Corrosion Behaviour and J774A.1 Macrophage Response to Hyaluronic Acid Functionalization of Electrochemically Reduced Graphene Oxide on Biomedical Grade CoCr
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalization of Electrochemically Reduced Graphene Oxide with Hyaluronic Acid
2.2.1. Electrochemical Reduction of Graphene Oxide (ErGO)
2.2.2. Functionalization with Hyaluronic Acid
2.2.3. Electrochemical Characterization of ErGO and ErGOHA on CoCr
2.3. Biocompatibility Response—Macrophages Cultures Assays
2.3.1. Cell Fixation and Optical Microscopy
2.3.2. Measurement of Mitochondrial Activity
2.3.3. Measurement of Lactate Dehydrogenase Activity
2.3.4. Inflammatory Response of J774A.1 Macrophage to CoCrErGOHA, CoCrErGO, and CoCr
2.3.5. Statistical Analysis of the Biocompatibility and Inflammatory Response Assays
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Mischler, S.; Muñoz, A.I. Wear of CoCrMo Alloys Used in Metal-on-Metal Hip Joints: A Tribocorrosion Appraisal. Wear 2013, 297, 1081–1094. [Google Scholar] [CrossRef]
- Bijukumar, D.R.; Segu, A.; Souza, J.C.M.; Li, X.; Barba, M.; Mercuri, L.G.; Jacobs, J.J.; Mathew, M.T. Systemic and Local Toxicity of Metal Debris Released from Hip Prostheses: A Review of Experimental Approaches. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 951–963. [Google Scholar] [CrossRef]
- Ingham, E.; Fisher, J. The Role of Macrophages in Osteolysis of Total Joint Replacement. Biomaterials 2005, 26, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Nich, C.; Goodman, S.B. Role of Macrophages in the Biological Reaction to Wear Debris from Joint Replacements. J. Autom. Inf. Sci. 2014, 24, 259–265. [Google Scholar] [CrossRef]
- Wimmer, M.A.; Sprecher, C.; Hauert, R.; Täger, G.; Fischer, A. Tribochemical Reaction on Metal-on-Metal Hip Joint Bearings: A Comparison between in-Vitro and in-Vivo Results. Wear 2003, 255, 1007–1014. [Google Scholar] [CrossRef]
- Mathew, M.T.; Nagelli, C.; Pourzal, R.; Fischer, A.; Laurent, M.P.; Jacobs, J.J.; Wimmer, M.A. Tribolayer Formation in a Metal-on-Metal (MoM) Hip Joint: An Electrochemical Investigation. J. Mech. Behav. Biomed. Mater. 2014, 29, 199–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Pourzal, R.; Wimmer, M.A.; Jacobs, J.J.; Fischer, A.; Marks, L.D. Graphitic Tribological Layers in Metal-on-Metal Hip Replacements. Science 2011, 334, 1687–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Zhang, W.; Lee, S.; McNear, K.L.; Chung, T.F.; Lee, S.; Lee, K.; Crist, S.A.; Ratliff, T.L.; Zhong, Z.; Chen, Y.P.; et al. Use of Graphene as Protection Film in Biological Environments. Sci. Rep. 2014, 4, 4097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Argumánez, A.; Llorente, I.; Caballero-Calero, O.; González, Z.; Menéndez, R.; Escudero, M.L.; García-Alonso, M.C. Electrochemical Reduction of Graphene Oxide on Biomedical Grade CoCr Alloy. Appl. Surf. Sci. 2019, 465, 1028–1036. [Google Scholar] [CrossRef]
- Escudero, M.L.; Llorente, I.; Pérez-Maceda, B.T.; José-Pinilla, S.S.; Sánchez-López, L.; Lozano, R.M.; Aguado-Henche, S.; de Arriba, C.C.; Alobera-Gracia, M.A.; García-Alonso, M.C. Electrochemically Reduced Graphene Oxide on CoCr Biomedical Alloy: Characterization, Macrophage Biocompatibility and Hemocompatibility in Rats with Graphene and Graphene Oxide. Mater. Sci. Eng. C 2020, 109, 110522. [Google Scholar] [CrossRef]
- Mazzucco, D.; Scott, R.; Spector, M. Composition of Joint Fluid in Patients Undergoing Total Knee Replacement and Revision Arthroplasty: Correlation with Flow Properties. Biomaterials 2004, 25, 4433–4445. [Google Scholar] [CrossRef]
- Nich, C.; Takakubo, Y.; Pajarinen, J.; Ainola, M.; Salem, A.; Sillat, T.; Rao, A.J.; Raska, M.; Tamaki, Y.; Takagi, M.; et al. Macrophages—Key Cells in the Response to Wear Debris from Joint Replacements. J. Biomed. Mater. Res. Part A 2013, 101, 3033–3045. [Google Scholar] [CrossRef] [Green Version]
- Perez-Maceda, B.; López-Fernández, M.; Díaz, I.; Kavanaugh, A.; Billi, F.; Escudero, M.; García-Alonso, M.; Lozano, R. Macrophage Biocompatibility of CoCr Wear Particles Produced under Polarization in Hyaluronic Acid Aqueous Solution. Materials 2018, 11, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for Modifying Current Cytotoxicity Testing Standards for Biodegradable Magnesium-Based Materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.M.; Pérez-Maceda, B.T.; Carboneras, M.; Onofre-Bustamante, E.; García-Alonso, M.C.; Escudero, M.L. Response of MC3T3-E1 osteoblasts, L929 fibroblasts, and J774 macrophages to fluoride surface-modified AZ31 magnesium alloy. J. Biomed. Mater. Res. Part A 2013, 101, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The Analysis of Electrode Impedances Complicated by the Presence of a Constant Phase Element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Time-Constant Dispersion. In Electrochemical Impedance Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 233–263. ISBN 978-0-470-38158-8. [Google Scholar]
- Horkay, F.; Basser, P.J.; Londono, D.J.; Hecht, A.-M.; Geissler, E. Ions in Hyaluronic Acid Solutions. J. Chem. Phys. 2009, 131, 184902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranova, N.S.; Nilebäck, E.; Haller, F.M.; Briggs, D.C.; Svedhem, S.; Day, A.J.; Richter, R.P. The Inflammation-Associated Protein TSG-6 Cross-Links Hyaluronan via Hyaluronan-Induced TSG-6 Oligomers. J. Biol. Chem. 2011, 286, 25675–25686. [Google Scholar] [CrossRef] [Green Version]
- Richter, R.P.; Hock, K.K.; Burkhartsmeyer, J.; Boehm, H.; Bingen, P.; Wang, G.; Steinmetz, N.F.; Evans, D.J.; Spatz, J.P. Membrane-Grafted Hyaluronan Films: A Well-Defined Model System of Glycoconjugate Cell Coats. J. Am. Chem. Soc. 2007, 129, 5306–5307. [Google Scholar] [CrossRef]
- De Gennes, P.-G. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NY, USA, 1979; ISBN 978-0-8014-1203-5. [Google Scholar]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, B.; Rajappa, M.; Mallika, V.; Shukla, D.K.; Kumar, S. TNF-α/IL-10 Ratio and C-Reactive Protein as Markers of the Inflammatory Response in CAD-Prone North Indian Patients with Acute Myocardial Infarction. Clin. Chim. Acta 2009, 408, 14–18. [Google Scholar] [CrossRef]
- Qu, Y.; He, F.; Yu, C.; Liang, X.; Liang, D.; Ma, L.; Zhang, Q.; Lv, J.; Wu, J. Advances on Graphene-Based Nanomaterials for Biomedical Applications. Mater. Sci. Eng. C 2018, 90, 764–780. [Google Scholar] [CrossRef]
- Yue, H.; Wei, W.; Yue, Z.; Wang, B.; Luo, N.; Gao, Y.; Ma, D.; Ma, G.; Su, Z. The Role of the Lateral Dimension of Graphene Oxide in the Regulation of Cellular Responses. Biomaterials 2012, 33, 4013–4021. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.Y.; Xia, T.; Liu, S. Crucial Role of Lateral Size for Graphene Oxide in Activating Macrophages and Stimulating Pro-Inflammatory Responses in Cells and Animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef] [PubMed]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing Biocompatibility of Graphene Oxide-Based Nanocarriers: A Review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-B.; Duong, C.-T.; Chang, H.-G.; Sharma, A.R.; Thompson, M.S.; Park, S.; Kwak, B.-C.; Kim, T.-Y.; Lee, S.-S.; Park, S. Role of Hyaluronic Acid and Phospholipid in the Lubrication of a Cobalt-Chromium Head for Total Hip Arthroplasty. Biointerphases 2014, 9, 031007. [Google Scholar] [CrossRef] [Green Version]

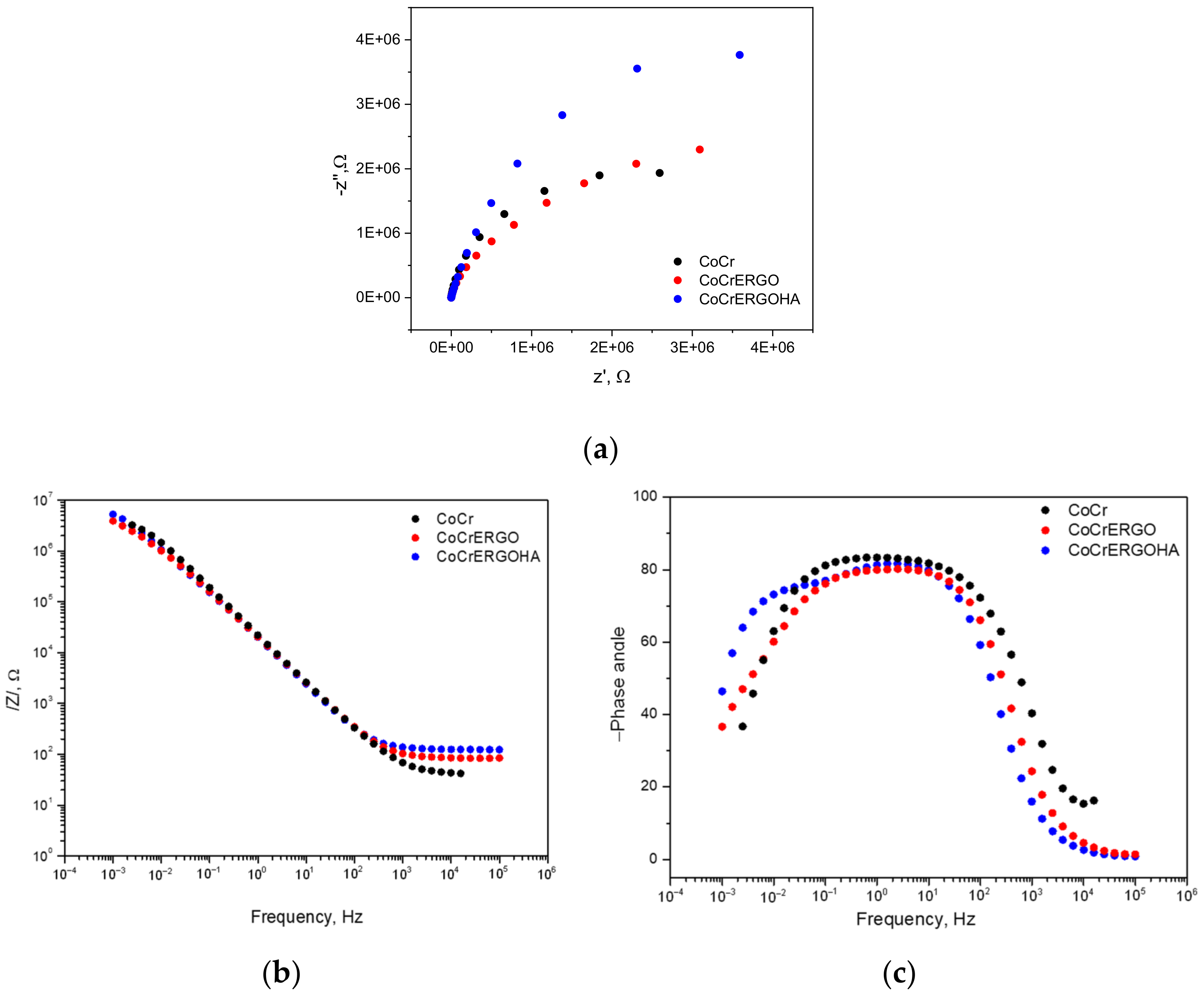
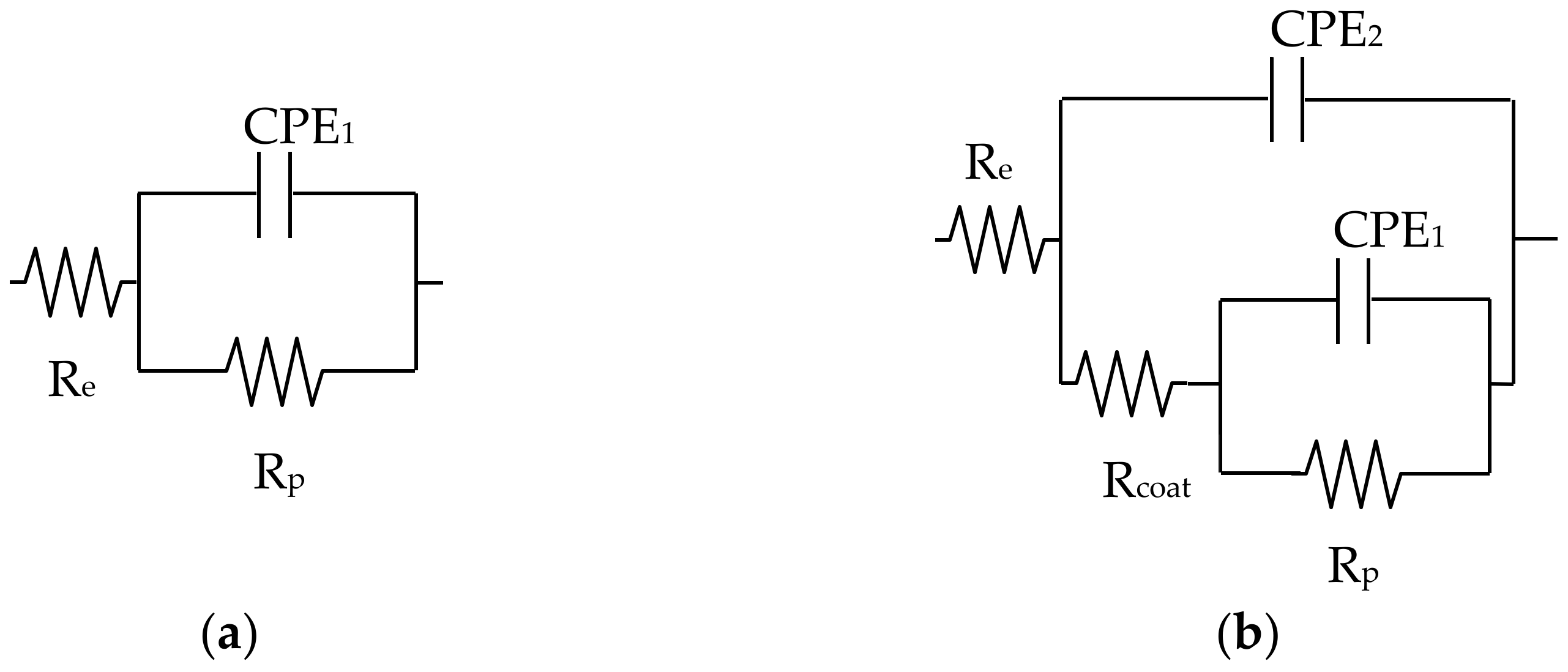



| Surface | Time, d | Re ± Error %, Ω | Rcoat ± Error %, MΩ | CPE2 ± Error %, µsn/Ω | n2 ± Error % | Rp ± Error %, MΩ | CPE1 ± Error %, µsn/Ω | n1 ± Error % | Chi2 | CCPE1, µF | CCPE2, µF | Cexp µF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CoCr | 0 | 87.4 ± 3.6 | 0.2 ± 6.5 | 14.7 ± 4.4 | 0.91 ± 1.16 | 0.0153 | 1.7 | 6.5 | ||||
| 7 | 44.6 ± 2.0 | 4.4 ± 4.1 | 8.2 ± 1.1 | 0.92 ± 0.25 | 0.0021 | 1.0 | 3.3 | |||||
| CoCrErGO | 0 | 83.9 ± 1.3 | 2.3 ± 3.6 | 9.3 ± 1.1 | 0.90 ± 0.28 | 0.0017 | 0.6 | 3.9 | ||||
| 7 | 85.1 ± 0.5 | 2.3 ± 7.0 | 9.4 ± 0.6 | 0.90 ± 0.14 | 3.8 ± 12.8 | 10.1 ± 36.0 | 0.78 ± 8.90 | 0.0002 | 0.62 | 3.4 | ||
| CoCrErGOHA | 0 | 122.1 ± 0.6 | 0.3 ± 12.9 | 11.0 ± 1.2 | 0.90 ± 0.27 | 2.0 ± 6.7 | 6.6 ± 14.2 | 0.64 ± 5.21 | 0.0004 | 0.7 | 4.1 | |
| 7 | 125.6 ± 0.6 | 0.8 ± 27.9 | 9.4 ± 1.19 | 0.92 ± 0.27 | 11.0 ± 7.8 | 1.9 ± 15.0 | 0.71 ± 5.43 | 0.0004 | 1.0 | 3.9 |
| Cytokines | No Material | CoCr | CoCrErGO | CoCrErGOHA |
|---|---|---|---|---|
| TNF-α | 96.73 (±11.3) | 80.46 (±12.6) | 110.34 (±50.4) | 130.28 (±11.1) |
| IL-10 | 160.97 (±31.9) | 85.21 (±61.6) | 84.65 (±43.3) | 139.44 (±60.6) |
| TNF-α/IL-10 | 0.61 | 0.94 | 1.30 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chico, B.; Pérez-Maceda, B.T.; San José, S.; Escudero, M.L.; García-Alonso, M.C.; Lozano, R.M. Corrosion Behaviour and J774A.1 Macrophage Response to Hyaluronic Acid Functionalization of Electrochemically Reduced Graphene Oxide on Biomedical Grade CoCr. Metals 2021, 11, 1078. https://doi.org/10.3390/met11071078
Chico B, Pérez-Maceda BT, San José S, Escudero ML, García-Alonso MC, Lozano RM. Corrosion Behaviour and J774A.1 Macrophage Response to Hyaluronic Acid Functionalization of Electrochemically Reduced Graphene Oxide on Biomedical Grade CoCr. Metals. 2021; 11(7):1078. https://doi.org/10.3390/met11071078
Chicago/Turabian StyleChico, Belén, Blanca Teresa Pérez-Maceda, Sara San José, María Lorenza Escudero, María Cristina García-Alonso, and Rosa María Lozano. 2021. "Corrosion Behaviour and J774A.1 Macrophage Response to Hyaluronic Acid Functionalization of Electrochemically Reduced Graphene Oxide on Biomedical Grade CoCr" Metals 11, no. 7: 1078. https://doi.org/10.3390/met11071078
APA StyleChico, B., Pérez-Maceda, B. T., San José, S., Escudero, M. L., García-Alonso, M. C., & Lozano, R. M. (2021). Corrosion Behaviour and J774A.1 Macrophage Response to Hyaluronic Acid Functionalization of Electrochemically Reduced Graphene Oxide on Biomedical Grade CoCr. Metals, 11(7), 1078. https://doi.org/10.3390/met11071078






