Role of the Jet Angle, Particle Size, and Particle Concentration in the Degradation Behavior of Carbon Steel under Slow Slurry Erosion-Corrosion Conditions
Abstract
:1. Introduction
2. Experimental Part
2.1. Metallic Substrate
2.2. Corrosive Solution and Abradant
2.3. Electrochemical Parameters
2.4. Flow-Corrosion and Erosion-Corrosion Experiments
3. Results and Discussions
3.1. Flow Corrosion Rate
3.2. EC Rate versus Impact Angle and Particle Velocity
3.3. Particle Size
3.4. Particles Concentration
3.5. Erosion Corrosion Morphologies
- (i)
- (ii)
- the cleaning process before the weighing (including but not limited to oxide removal);
- (iii)
- the analysis scale used in this work.
4. Conclusions
- -
- The electrochemical reactivity of carbon steel only depends on the solution flow. In aerated solution, experiments operated under jet conditions presented more anodic behavior and higher cathodic currents due to the higher contribution of the dissolved oxygen reduction. When particles were added to the jet flow, no significant modifications of the electrochemical behavior of SM400B were recorded, while Tafel fits analysis reported different flow corrosion rates.
- -
- Maximum erosion-corrosion rate was found for a jet angle at 45°, whereas a minimum of EC was recorded with a jet angle of 90° (angle range between 30° and 90°). An increase in particle percentage in solution led to a straight raise in the mass loss. A slowdown of EC was recorded because of the high solution density that impeded the particle’s impingement and modified the flow dynamic at the sample surface. The concentration threshold value (about 100 g/L for the particle size of 181 µm) was determined using Equation (4).
- -
- An increase in the particle size promoted the EC rate. A linear trend was detected with fixed particle mass, while an exponential growth was found for a set number of particles in the solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Z.B.; Zheng, Y.G.; Zhou, X.; He, S.Y.; Sun, W.H.; Wang, J.Q. Determination of the critical flow velocities for erosion–corrosion of passive materials under impingement by NaCl solution containing sand. Corros. Sci. 2014, 88, 187–196. [Google Scholar] [CrossRef]
- Watson, S.W.; Friedersdorf, F.J.; Madsen, B.W.; Cramer, S.D. Methods of measuring wear-corrosion synergism. Wear 1995, 181, 476–484. [Google Scholar] [CrossRef]
- Alam, T.; Islam, M.A.; Farhat, Z.N. Slurry Erosion of Pipeline Steel: Effect of Velocity and Microstructure. J. Tribol. 2016, 138. [Google Scholar] [CrossRef]
- Buszko, M.H.; Krella, A.K. Slurry Erosion–Design of Test Devices. Adv. Mater. Sci. 2017, 17, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Truscott, G.F. A literature survey on abrasive wear in hydraulic machinery. Wear 1972, 20, 29–50. [Google Scholar] [CrossRef]
- Javaheri, V.; Porter, D.; Kuokkala, V.-T. Slurry erosion of steel–Review of tests, mechanisms and materials. Wear 2018, 408–409, 248–273. [Google Scholar] [CrossRef]
- Desale, G.R.; Gandhi, B.K.; Jain, S.C. Effect of erodent properties on erosion wear of ductile type materials. Wear 2006, 261, 914–921. [Google Scholar] [CrossRef]
- Wood, R.J.K.; Jones, T.F.; Ganeshalingam, J.; Miles, N.J. Comparison of predicted and experimental erosion estimates in slurry ducts. Wear 2004, 256, 937–947. [Google Scholar] [CrossRef]
- Aguirre, J.; Walczak, M.; Rohwerder, M. The mechanism of erosion-corrosion of API X65 steel under turbulent slurry flow: Effect of nominal flow velocity and oxygen content. Wear 2019, 438–439, 203053. [Google Scholar] [CrossRef]
- Zhang, Y.; Reuterfors, E.P.; McLaury, B.S.; Shirazi, S.A.; Rybicki, E.F. Comparison of computed and measured particle velocities and erosion in water and air flows. Wear 2007, 263, 330–338. [Google Scholar] [CrossRef]
- Peng, W.; Cao, X. Numerical simulation of solid particle erosion in pipe bends for liquid–solid flow. Powder Technol. 2016, 294, 266–279. [Google Scholar] [CrossRef]
- Owen, J.; Ducker, E.; Huggan, M.; Ramsey, C.; Neville, A.; Barker, R. Design of an elbow for integrated gravimetric, electrochemical and acoustic emission measurements in erosion-corrosion pipe flow environments. Wear 2019, 428–429, 76–84. [Google Scholar] [CrossRef]
- Levy, A.V.; Chik, P. The effects of erodent composition and shape on the erosion of steel. Wear 1983, 89, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.L.; Dewangan, S.K.; Sharma, A. A review on particulate slurry erosive wear of industrial materials: In context with pipeline transportation of mineral−slurry. Part. Sci. Technol. 2017, 35, 103–118. [Google Scholar] [CrossRef]
- Kesana, N.R.; Throneberry, J.M.; McLaury, B.S.; Shirazi, S.A.; Rybicki, E.F. Effect of Particle Size and Liquid Viscosity on Erosion in Annular and Slug Flow. J. Energy Resour. Technol. 2014, 136. [Google Scholar] [CrossRef]
- Kowsari, K.; James, D.F.; Papini, M.; Spelt, J.K. The effects of dilute polymer solution elasticity and viscosity on abrasive slurry jet micro-machining of glass. Wear 2014, 309, 112–119. [Google Scholar] [CrossRef]
- Levin, M.; Wiklund, P.; Leygraf, C. Bioorganic compounds as copper corrosion inhibitors in hydrocarbon media. Corros. Sci. 2012, 58, 104–114. [Google Scholar] [CrossRef]
- Tilly, G.P. A two stage mechanism of ductile erosion. Wear 1973, 23, 87–96. [Google Scholar] [CrossRef]
- Islam, M.A.; Farhat, Z.N. Effect of impact angle and velocity on erosion of API X42 pipeline steel under high abrasive feed rate. Wear 2014, 311, 180–190. [Google Scholar] [CrossRef]
- Clark, H.M.; Wong, K.K. Impact angle, particle energy and mass loss in erosion by dilute slurries. Wear 1995, 186, 454–464. [Google Scholar] [CrossRef]
- Smith, A.J.; Stratmann, M.; Hassel, A.W. Investigation of the effect of impingement angle on tribocorrosion using single impacts. Electrochim. Acta 2006, 51, 6521–6526. [Google Scholar] [CrossRef]
- Chen, Z.X.; Hu, H.X.; Zheng, Y.G.; Guo, X.M. Effect of groove microstructure on slurry erosion in the liquid-solid two-phase flow. Wear 2021, 466, 203561. [Google Scholar] [CrossRef]
- Hogg, R. A Spheroid Model for the Role of Shape in Particle Size Analysis. KONA Powder Part. J. 2015, 32, 227–235. [Google Scholar] [CrossRef] [Green Version]
- López, D.M.; Falleiros, N.A.; Tschiptschin, A.P. Use of Electrochemical Tests for Assessment of the Effect of Erosive Particle Size on the Erosion-Corrosion Behaviour of AISI 304L Austenitic Stainless Steel. Mater. Res. 2016, 19, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Zhang, G.A.; Guo, X.P. Erosion–corrosion at different locations of X65 carbon steel elbow. Corros. Sci. 2014, 85, 318–330. [Google Scholar] [CrossRef]
- Liu, X.; MacDonald, D.D.; Wang, M.; Xu, Y. Effect of dissolved oxygen, temperature, and pH on polarization behavior of carbon steel in simulated concrete pore solution. Electrochim. Acta 2021, 366, 137437. [Google Scholar] [CrossRef]
- Bateni, M.R.; Szpunar, J.A.; Wang, X.; Li, D.Y. Wear and corrosion wear of medium carbon steel and 304 stainless steel. Wear 2006, 260, 116–122. [Google Scholar] [CrossRef]
- Romaine, A.; Crozet, M.; Mary, N.; Normand, B.; Chassagne, M.; Dufour, F. Importance of the surface and environmental conditions on the corrosion behavior of brass, steel and brass coated steel wires and brass coated steel cords. Corros. Sci. 2020, 177, 108966. [Google Scholar] [CrossRef]
- Kapałka, A.; Fóti, G.; Comninellis, C. Determination of the Tafel slope for oxygen evolution on boron-doped diamond electrodes. Electrochem. Commun. 2008, 10, 607–610. [Google Scholar] [CrossRef]
- Babić, R.; Metikoš-Huković, M. Oxygen reduction on stainless steel. J. Appl. Electrochem. 1993, 23, 352–357. [Google Scholar] [CrossRef]
- Bockris, J.O.; Drazic, D.; Despic, A.R. The electrode kinetics of the deposition and dissolution of iron. Electrochim. Acta 1961, 4, 325–361. [Google Scholar] [CrossRef]
- Foroulis, Z.A. The Kinetics of Anodic Dissolution of Iron in High Purity Water. Corros. Eng. 1979, 28, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Stern, M.; Weisert, E.D. Experimental observations on the relation between polarization resistance and corrosion rate. Proc. Am. Soc. Test. Mater. 1959, 18, 1280. [Google Scholar]
- Mansfeld, F.; Oldham, K.B. A modification of the Stern—Geary linear polarization equation. Corros. Sci. 1971, 11, 787–796. [Google Scholar] [CrossRef]
- Angst, U.; Büchler, M. On the applicability of the Stern–Geary relationship to determine instantaneous corrosion rates in macro-cell corrosion. Mater. Corros. 2015, 66, 1017–1028. [Google Scholar] [CrossRef]
- García-Galvan, F.R.; Fajardo, S.; Barranco, V.; Feliu, S. Experimental Apparent Stern–Geary Coefficients for AZ31B Mg Alloy in Physiological Body Fluids for Accurate Corrosion Rate Determination. Metals 2021, 11, 391. [Google Scholar] [CrossRef]
- Hloch, S.; Srivastava, M.; Nag, A.; Müller, M.; Hromasová, M.; Svobodová, J.; Kruml, T.; Chlupová, A. Effect of pressure of pulsating water jet moving along stair trajectory on erosion depth, surface morphology and microhardness. Wear 2020, 452, 203278. [Google Scholar] [CrossRef]
- Hojo, H.; Tsuda, K.; Yabu, T. Erosion damage of polymeric material by slurry. Wear 1986, 112, 17–28. [Google Scholar] [CrossRef]
- Clark, H.M. The influence of the flow field in slurry erosion. Wear 1992, 152, 223–240. [Google Scholar] [CrossRef]
- Niu, C.-S.T.Y. Simulation of Erosion by the Dilute Particulate Flow Impact. Numer. Heat Transf. Part Appl. 2000, 37, 167–187. [Google Scholar] [CrossRef]
- Fan, J.M.; Fan, C.M.; Wang, J. Flow Dynamic Simulation of Micro Abrasive Water Jet. Solid State Phenom. 2011, 175, 171–176. [Google Scholar] [CrossRef]
- Madsen, B.W. Measurement of erosion-corrosion synergism with a slurry wear test apparatus. Wear 1988, 123, 127–142. [Google Scholar] [CrossRef]
- Al-Bukhaiti, M.A.; Ahmed, S.M.; Badran, F.M.F.; Emara, K.M. Effect of impingement angle on slurry erosion behaviour and mechanisms of 1017 steel and high-chromium white cast iron. Wear 2007, 262, 1187–1198. [Google Scholar] [CrossRef]
- Wood, R.J.K. Erosion–corrosion interactions and their effect on marine and offshore materials. Wear 2006, 261, 1012–1023. [Google Scholar] [CrossRef]
- Haugen, K.; Kvernvold, O.; Ronold, A.; Sandberg, R. Sand erosion of wear-resistant materials: Erosion in choke valves. Wear 1995, 186, 179–188. [Google Scholar] [CrossRef]
- Rajahram, S.S.; Harvey, T.J.; Wood, R.J.K. Electrochemical investigation of erosion–corrosion using a slurry pot erosion tester. Tribol. Int. 2011, 44, 232–240. [Google Scholar] [CrossRef]
- Liebhard, M.; Levy, A. The effect of erodent particle characteristics on the erosion of metals. Wear 1991, 151, 381–390. [Google Scholar] [CrossRef]
- Torrance, A.A. An explanation of the hardness differential needed for abrasion. Wear 1981, 68, 263–266. [Google Scholar] [CrossRef]
- Finnie, I. Erosion of surfaces by solid particles. Wear 1960, 3, 87–103. [Google Scholar] [CrossRef]
- Finnie, I.; McFadden, D.H. On the velocity dependence of the erosion of ductile metals by solid particles at low angles of incidence. Wear 1978, 48, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Divakar, M.; Agarwal, V.K.; Singh, S.N. Effect of the material surface hardness on the erosion of AISI316. Wear 2005, 259, 110–117. [Google Scholar] [CrossRef]
- Gadhikar, A.A.; Sharma, A.; Goel, D.B.; Sharma, C.P. Effect of carbides on erosion resistance of 23–8-N steel. Bull. Mater. Sci. 2014, 37, 315–319. [Google Scholar] [CrossRef]
- McCabe, L.P.; Sargent, G.A.; Conrad, H. Effect of microstructure on the erosion of steel by solid particles. Wear 1985, 105, 257–277. [Google Scholar] [CrossRef]
- Desale, G.R.; Gandhi, B.K.; Jain, S.C. Particle size effects on the slurry erosion of aluminium alloy (AA 6063). Wear 2009, 266, 1066–1071. [Google Scholar] [CrossRef]
- Iwai, Y.; Nambu, K. Slurry wear properties of pump lining materials. Wear 1997, 210, 211–219. [Google Scholar] [CrossRef]
- Lynn, R.S.; Wong, K.K.; Clark, H.M. On the particle size effect in slurry erosion. Wear 1991, 149, 55–71. [Google Scholar] [CrossRef]
- Gandhi, B.K.; Singh, S.N.; Seshadri, V. Study of the parametric dependence of erosion wear for the parallel flow of solid–liquid mixtures. Tribol. Int. 1999, 32, 275–282. [Google Scholar] [CrossRef]
- Zitoun, K.B.; Sastry, S.K.; Guezennec, Y. Investigation of three dimensional interstitial velocity, solids motion, and orientation in solid–liquid flow using particle tracking velocimetry. Int. J. Multiph. Flow. 2001, 27, 1397–1414. [Google Scholar] [CrossRef]
- Badr, H.M.; Habib, M.A.; Ben-Mansour, R.; Said, S.A.M. Numerical investigation of erosion threshold velocity in a pipe with sudden contraction. Comput. Fluids 2005, 34, 721–742. [Google Scholar] [CrossRef]
- Garg, V.K.; Jayaraj, S. Boundary Layer Analysis for Two-Dimensional Slot Jet Impingement on Inclined Plates. J. Heat Transf. 1988, 110, 577–582. [Google Scholar] [CrossRef]
- Humphrey, J.A.C. Fundamentals of fluid motion in erosion by solid particle impact. Int. J. Heat Fluid Flow 1990, 11, 170–195. [Google Scholar] [CrossRef]
- Benchaita, M.T.; Griffith, P.; Rabinowicz, E. Erosion of Metallic Plate by Solid Particles Entrained in a Liquid Jet. J. Eng. Ind. 1983, 105, 215–222. [Google Scholar] [CrossRef]
- Shademan, M.; Balachandar, R.; Roussinova, V.; Barron, R. Round impinging jets with relatively large stand-off distance. Phys. Fluids 2016, 28, 075107. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. International Union of Pure and Applied Chemistry. In Standard Potentials in Aqueous Solution, 1st ed.; Dekker, M., Ed.; New York: New York, NY, USA, 1985. [Google Scholar]
- Tuzson, J.J. Laboratory Slurry Erosion Tests and Pump Wear Rate Calculations. J. Fluids Eng. 1984, 106, 135–140. [Google Scholar] [CrossRef]
- Roco, M.C.; Nair, P.; Addie, G.R. Test Approach for Dense Slurry Erosion, Slurry Eros. Uses Appl. Test Methods 1987. [Google Scholar] [CrossRef]
- Blatt, W.; Kohley, T.; Lotz, U.; Heitz, E. The Influence of Hydrodynamics on Erosion-Corrosion in Two-Phase Liquid-Particle Flow. Corrosion 1989, 45, 793–804. [Google Scholar] [CrossRef]
- Elvery, D.G.; Bremhorst, K. Wall Pressure and Effective Wall Shear Stresses in Heat Exchanger Tube Inlets With Application to Erosion-Corrosion. J. Fluids Eng. 1997, 119, 948–953. [Google Scholar] [CrossRef]
- Krella, A.K.; Zakrzewska, D.E.; Buszko, M.H.; Marchewicz, A. Effect of Thermal Treatment and Erosion Aggressiveness on Resistance of S235JR Steel to Cavitation and Slurry. Materials 2021, 14, 1456. [Google Scholar] [CrossRef]
- Dalbert, V.; Mary, N.; Normand, B.; Verdu, C.; Douillard, T.; Saedlou, S. The effects of microstructures and repassivation kinetics on the tribocorrosion resistance of ferrite and ferrite-martensite stainless steels. Wear 2019, 420–421, 245–256. [Google Scholar] [CrossRef]
- Rodríguez, E.; Flores, M.; Pérez, A.; Mercado-Solis, R.D.; González, R.; Rodríguez, J.; Valtierra, S. Erosive wear by silica sand on AISI H13 and 4140 steels. Wear 2009, 267, 2109–2115. [Google Scholar] [CrossRef]
- Ojala, N.; Valtonen, K.; Antikainen, A.; Kemppainen, A.; Minkkinen, J.; Oja, O.; Kuokkala, V.-T. Wear performance of quenched wear resistant steels in abrasive slurry erosion. Wear 2016, 354–355, 21–31. [Google Scholar] [CrossRef]
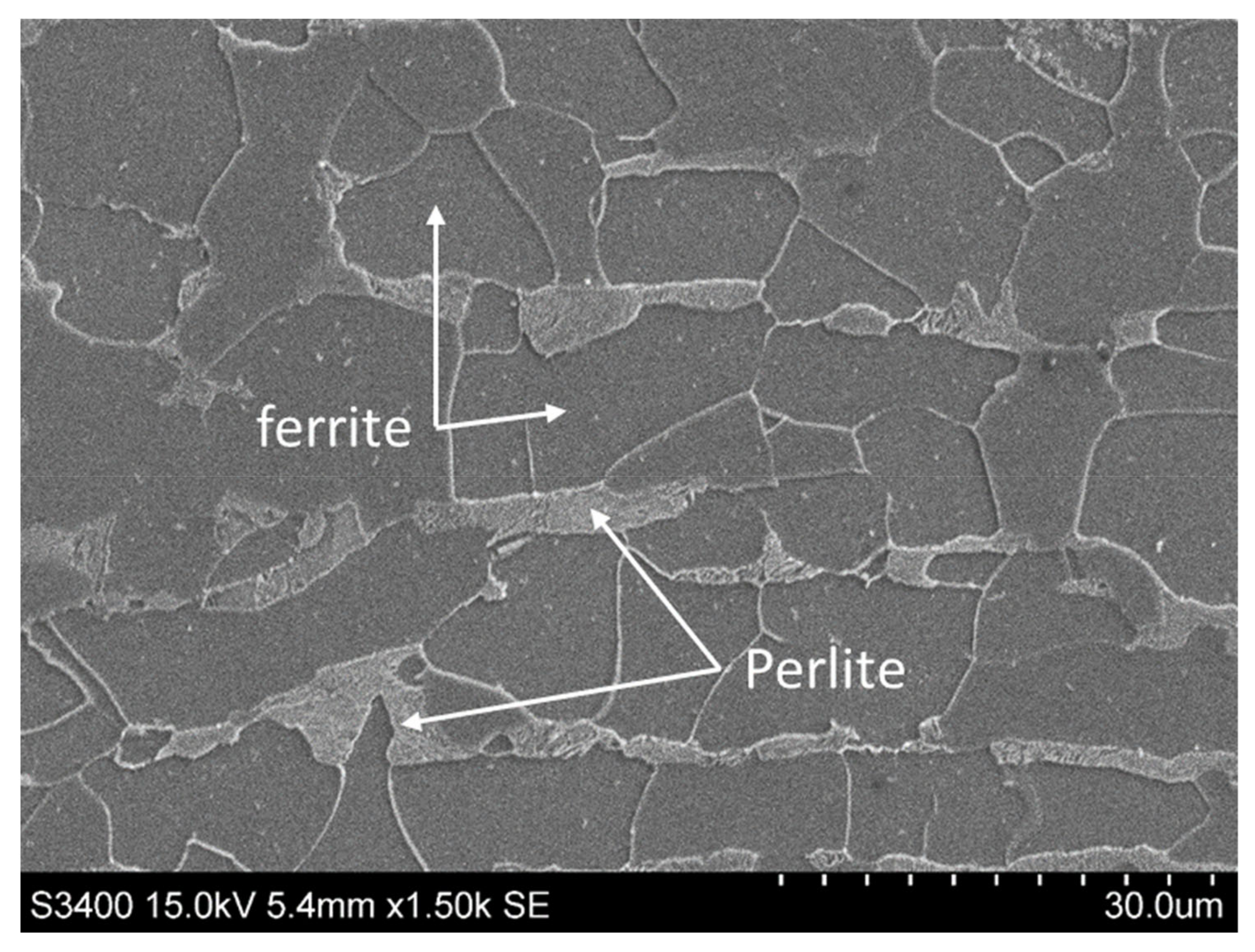















| Elements | C | Si | Mn | Cr | P | S | Fe |
|---|---|---|---|---|---|---|---|
| sample | 0.14 | 0.21 | 0.85 | 0.02 | 0.012 | 0.006 | Bal. |
| Standard | <0.2 | <0.35 | 0.6–1.4 | Not specified | <0.035 | <0.035 |
| Sample Condition (90° + 45°) | Erosion-Corrosion Rate by Electrochemical Measurements | Weighing | |||||
|---|---|---|---|---|---|---|---|
| Ecorr V/MSE | Jcorr µA/cm2 | βc mV/dec | βa mV/dec | Corrosion Rate mm/year | Mass Loss mg × cm−2 × h−1 | Mass Loss mg × cm−2 × h−1 | |
| 0 m × s−1 (90°, 0 g × L−1) | −1.09 | 2 | 115 | 92 | 0.02 | 0.02 | Not detectable |
| 2.6 m × s−1 (90°, 0 g × L−1) | −0.86 | 88 | 297 | 171 | 1.15 | 0.08 | 0.29 |
| 4.8 m × s−1 (90°, 0 g × L−1) | −0.84 | 137 | 271 | 190 | 1.65 | 0.14 | 0.39 |
| 181 µm (90°, 10 g × L−1) | −0.88 | 156 | 260 | 186 | 1.87 | 0.16 | 0.64 |
| 219 µm (90°, 10 g × L−1) | −0.87 | 181 | 322 | 234 | 2.18 | 0.18 | 0.83 |
| Jet Angle (°) | Erosion Corrosion Rate | Wmax (mg × cm−2 × h−1) | C1/2 (g/L) | R (Coefficient of Correlation) |
|---|---|---|---|---|
| 90 | 10 ± 3 | 129 ± 20 | 0.96 | |
| 45 | 3D profile | 12 ± 2 | 95 ± 20 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasse, C.; Mary, N.; Abe, H.; Watanabe, Y.; Normand, B. Role of the Jet Angle, Particle Size, and Particle Concentration in the Degradation Behavior of Carbon Steel under Slow Slurry Erosion-Corrosion Conditions. Metals 2021, 11, 1152. https://doi.org/10.3390/met11081152
Rasse C, Mary N, Abe H, Watanabe Y, Normand B. Role of the Jet Angle, Particle Size, and Particle Concentration in the Degradation Behavior of Carbon Steel under Slow Slurry Erosion-Corrosion Conditions. Metals. 2021; 11(8):1152. https://doi.org/10.3390/met11081152
Chicago/Turabian StyleRasse, Charles, Nicolas Mary, Hiroshi Abe, Yutaka Watanabe, and Bernard Normand. 2021. "Role of the Jet Angle, Particle Size, and Particle Concentration in the Degradation Behavior of Carbon Steel under Slow Slurry Erosion-Corrosion Conditions" Metals 11, no. 8: 1152. https://doi.org/10.3390/met11081152
APA StyleRasse, C., Mary, N., Abe, H., Watanabe, Y., & Normand, B. (2021). Role of the Jet Angle, Particle Size, and Particle Concentration in the Degradation Behavior of Carbon Steel under Slow Slurry Erosion-Corrosion Conditions. Metals, 11(8), 1152. https://doi.org/10.3390/met11081152








