Microstructure and Corrosion Behavior of Extruded Mg-Sn-Y Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Microstructural Characterization
2.4. Microhardness
2.5. Electrochemical Behavior
3. Results
3.1. Microstructural Characterization
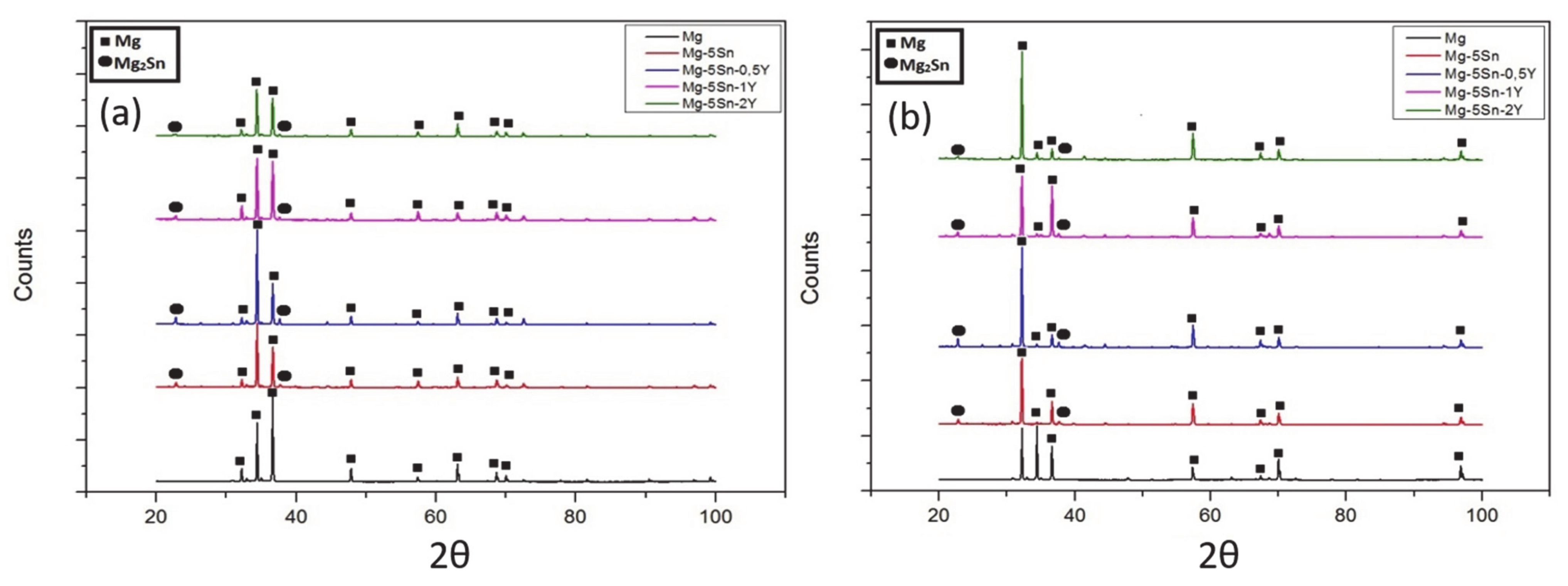
3.1.1. X-ray Diffraction
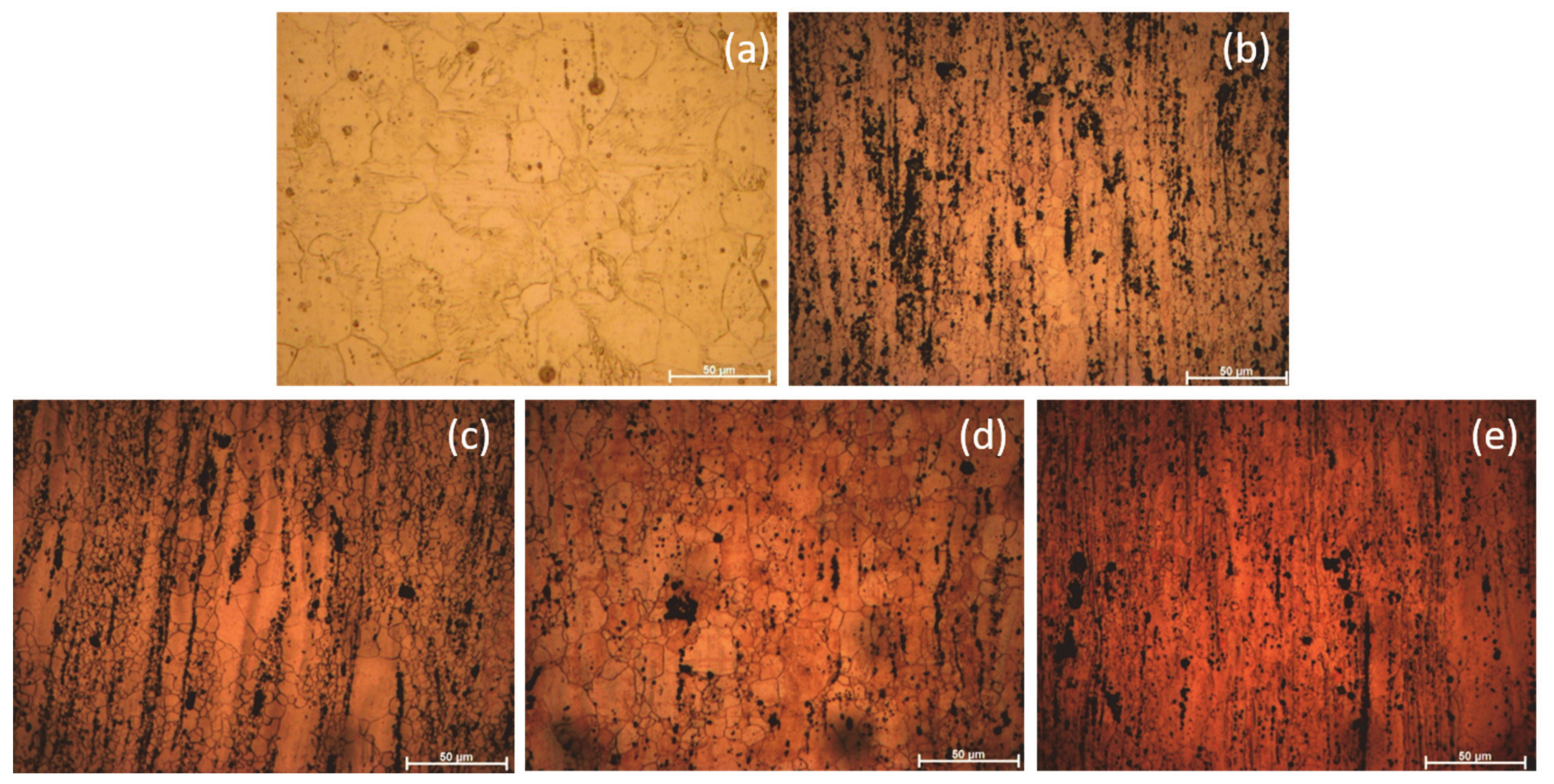
3.1.2. Optical Microscopy
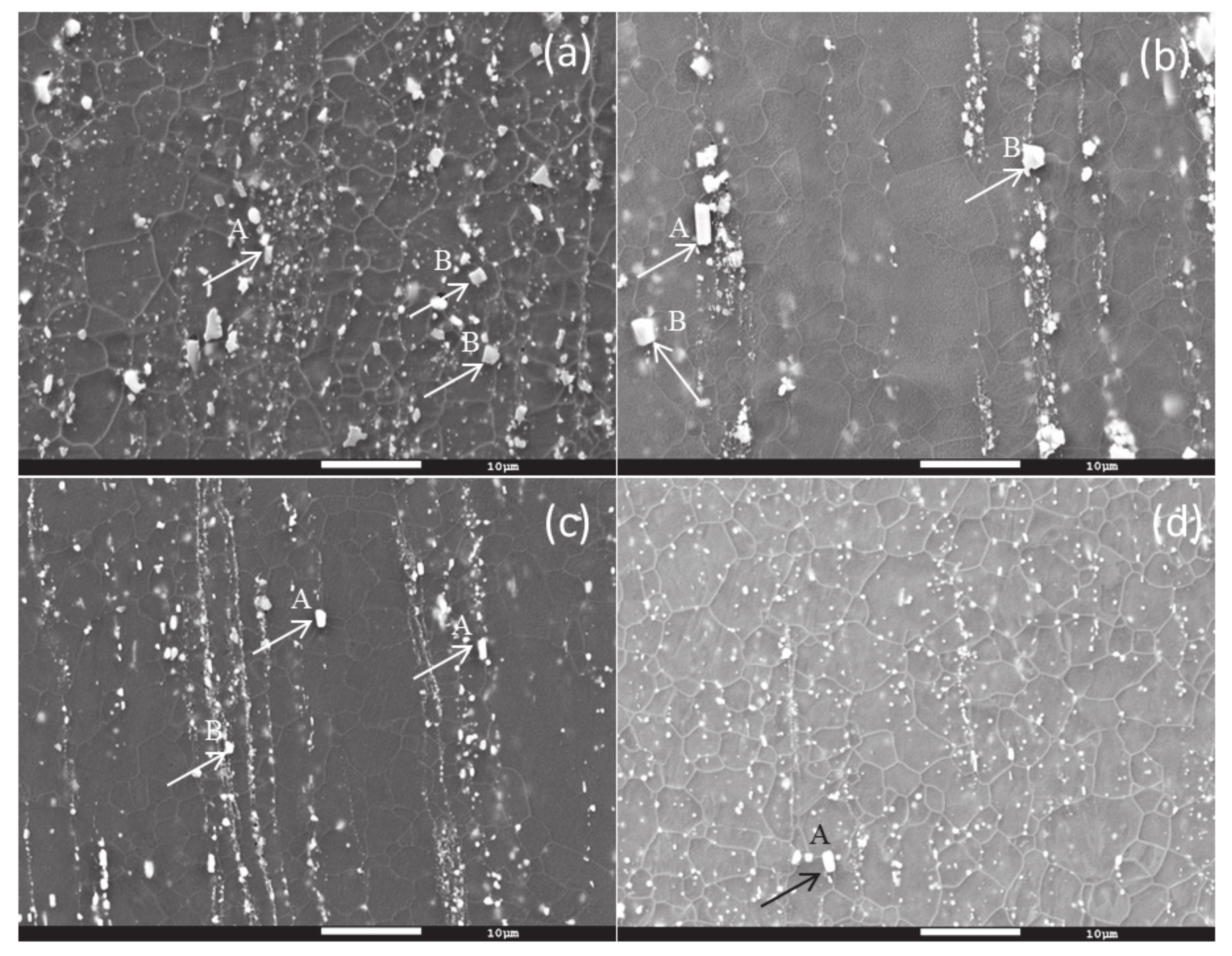
3.1.3. Scanning Electron Microscopy
3.1.4. Energy Dispersive Spectroscopy
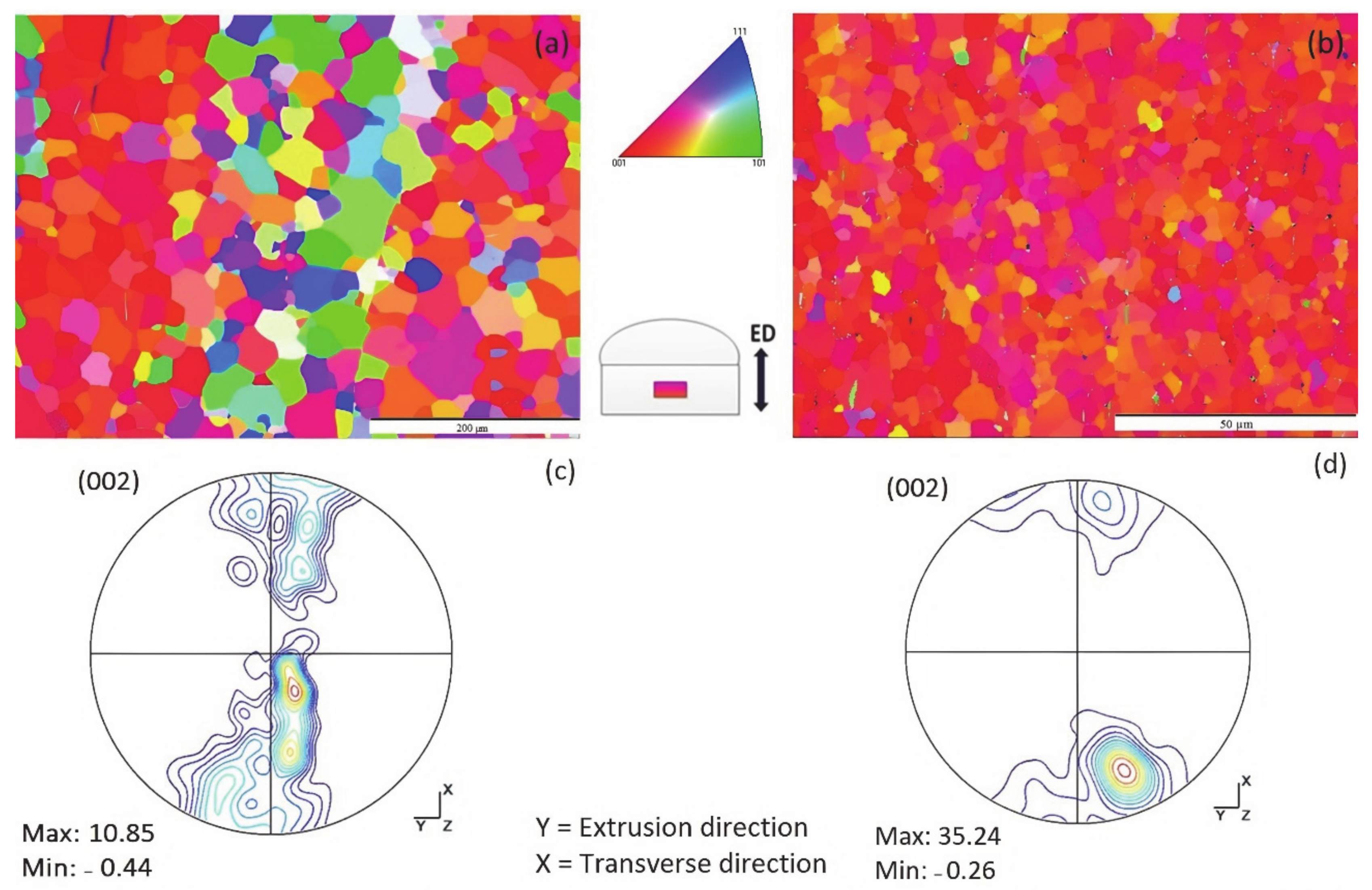
3.1.5. Electron Back-Scattered Diffraction
3.2. Microhardness
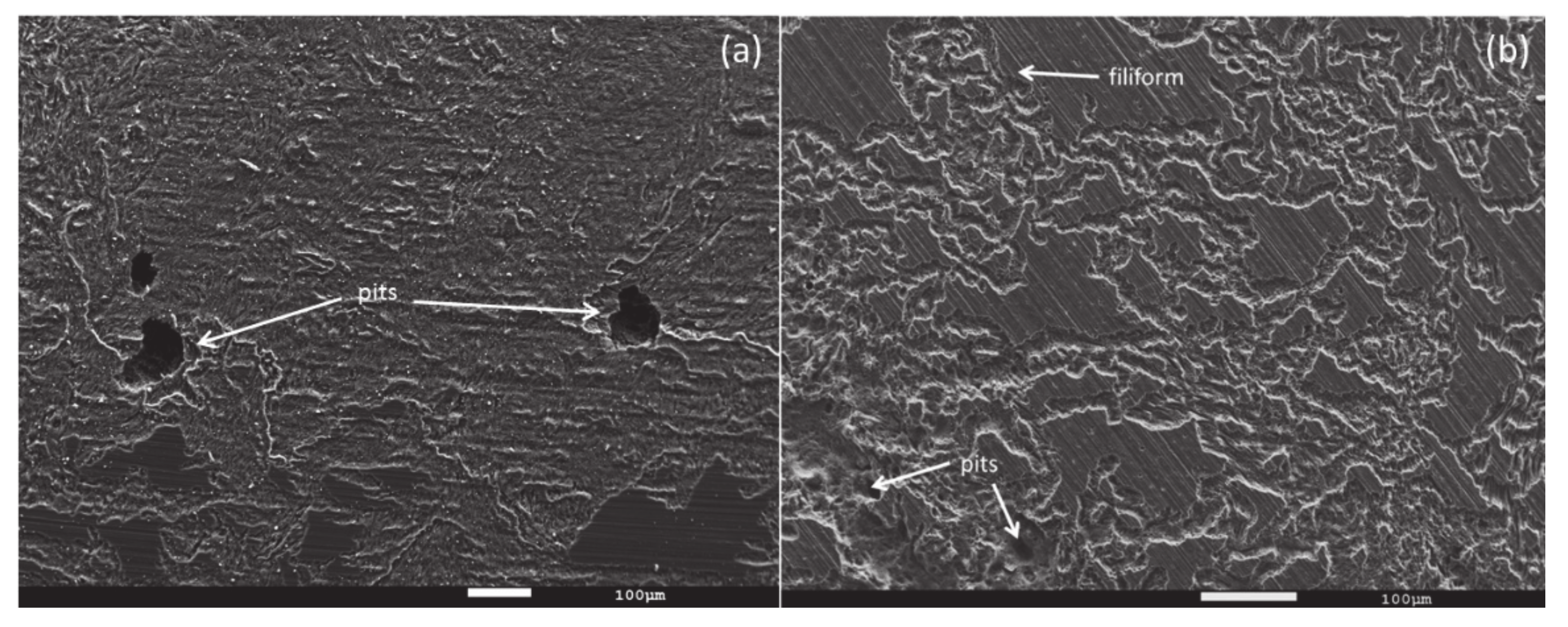
3.3. Corrosion Behavior
3.3.1. Weight Loss
3.3.2. Potentiodynamic Polarization Test
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, A.A. Magnesium casting technology for structural applications. J. Magnes. Alloy. 2013, 1, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Avedesian, M.M.; Baker, H. ASM International Handbook Committee: Magnesium and Magnesium Alloys; ASM International: Novelty, OH, USA, 1999; ISBN 9780871706577. [Google Scholar]
- Son, H.T.; Lee, J.B.; Jeong, H.G.; Konno, T.J. Effects of Al and Zn additions on mechanical properties and precipitation behaviors of Mg-Sn alloy system. Mater. Lett. 2011, 65, 1966–1969. [Google Scholar] [CrossRef]
- Bowles, A.L.; Dieringa, H.; Blawert, C.; Hort, N.; Kainer, K.U. Investigations in the Magnesium-Tin System. Mater. Sci. Forum 2005, 488–489, 135–138. [Google Scholar] [CrossRef]
- Hou, L.; Li, Z.; Zhao, H.; Pan, Y.; Pavlinich, S.; Liu, X.; Li, X.; Zheng, Y.; Li, L. Microstructure, Mechanical Properties, Corrosion Behavior and Biocompatibility of As-Extruded Biodegradable Mg–3Sn–1Zn–0.5Mn Alloy. J. Mater. Sci. Technol. 2016, 32, 874–882. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, F.; Zhao, S.; Pan, H.; Song, K.; Tang, A. Preparation and characterization of as-extruded Mg–Sn alloys for orthopedic applications. Mater. Des. 2015, 70, 60–67. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Lipov, J.; Ruml, T. Structure, mechanical properties, corrosion behavior and cytotoxicity of biodegradable Mg-X (X = Sn, Ga, In) alloys. Mater. Sci. Eng. C 2013, 33, 2421–2432. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Arvind Singh, R.; Thenambika, V.; Kirubanidhi, J.; Gupta, M. Impression Creep Characteristics of Mg-Sn-Ag Alloy. Appl. Mech. Mater. 2016, 854, 33–37. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Weiyang, J.; Qiao, B.; Wang, Y.; Li, Y.; Jiang, D. A novel biodegradable Mg-1Zn-0.5Sn alloy: Mechanical properties, corrosion behavior, biocompatibility, and antibacterial activity. J. Magnes. Alloy. 2020, 8, 374–386. [Google Scholar] [CrossRef]
- Zhen, Z.; Xi, T.; Zheng, Y.; Li, L.; Li, L. InVitro study on Mg-Sn-Mn Alloy as biodegradable metals. J. Mater. Sci. Technol. 2014, 30, 675–685. [Google Scholar] [CrossRef]
- Tang, W.N.; Park, S.S.; You, B.S. Effect of the Zn content on the microstructure and mechanical properties of indirect-extruded Mg–5Sn–xZn alloys. Mater. Des. 2011, 32, 3537–3543. [Google Scholar] [CrossRef]
- Fang, C.; Wen, Z.; Liu, X.; Hao, H.; Chen, G.; Zhang, X. Microstructures and mechanical properties of Mg2Sn-nanophase reinforced Mg–Mg2Sn composite. Mater. Sci. Eng. A 2017, 684, 229–232. [Google Scholar] [CrossRef]
- Zhang, J.H.; Liu, H.F.; Sun, W.; Lu, H.Y.; Tang, D.X.; Meng, J. Influence of structure and lonic radius on solubility limit in the Mg-Re systems. Mater. Sci. Forum 2007, 561–565, 143–146. [Google Scholar] [CrossRef]
- Sugamata, M.; Hanawa, S.; Kaneko, J. Structures and mechanical properties of rapidly solidified Mg-Y based alloys. Mater. Sci. Eng. A 1997, 226–228, 861–866. [Google Scholar] [CrossRef]
- Gerischer, H. Elektrodenpotentiale: Tables of Standard Electrode Potentials. Hrsg. von G. Milazzo und S. Caroli. John Wiley & Sons: Chichester, New York, 1978. XVI, 421 S., geb. £ 17,50. Nachr. Chem. Tech. Lab. 1978, 26, 661. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Deng, X.; Hongwei, L.; Yongjun, L.; Minglong, M.; Ning, L.; Wang, Y. Corrosion behavior of Mg–Y alloy in NaCl aqueous solution. Prog. Nat. Sci. Mater. Int. 2012, 22, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, K.; Li, X.; Deng, X.; Li, Y.; Ma, M.; Shi, Y. Effect of solid-solution treatment on corrosion and electrochemical behaviors of Mg-15Y alloy in 3.5 wt.% NaCl solution. J. Rare Earths 2012, 30, 1158–1167. [Google Scholar] [CrossRef]
- Sudholz, A.D.; Gusieva, K.; Chen, X.B.; Muddle, B.C.; Gibson, M.A.; Birbilis, N. Electrochemical behaviour and corrosion of Mg–Y alloys. Corros. Sci. 2011, 53, 2277–2282. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, B.; Yang, H.; Yang, Q.; Xia, X.; Pan, F. High temperature oxidation behavior of Mg-Y-Sn, Mg-Y, Mg-Sn alloys and its effect on corrosion property. Appl. Surf. Sci. 2015, 353, 1013–1022. [Google Scholar] [CrossRef]
- Gupta, M.; Lai, M.O.; Saravanaranganathan, D. Synthesis, microstructure and properties characterization of disintegrated melt deposited Mg/SiC composites. J. Mater. Sci. 2000, 35, 2155–2165. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Knoop and Vickers Hardness of Materials. E384-11e1. Available online: http://www.astm.org/cgi-bin/resolver.cgi?E384-11e1 (accessed on 4 September 2018).
- Zhao, H.D.; Qin, G.W.; Ren, Y.P.; Pei, W.L.; Chen, D.; Guo, Y. Microstructure and tensile properties of as-extruded Mg-Sn-Y alloys. Trans. Nonferr. Met. Soc. China 2010, 20, s493–s497. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Sankaranarayanan, S.; Koh, S.P.X.; Gupta, M. Effect of Ag and Cu trace additions on the microstructural evolution and mechanical properties of Mg-5Sn alloy. J. Alloys Compd. 2013, 565, 56–65. [Google Scholar] [CrossRef]
- She, J.; Pan, F.; Zhang, J.; Tang, A.; Luo, S.; Yu, Z.; Song, K.; Rashad, M. Microstructure and mechanical properties of Mg-Al-Sn extruded alloys. J. Alloys Compd. 2016, 657, 893–905. [Google Scholar] [CrossRef]
- Kevorkijan, V.; Davor Skapin, S. Studies of Mg2Sn-based composites. Assoc. Met. Eng. Serb. 2010, 16, 47–61. [Google Scholar]
- Liu, X.; Shan, D.; Song, Y.; Chen, R.; Han, E. Influences of the quantity of Mg2Sn phase on the corrosion behavior of Mg–7Sn magnesium alloy. Electrochim. Acta 2011, 56, 2582–2590. [Google Scholar] [CrossRef]
- ASM International. ASM Handbook, Volume 3, Alloy Phase Diagrams; ASM International: Novelty, OH, USA, 2004; Volume 7, ISBN 0871703777. [Google Scholar]
- Zhao, C.; Chen, X.; Pan, F.; Gao, S.; Zhao, D.; Liu, X. Effect of Sn content on strain hardening behavior of as-extruded Mg-Sn alloys. Mater. Sci. Eng. A 2018, 713, 244–252. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Liao, J.; Hotta, M.; Yamamoto, N. Corrosion behavior of fine-grained AZ31B magnesium alloy. Corros. Sci. 2012, 61, 208–214. [Google Scholar] [CrossRef]
- Chen, D.; Ren, Y.P.; Guo, Y.; Pei, W.L.; Zhao, H.D.; Qln, G.W. Microstructures and tensile properties of as-extruded Mg-Sn binary alloys. Trans. Nonferr. Met. Soc. China 2010, 20, 1321–1325. [Google Scholar] [CrossRef]
- Ha, H.Y.; Kang, J.Y.; Yang, J.; Yim, C.D.; You, B.S. Role of Sn in corrosion and passive behavior of extruded Mg-5 wt%Sn alloy. Corros. Sci. 2016, 102, 355–362. [Google Scholar] [CrossRef]
- Liu, X.; Shan, D.; Song, Y.; Han, E. Influence of yttrium element on the corrosion behaviors of Mg—Y binary magnesium alloy. J. Magnes. Alloy. 2017, 5, 26–34. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, C.; Shen, L.; Zhao, Z.; Peng, S.; Shuai, C. In-situ deposition of apatite layer to protect Mg-based composite fabricated via laser additive manufacturing. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]


| Material | Section | (I/Imax) Plane | ||
|---|---|---|---|---|
| (101̅0) Prismatic | (0002) Basal | (101̅1) Pyramidal | ||
| Mg | T | 0.9602 | 1.0000 | 0.6295 |
| L | 0.1546 | 0.6959 | 1.0000 | |
| Mg-5Sn | T | 1.0000 | 0.0407 | 0.3407 |
| L | 0.1329 | 1.0000 | 0.6379 | |
| Mg-5Sn-0.5Y | T | 1.0000 | 0.0351 | 0.1285 |
| L | 0.759 | 1.0000 | 0.4367 | |
| Mg-5Sn-1Y | T | 1.0000 | 0.0581 | 0.8495 |
| L | 0.2355 | 1.0000 | 0.9500 | |
| Mg-5Sn-2Y | T | 1.0000 | 0.0649 | 0.1018 |
| L | 0.1686 | 1.0000 | 0.9014 | |
| Material/Alloy | Hardness (hv 500) | Grain Size (µm) |
|---|---|---|
| Pure Mg | 33.8 | 22.42 |
| Mg-5Sn | 51.75 | 3.62 |
| Mg-5Sn-0.5Y | 46.05 | 7.21 |
| Mg-5Sn-1Y | 47.65 | 5.92 |
| Mg-5Sn-2Y | 48.23 | 3.65 |
| Material | Oblong/ Rod (A) | Polygonal (B) |
|---|---|---|
| Mg-5Sn | (93)Mg-(7)Sn | (76)Mg-(24)Sn |
| Mg-5Sn-0.5Y | (88)Mg-(6)Sn-(5)Y | (49)Mg-(26)Sn-(24)Y |
| Mg-5Sn-1Y | (71)Mg-(15)Sn-(13)Y | (55)Mg-(23)Sn-(21)Y |
| Mg-5Sn-2Y | (56)Mg-(2)Sn-(41)Y | (41)Mg-(29)Sn-(29)Y |
| Material | Ecorr (V vs. Ag/AgCl) | icorr (mAcm−2) |
| Mg-5Sn | −1.57 | 0.2 |
| Mg-5Sn-0.5Y | −1.425 | 0.04 |
| Mg-5Sn-1Y | −1.55 | 0.15 |
| Mg-5Sn-2Y | −1.51 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panemangalore, D.B.; Shabadi, R.; Gupta, M.; Lesven, L. Microstructure and Corrosion Behavior of Extruded Mg-Sn-Y Alloys. Metals 2021, 11, 1095. https://doi.org/10.3390/met11071095
Panemangalore DB, Shabadi R, Gupta M, Lesven L. Microstructure and Corrosion Behavior of Extruded Mg-Sn-Y Alloys. Metals. 2021; 11(7):1095. https://doi.org/10.3390/met11071095
Chicago/Turabian StylePanemangalore, Devadas Bhat, Rajashekhara Shabadi, Manoj Gupta, and Ludovic Lesven. 2021. "Microstructure and Corrosion Behavior of Extruded Mg-Sn-Y Alloys" Metals 11, no. 7: 1095. https://doi.org/10.3390/met11071095
APA StylePanemangalore, D. B., Shabadi, R., Gupta, M., & Lesven, L. (2021). Microstructure and Corrosion Behavior of Extruded Mg-Sn-Y Alloys. Metals, 11(7), 1095. https://doi.org/10.3390/met11071095









