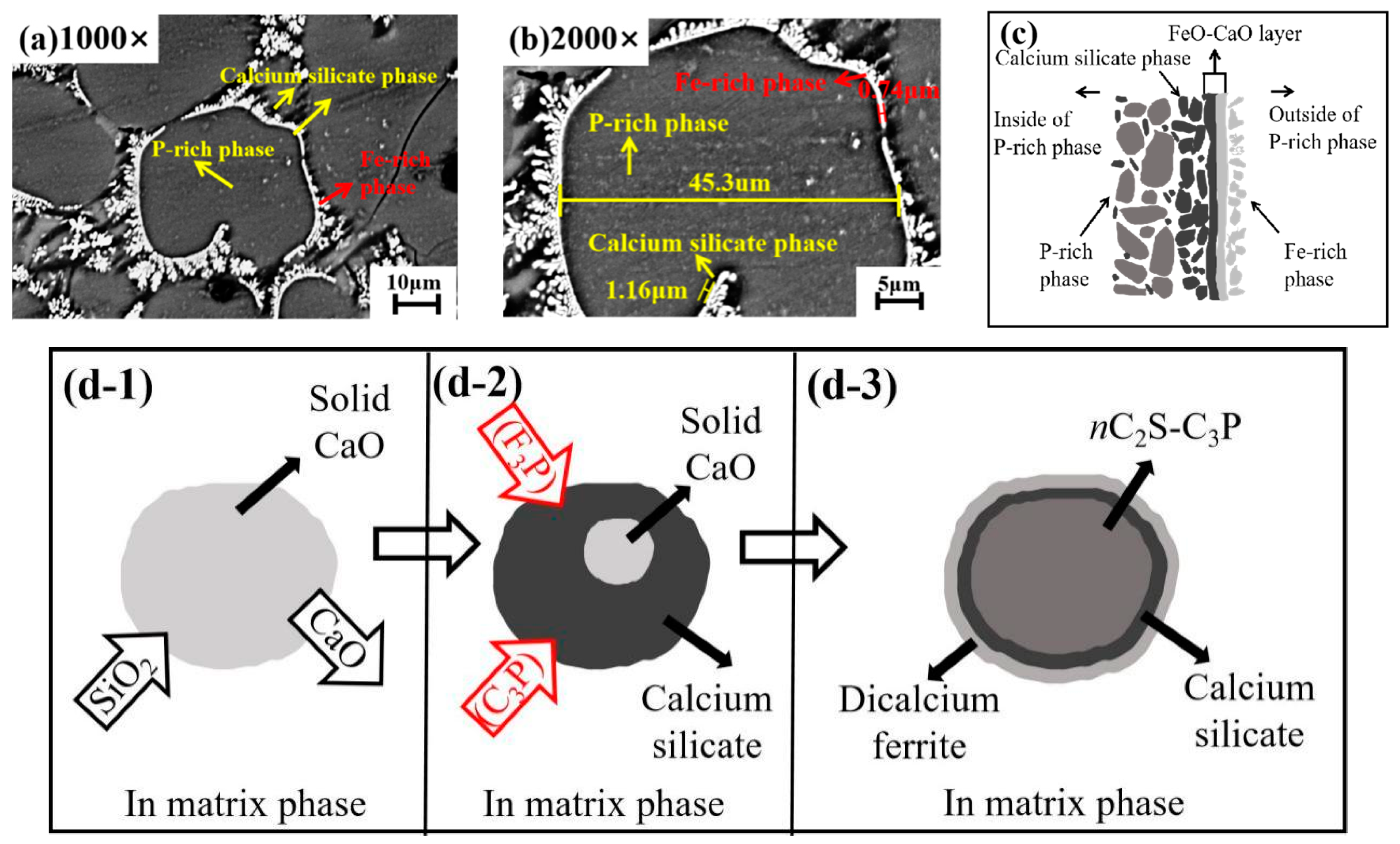
3.4. Analysis of the P-Rich Phase in Dephosphorization Slag at 1405 °C
4. Discussion
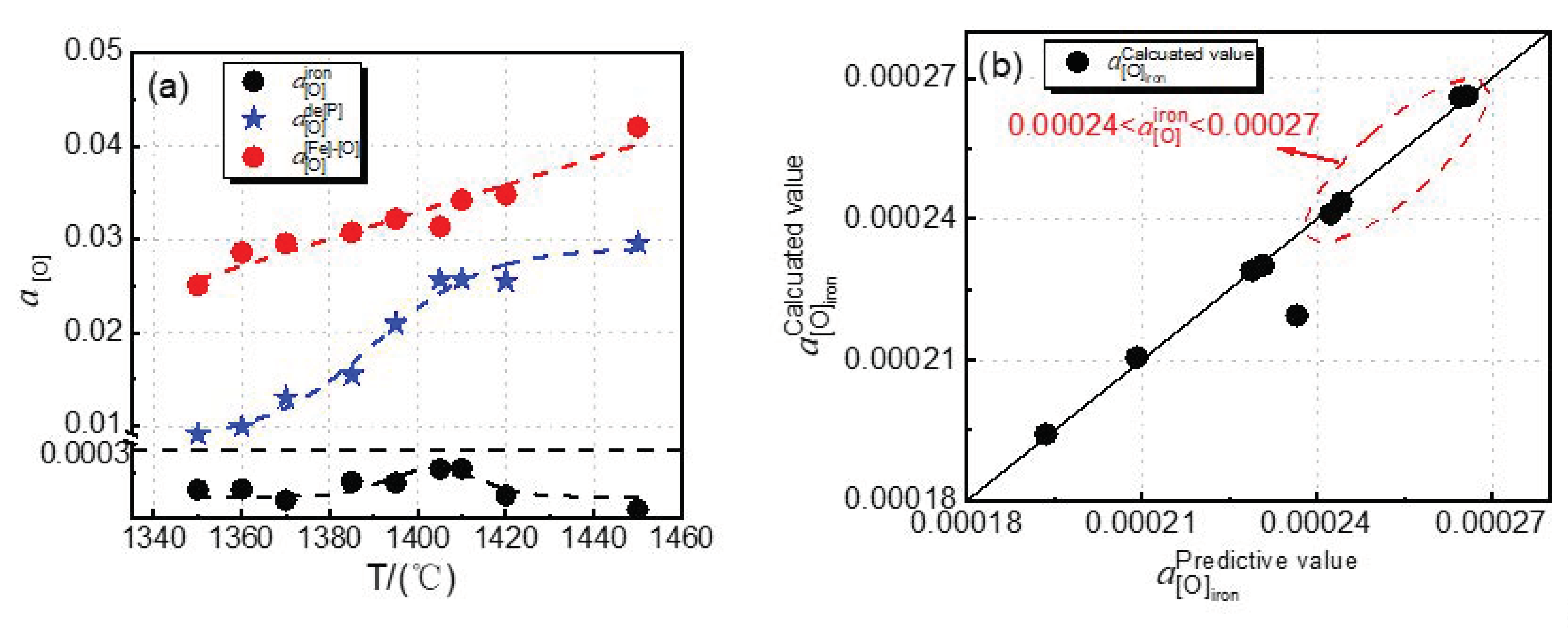
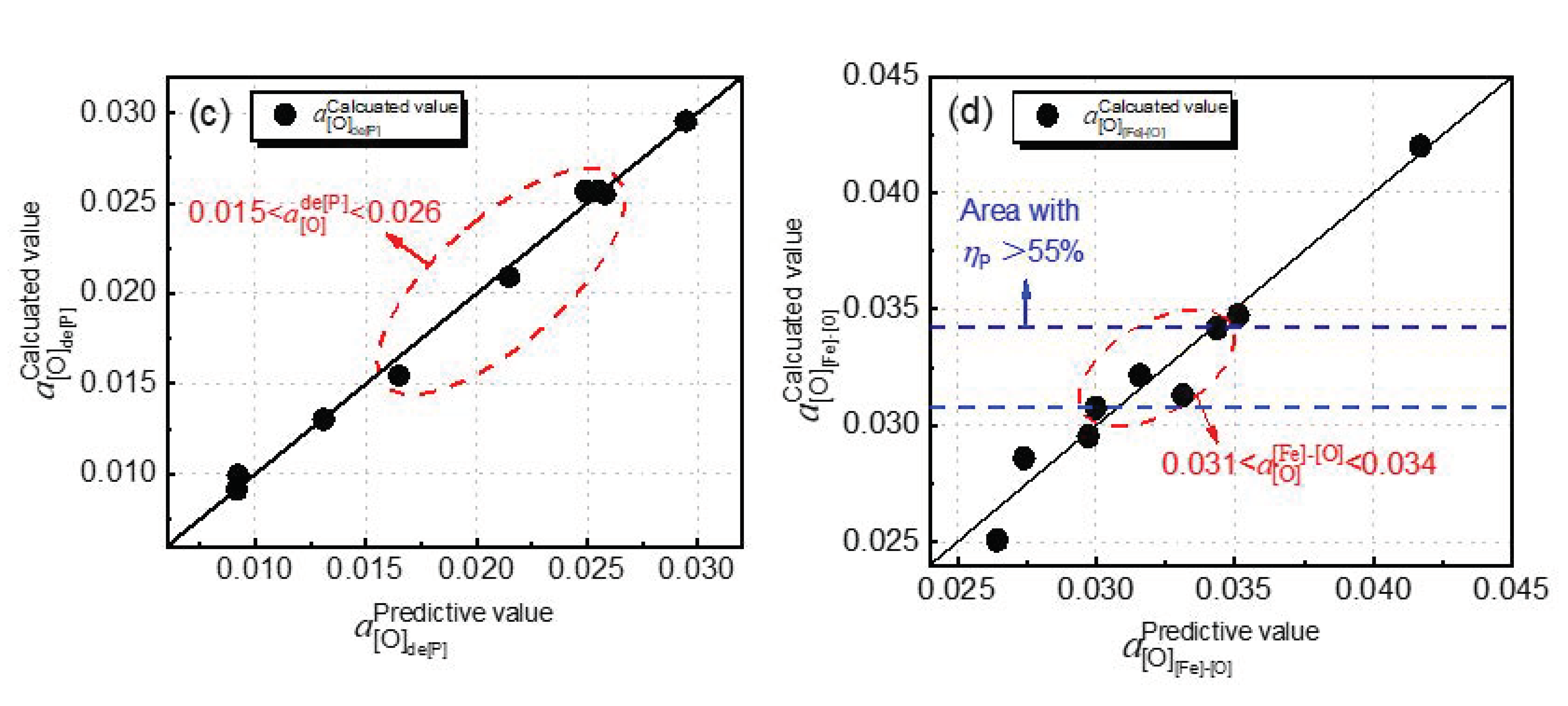
4.1. Effect of Dephosphorization Endpoint Temperature on and
4.2. Effect of Dephosphorization Endpoint Temperature on the Oxygen Activity on Hot Metal Surface, the Phosphorus Oxidation Equilibrium Oxygen Activity, and the Iron Oxidation Equilibrium Oxygen Activity at Slag–Hot Metal Interface
4.3. Effect of Dephosphorization Endpoint Temperature on Phosphorus Capacity and Phosphorus Distribution Ratio of Dephosphorization Slag
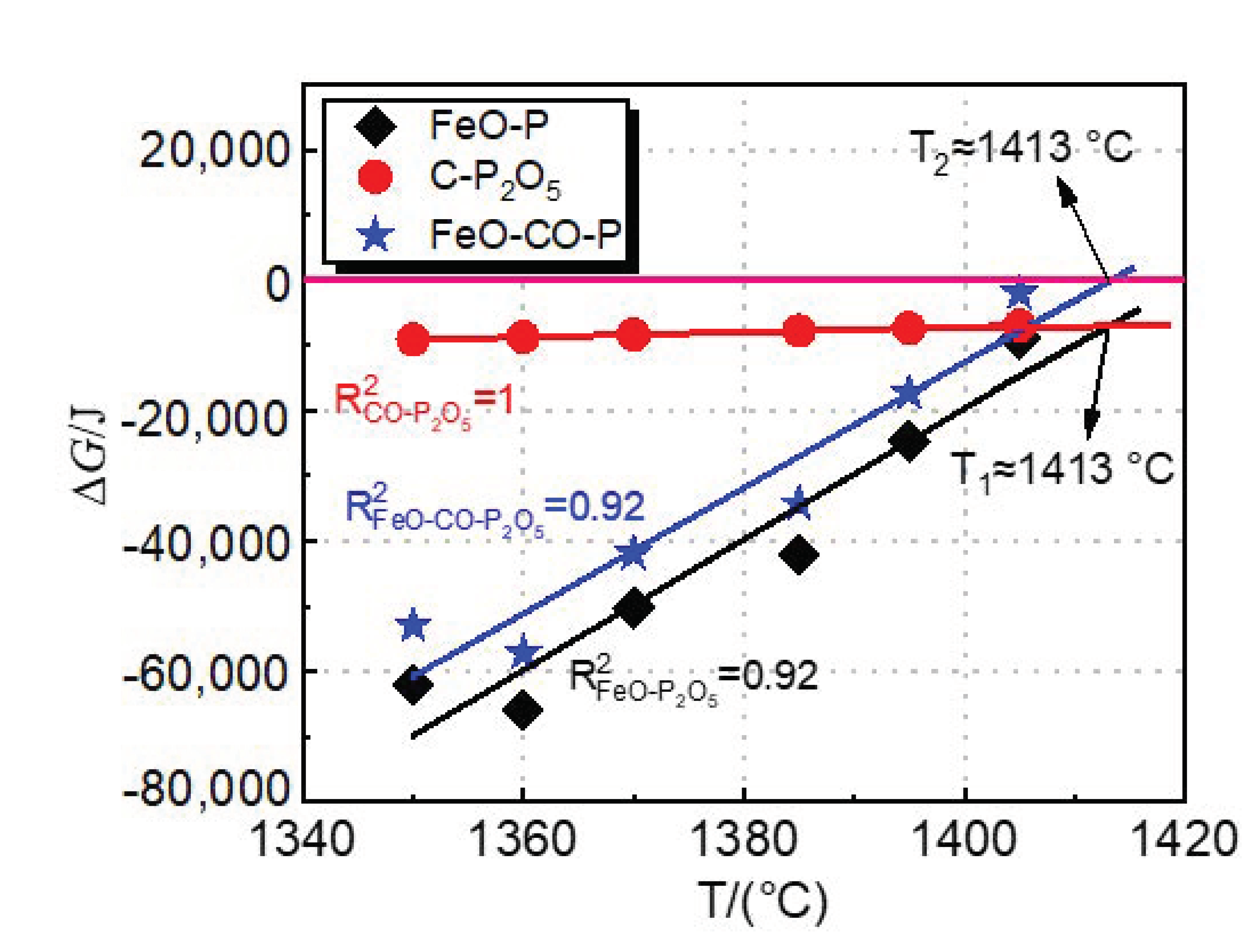
4.4. Effect of Dephosphorization Endpoint Temperature on Dephosphorization, Rephosphorization, and the Optimum Temperature of Deslagging
5. Conclusions
- (1)
- In the temperature range of 1350–1450 °C, with increasing dephosphorization endpoint temperature, the dephosphorization ratio and phosphorus distribution ratio first increase and then decrease. The phosphorus content in hot metal at the end of dephosphorization first decreases and then increases. The optimum dephosphorization temperature is in the range of 1385–1410 °C, with the dephosphorization ratio higher than 55%, the P2O5 content in the dephosphorization slag of 3.93–4.17%, and the logLP value of 1.76–2.09.
- (2)
- Dephosphorization slag is mainly composed of the gray massive P-rich phase, gray white Fe-rich phase, and black calcium silicate phase. The path of phosphorus in hot metal entering the P-rich phase of dephosphorization slag can be reasonably inferred as: hot metal → Fe-rich phase → P-rich phase in dephosphorization slag.
- (3)
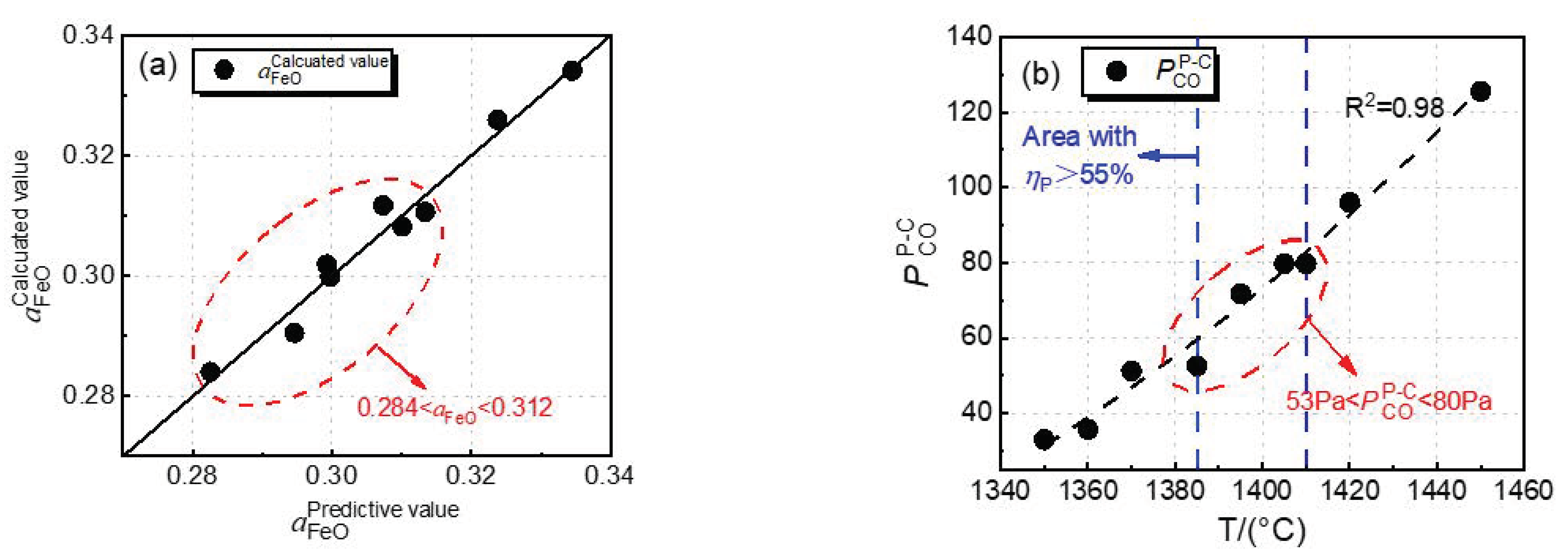
- is decided by the dephosphorization endpoint temperature and basic oxide content in dephosphorization slag, and has multiple linear correlation with them. The of selective oxidation reaction of carbon and phosphorus has a good exponential relationship with the endpoint temperature of dephosphorization. It is beneficial to converter dephosphorization when the temperature is 1385–1410 °C, the value of is 53–80 Pa, and value is 0.284–0.312.
- (4)
- The oxygen activity on the surface of hot metal of , the oxygen activity of the phosphorus oxidation at the slag–hot metal interface of , and the oxygen activity of the iron oxidation at the slag–hot metal interface of all increase with increasing the dephosphorization endpoint temperature in the range of 1350–1405 °C. The temperature has the greater effect on the phosphorus oxygen equilibrium of the slag–hot metal interface in the temperature range of 1350–1405 °C, and the temperature has a greater effect on the iron oxygen equilibrium in the slag in the temperature range of 1405–1450 °C. In the temperature range of 1350–1450 °C, owing to , plays the most important role on the dephosphorization in the double slag steelmaking process.
- (5)
- Under the present industrial experimental conditions, when the temperature is 1413 °C, the dephosphorization and rephosphorization reactions are in dynamic equilibrium. Considering the experimental results and thermodynamic calculation results of industrial experiments by the double slag dephosphorization process, the optimal temperature range for intermediate deslagging is about 1400–1420 °C.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogawa, Y.; Yano, M.; Kitamura, S.; Hirata, H. Development of the Continuous Dephosphorization and Decarburization Process Using BOF. Tetsu-to-Hagane 2001, 87, 21–28. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Li, J.; Feng, J.; Wang, F. Dephosphorization by Double-Slag Process in Converter Steelmaking. High Temp. Mater. Process. 2017, 37, 625–633. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Zhou, C.G.; Cai, K.S.; Wu, G.P.; Cai, Y.L. Practice on first deslagging process of double slag dephosphorization in 120 t top and bottom combined blown converter. Spec. Steel 2013, 34, 30–32. [Google Scholar]
- Ye, G.-F.; Yang, J.; Zhang, R.-H.; Yang, W.-K.; Sun, H. Behavior of phosphorus enrichment in dephosphorization slag at low temperature and low basicity. Int. J. Miner. Met. Mater. 2021, 28, 66–75. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yiming, T.; Takahashi, S.; Endo, R. Thermal Conductivity of 2CaO·SiO2 Bearing Solid Solution. ISIJ Int. 2017, 57, 1698–1702. [Google Scholar] [CrossRef]
- Kitamura, S.-Y.; Shibata, H.; Maruoka, N. Kinetic Model of Hot Metal Dephosphorization by Liquid and Solid Coexisting Slags. Steel Res. Int. 2008, 79, 586–590. [Google Scholar] [CrossRef]
- Suito, H.; Inoue, R. Behavior of phosphorous transfer from CaO-FetO-P2O5(-SiO2) slag to CaO particles. ISIJ Int. 2006, 46, 180–187. [Google Scholar] [CrossRef]
- Kitamura, S.Y.; Saito, S.; Utagawa, K.; Shibata, H.; Robertson, D.G. Mass transfer of P2O5 between liquid slag and solid solution of 2CaO·SiO2 and 3CaO·P2O5. ISIJ Int. 2009, 49, 1838–1844. [Google Scholar] [CrossRef]
- Xie, S.; Wang, W.; Luo, Z.; Huang, D. Mass Transfer Behavior of Phosphorus from the Liquid Slag Phase to Solid 2CaO·SiO2 in the Multiphase Dephosphorization Slag. Met. Mater. Trans. A 2016, 47, 1583–1593. [Google Scholar] [CrossRef]
- Kakimoto, S.; Kiyose, A.; Murao, R. Influence of P2O5 on dissolution behavior of lime in molten slag. ISIJ Int. 2017, 49, 1710–1717. [Google Scholar] [CrossRef]
- Wu, X.R.; Wang, P.; Li, L.S.; Wu, Z.J.; Chen, R.H. Distribution and enrichment of phosphorus in solidified BOF steelmaking slag. Ironmak. Steelmak. 2011, 38, 185–188. [Google Scholar] [CrossRef]
- Pahlevani, F.; Kitamura, S.Y.; Shibata, H.; Maruoka, N. Distribution of P2O5 between solid solution of 2CaO·SiO2-3CaO·P2O5 and liquid phase. ISIJ Int. 2010, 50, 822–829. [Google Scholar] [CrossRef]
- Tian, Z.H.; Li, B.H.; Zhang, X.M.; Jiang, Z.H. Double slag operation dephosphorization in BOF for producing low phosphorus steel. J. Iron Steel Res. Int. 2009, 16, 6–14. [Google Scholar] [CrossRef]
- Yang, X.; Sun, F.-M.; Yang, J.-L.; Liu, F.; Cheng, K.-S.; Wang, J.-H. Optimization of Low Phosphorus Steel Production with Double Slag Process in BOF. J. Iron Steel Res. Int. 2013, 20, 41–47. [Google Scholar] [CrossRef]
- Zhou, C.G.; Li, J.; Wu, H.; Yang, K.Z.; Cai, K.S.; Wu, G.P.; Cao, Y.L. Study of factors affecting liquid steel rephosphorization. Iron Steel Vanadium Titan. 2014, 35, 116–122. [Google Scholar]
- Han, X.; Li, J.; Hu, X.G.; Zhou, C.G.; Zhang, H.Y.; Guo, C. Effect of oxidability of final slag in converter on dephosphorization. Foundry Technol. 2015, 36, 412–415. [Google Scholar]
- Kitamura, S.Y.; Yonezawa, K.; Ogawa, Y.; Sasaki, N. Improvement of reaction efficiency in hot metal dephosphorization. Ironmak. Steelmak. 2002, 29, 121–124. [Google Scholar] [CrossRef]
- Zhou, C.G.; Li, J.; Wu, H.; Cai, K.S.; Wu, G.P.; Cao, Y.L. Study on the temperature of first deslagging of double slag dephosphorization in converter. Iron Steel 2014, 49, 24–28. [Google Scholar]
- Karbowniczek, M.; Cebula, E.K.; Reichel, J. Investigations of the dephosphorization of liquid iron solution containing chromium and nickel mater. Met. Mater. Trans. B 2012, 43, 554–561. [Google Scholar] [CrossRef]
- Hamano, T.; Fukagai, S.; Tsukihashi, F. Reaction mechanism between solid CaO and FeOx-CaO-SiO2-P2O5 slag at 1573 K. ISIJ Int. 2006, 46, 490–495. [Google Scholar] [CrossRef]
- Yang, X.; Matsuura, H.; Tsukihashi, F. Condensation of P2O5 at the interface between 2CaO center dot SiO2 and CaO-SiO2-FeOx-P2O5 slag. ISIJ Int. 2009, 49, 1298–1307. [Google Scholar] [CrossRef]
- Yang, X.; Matsuura, H.; Tsukihashi, F. Reaction Behavior of P2O5 at the interface between solid 2CaO center dot SiO2 and liquid CaO-SiO2-FeOx-P2O5 slags saturated with solid 5CaO center dot SiO2 center dot P2O5 at 1573 K. ISIJ Int. 2010, 50, 702–711. [Google Scholar] [CrossRef][Green Version]
- Xie, S.; Wang, W.; Huang, D.; Li, H.; Du, Y. Clarification of the Dissolution of Solid CaO and the Phosphorus-Enrichment Capability of Calcium Silicates in the Multiphase Slag Based on the Ion and Molecule Coexistence Theory. Steel Res. Int. 2018, 89. [Google Scholar] [CrossRef]
- Huang, X.H. Principles of Iron and Steel Metallurgy; Metallurgical Industry Press: Beijing, China, 2002; pp. 213–215. [Google Scholar]
- Ban-Ya, S. Mathematical Expression of Slag-Metal Reactions in Steelmaking Process by Quadratic Formalism Based on the Regular Solution Model. ISIJ Int. 1993, 33, 2–11. [Google Scholar] [CrossRef]
- Turkdogan, E.T.; Pearson, J. Activities of constituents of iron and steelmaking slags, Part 3-Phosphorus pentoxide. Iron Steel 1953, 175, 398–403. [Google Scholar]
- Yang, X.M.; Duan, J.P.; Shi, C.B.; Zhang, M.; Zhang, Y.L.; Wang, J.C. A thermodynamic model of phosphate distribution ratio between CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 slags and molten steel during a top–bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Met. Mater. Trans. B 2011, 42, 738–770. [Google Scholar] [CrossRef]
- Yang, W.; Yang, J.; Shi, Y.; Yang, Z.; Gao, F.; Zhang, R.; Ye, G. Effect of basicity on dephosphorization of hot metal with a low basicity slag at 1653 K. Ironmak. Steelmak. 2021, 48, 69–77. [Google Scholar] [CrossRef]
- Turkdogan, E.T. Assessment of P2O5 Activity Coefficients in Molten Slags. ISIJ Int. 2000, 40, 964–970. [Google Scholar] [CrossRef]
- Basu, S.; Lahiri, A.K.; Seetharaman, S. A Model for Activity Coefficient of P2O5 in BOF Slag and Phosphorus Distribution between Liquid Steel and Slag. ISIJ Int. 2007, 47, 1236–1238. [Google Scholar] [CrossRef][Green Version]
- Wu, X.; Bao, Y.P.; Yue, F.; Feng, J.; Tang, D.C. Study on factors to affect the product of carbon content and oxygen content at blowing end-point of BOF steelmaking. Res. Iron Steel 2010, 38, 26–29. [Google Scholar]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Duan, D.P.; Zhang, J. A thermodynamic model for predicting phosphate capacity of CaO-based slags during hot metal dephosphorization pretreatment process. Ironmak. Steelmak. 2017, 44, 437–454. [Google Scholar] [CrossRef]
- Wagner, C. The concept of the basicity of slags. Met. Mater. Trans. A 1975, 6, 405–409. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Duan, J.P.; Zhang, J. A thermodynamic model of phosphate capacity for CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 slags equilibrated with molten steel during a top–bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Met. Mater. Trans. B 2011, 42, 951–976. [Google Scholar] [CrossRef]
- Maruoka, N.; Ono, S.; Kitamura, S.Y. Equilibrium distribution ratio of phosphorus between solid iron and magnesio-wustite-saturated Al2O3-CaO-FetO-MgO-SiO2 slag at 1623K. ISIJ Int. 2013, 53, 1709–1714. [Google Scholar] [CrossRef]
- Roger, S.; Dong, Y.C.; Wu, Q.A. Uses of limes-based fluxes for simultaneous removal of phosphorus and sulfur in hot metal pretreatment. Scand. J. Met. 1990, 19, 98–109. [Google Scholar]
- Sobandi, A.; Katayama, H.G.; Momono, T. Activity of phosphorus oxide in CaO-MnO-SiO2-PO2.5(-MgO, FetO) slags. ISIJ Int. 1998, 38, 781–788. [Google Scholar] [CrossRef]
- Healy, G.W. A new look at phosphorus distribution. Iron Steel 1970, 208, 664–668. [Google Scholar]
- Suito, H.; Inoue, R. Phosphorus distribution between MgO-saturated CaO-FetO-SiO2-P2O5-MnO slags and liquid iron. Trans. ISIJ 1984, 24, 40–46. [Google Scholar] [CrossRef]
- Kawai, Y.; Takahashi, I.; Miyashita, Y.; Tachibana, K. For dephosphorization equilibrium between slag and molten steel in the converter furnace. Tetsu-to-Hagané 1977, 63, 156. [Google Scholar]
- Usui, T.; Yamada, K.; Kawai, Y.; Inoue, S.; Ishikawa, H.; Nimura, Y. Experiment of phosphorus and oxygen distribution between CaO-SiO2-MgO-FetO slag and liquid steel and estimation of phosphorus content at end point of top and bottom blowing converter. Tetsu-to-Hagané 1991, 77, 1641–1648. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.F.; Sommerville, I.D.; Toguri, J.M. Equation for the equilibrium distribution of phosphorus between basic slags and steel. Trans. Iron Steel Soc. 1985, 6, 29–35. [Google Scholar]
- Zhou, C.G.; Li, J.; Luo, K.M.; Han, X.; Zhang, Z.M.; Liu, Z.M.; Deng, C.F. First deslagging practice of double slag process for dephosphorization. Iron Steel Vanadium Titan. 2016, 37, 119–126. [Google Scholar]
- Zhang, T.X.; Song, J.S.; Zhou, D.D.; Liu, X.Y. Calculation and process test for dephosphorization parameter of 120t converter. Spec. Steel 2020, 41, 25–27. [Google Scholar]
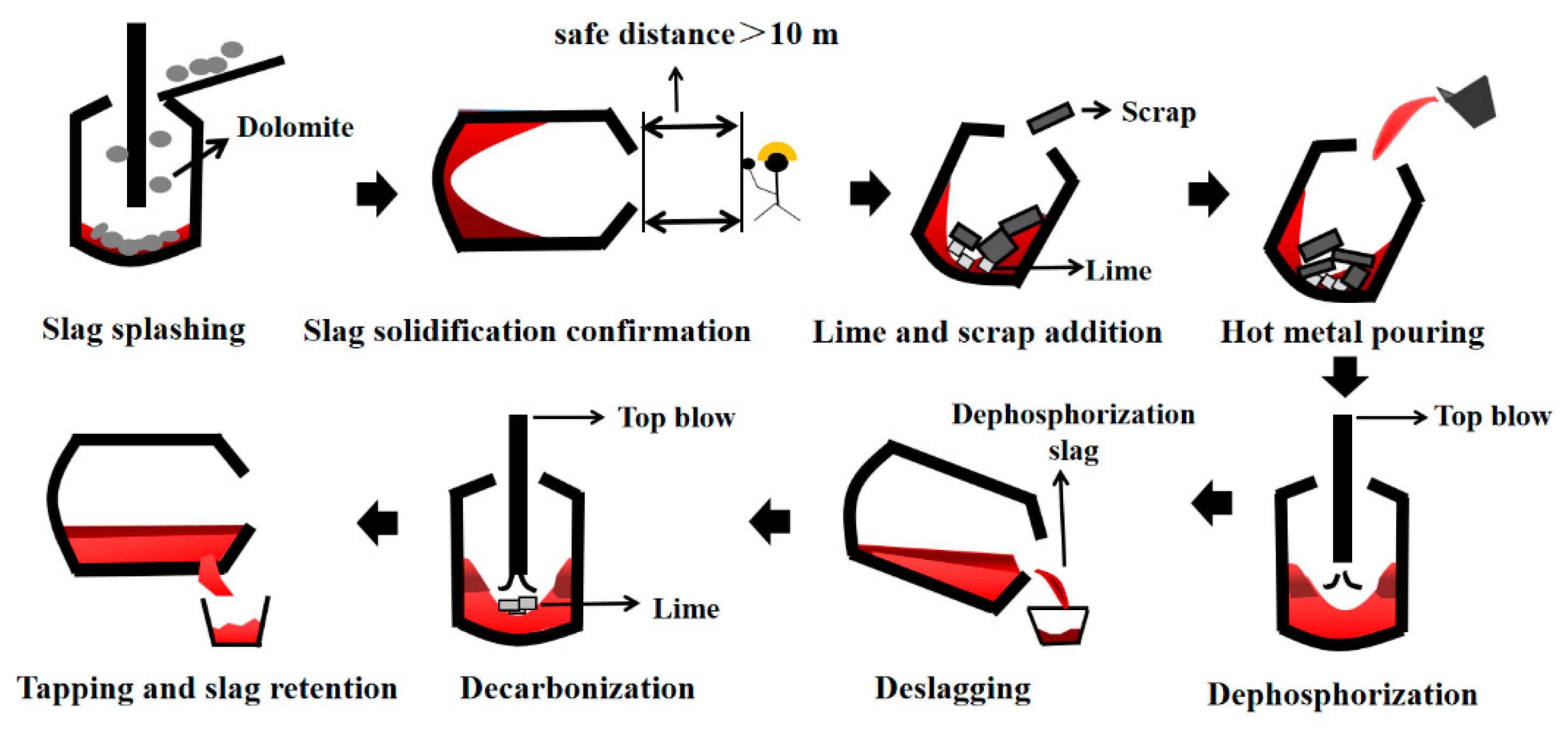
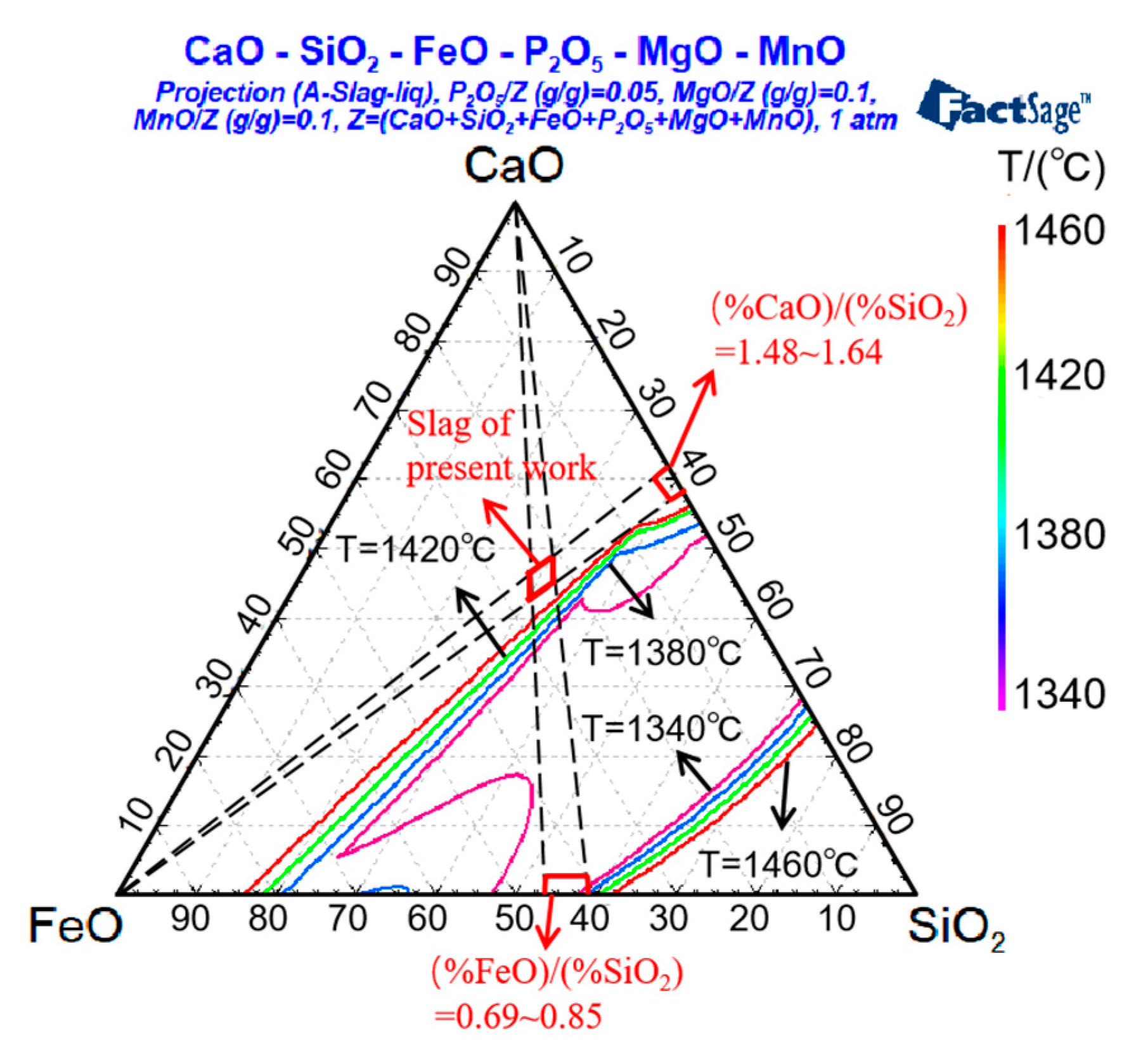
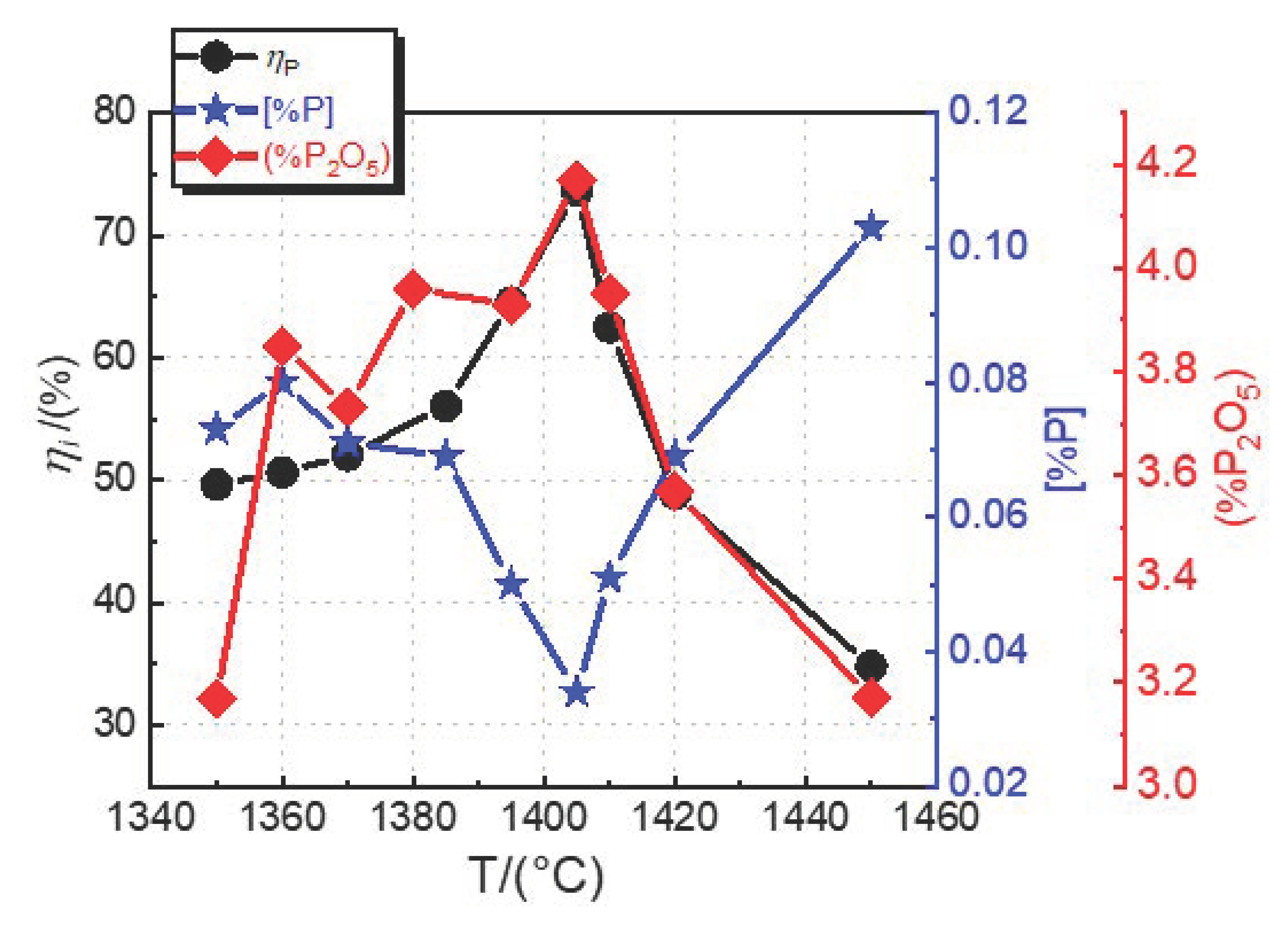
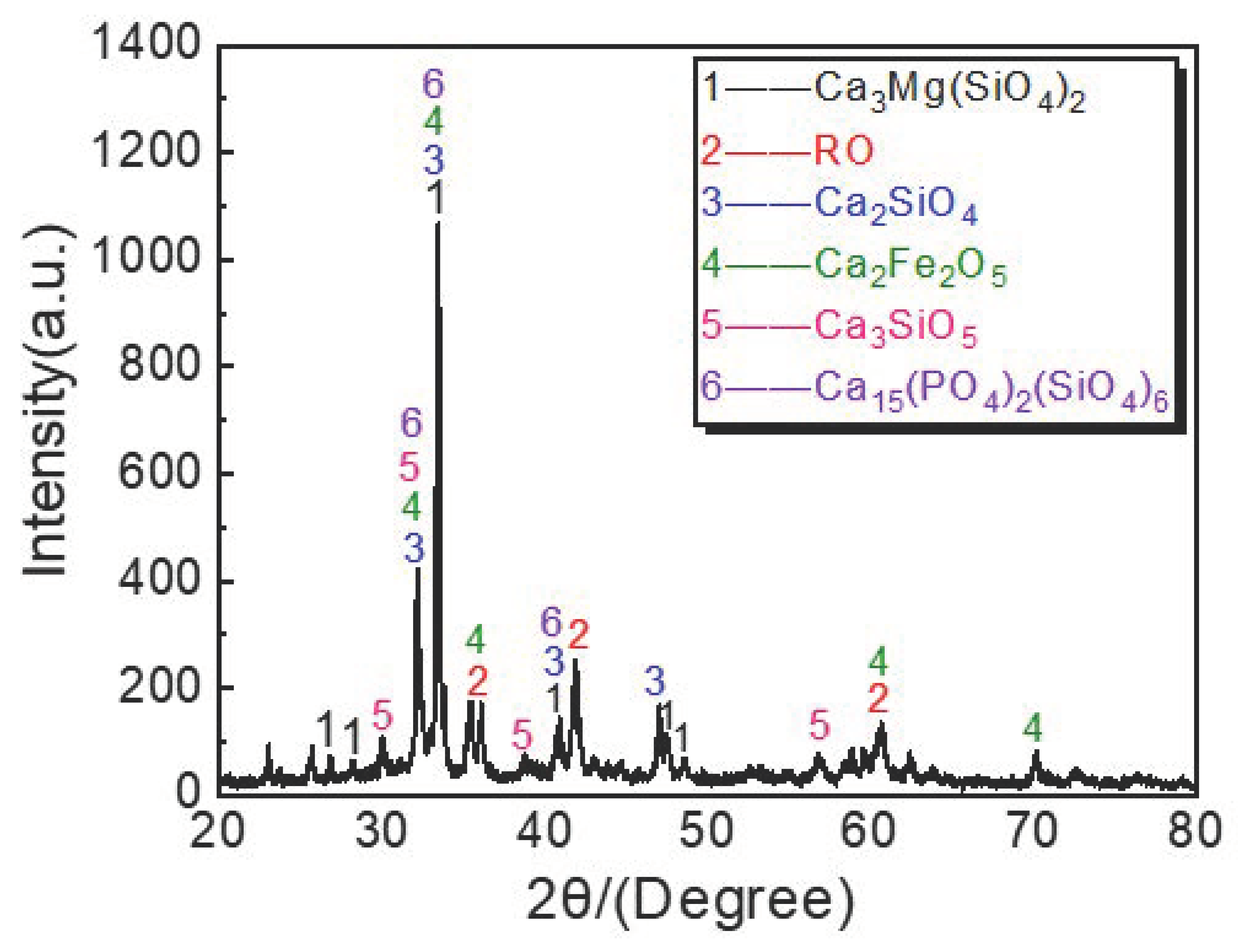


| Sample | C | Si | Mn | S | P | T/°C |
|---|---|---|---|---|---|---|
| T1350 | 4.65 | 0.29 | 0.18 | 0.03 | 0.145 | 1330 |
| T1360 | 4.65 | 0.36 | 0.25 | 0.01 | 0.162 | 1326 |
| T1370 | 4.65 | 0.34 | 0.29 | 0.03 | 0.148 | 1350 |
| T1385 | 4.65 | 0.32 | 0.29 | 0.01 | 0.157 | 1350 |
| T1395 | 4.65 | 0.41 | 0.28 | 0.01 | 0.141 | 1365 |
| T1405 | 4.65 | 0.29 | 0.27 | 0.01 | 0.129 | 1366 |
| T1410 | 4.65 | 0.31 | 0.25 | 0.02 | 0.136 | 1357 |
| T1420 | 4.65 | 0.40 | 0.31 | 0.04 | 0.135 | 1355 |
| T1450 | 4.65 | 0.39 | 0.29 | 0.03 | 0.158 | 1365 |
| Sample | C | Si | Mn | S | P | /% | T/°C |
|---|---|---|---|---|---|---|---|
| T1350 | 3.076 | 0.010 | 0.060 | 0.018 | 0.073 | 49.7 | 1350 |
| T1360 | 3.081 | 0.025 | 0.089 | 0.010 | 0.080 | 50.6 | 1360 |
| T1370 | 3.241 | 0.015 | 0.097 | 0.009 | 0.071 | 52.0 | 1370 |
| T1385 | 3.039 | 0.010 | 0.092 | 0.010 | 0.069 | 56.1 | 1385 |
| T1395 | 3.069 | 0.022 | 0.117 | 0.018 | 0.050 | 64.5 | 1395 |
| T1405 | 2.942 | 0.009 | 0.167 | 0.018 | 0.034 | 73.6 | 1405 |
| T1410 | 2.943 | 0.012 | 0.143 | 0.017 | 0.051 | 62.5 | 1410 |
| T1420 | 3.253 | 0.017 | 0.138 | 0.012 | 0.069 | 48.9 | 1420 |
| T1450 | 3.488 | 0.015 | 0.147 | 0.019 | 0.103 | 34.8 | 1450 |
| Sample | CaO | SiO2 | MgO | MnO | Al2O3 | P2O5 | FeO | B/- |
|---|---|---|---|---|---|---|---|---|
| T1350 | 35.96 | 22.77 | 7.24 | 9.37 | 2.52 | 3.17 | 18.97 | 1.58 |
| T1360 | 34.87 | 21.53 | 9.05 | 9.76 | 2.56 | 3.85 | 18.38 | 1.62 |
| T1370 | 34.66 | 22.51 | 8.15 | 9.25 | 2.83 | 3.73 | 18.87 | 1.54 |
| T1385 | 35.22 | 21.60 | 8.58 | 10.01 | 3.59 | 3.96 | 17.04 | 1.63 |
| T1395 | 34.88 | 22.48 | 8.98 | 9.92 | 2.86 | 3.93 | 16.95 | 1.55 |
| T1405 | 37.30 | 22.71 | 8.23 | 8.49 | 2.27 | 4.17 | 16.83 | 1.64 |
| T1410 | 35.73 | 22.91 | 8.94 | 8.29 | 3.05 | 3.95 | 17.13 | 1.56 |
| T1420 | 35.51 | 23.71 | 9.18 | 8.80 | 2.85 | 3.57 | 16.38 | 1.50 |
| T1450 | 35.28 | 23.84 | 9.35 | 8.59 | 2.23 | 3.17 | 17.54 | 1.48 |
| Position | Ca | Si | Mn | Mg | P | Al | Fe | O |
|---|---|---|---|---|---|---|---|---|
| 1-1 | 34.77 | 12.94 | 2.70 | 2.03 | 4.34 | 0.16 | 1.62 | 41.44 |
| 1-2 | 35.98 | 12.37 | 2.90 | 2.08 | 4.25 | 0.12 | 1.26 | 41.04 |
| 1-3 | 37.00 | 13.93 | 3.63 | 1.36 | 4.42 | 0.01 | 2.22 | 37.43 |
| 2-1 | 29.68 | 13.23 | 3.39 | 2.94 | 3.61 | 1.65 | 2.65 | 42.85 |
| 2-2 | 35.25 | 13.80 | 2.18 | 1.99 | 3.51 | 0.14 | 1.58 | 41.55 |
| 2-3 | 29.06 | 13.27 | 3.78 | 2.19 | 3.20 | 2.13 | 3.16 | 43.21 |
| 3 | 30.56 | 12.55 | 6.02 | 2.49 | 2.20 | 0.85 | 5.80 | 39.53 |
| 4 | 2.32 | 0.92 | 3.55 | 1.35 | 0.17 | 0.43 | 82.14 | 9.12 |
| 5 | 20.36 | 10.22 | 7.33 | 2.92 | 1.14 | 2.03 | 24.06 | 31.94 |
| 6 | 27.08 | 17.79 | 3.19 | 3.33 | 1.44 | 2.56 | 4.15 | 40.46 |
| Scholar | Slag | Empirical Formulas |
|---|---|---|
| Yang [28] | CaO-FeO-SiO2-MgO-Al2O3 | |
| Maruoka [35] | CaO-FeO-SiO2-MgO-Al2O3 | |
| Selin [36] | CaO-SiO2-CaF2 | |
| Sobandi [37] | CaO-MnO-SiO2-PO2.5(-MgO, FetO) |
| Oxide | CaO | SiO2 | MnO | P2O5 | FeO | Fe2O3 | MgO | Al2O3 |
|---|---|---|---|---|---|---|---|---|
| 1.00 | 0.46 | 0.59 | 0.40 | 0.51 | 0.48 | 0.78 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Yang, J.; Lu, X.; Liu, W.; Ye, G.; Zhang, R.; Yang, W. Dephosphorization in Double Slag Converter Steelmaking Process at Different Temperatures by Industrial Experiments. Metals 2021, 11, 1030. https://doi.org/10.3390/met11071030
Sun H, Yang J, Lu X, Liu W, Ye G, Zhang R, Yang W. Dephosphorization in Double Slag Converter Steelmaking Process at Different Temperatures by Industrial Experiments. Metals. 2021; 11(7):1030. https://doi.org/10.3390/met11071030
Chicago/Turabian StyleSun, Han, Jian Yang, Xinwu Lu, Wanshan Liu, Gefan Ye, Runhao Zhang, and Wenkui Yang. 2021. "Dephosphorization in Double Slag Converter Steelmaking Process at Different Temperatures by Industrial Experiments" Metals 11, no. 7: 1030. https://doi.org/10.3390/met11071030
APA StyleSun, H., Yang, J., Lu, X., Liu, W., Ye, G., Zhang, R., & Yang, W. (2021). Dephosphorization in Double Slag Converter Steelmaking Process at Different Temperatures by Industrial Experiments. Metals, 11(7), 1030. https://doi.org/10.3390/met11071030







