In-Situ Hollow Sample Setup Design for Mechanical Characterisation of Gaseous Hydrogen Embrittlement of Pipeline Steels and Welds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In-Situ Gaseous H2 Setup Design and Testing
2.2.1. Setup Design
2.2.2. In-Situ H2 Test Procedure
2.3. Modelling Support
2.3.1. Modelling H Diffusion
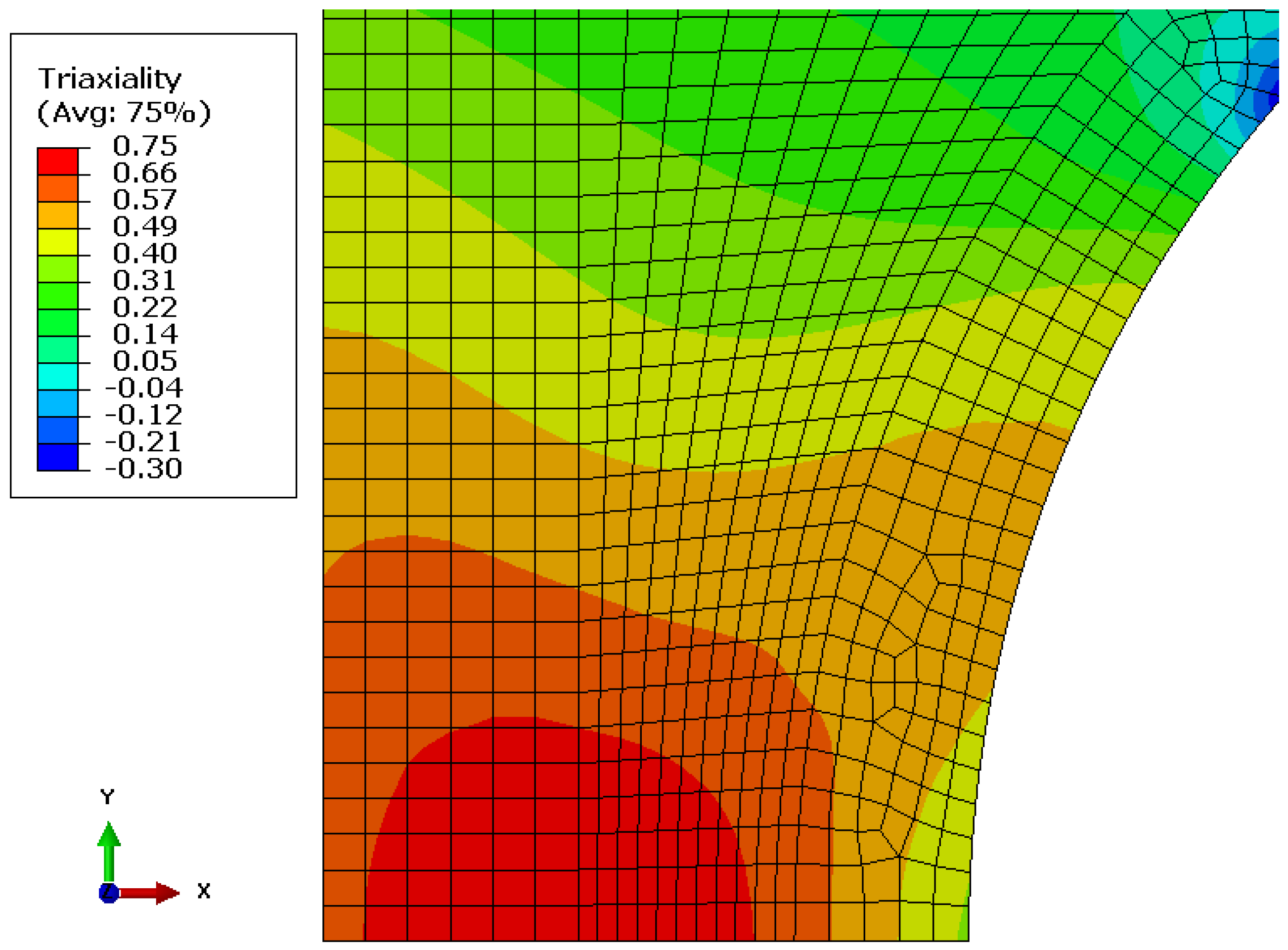
2.3.2. Modelling Deformation and Stress Distribution in the Notch
2.4. Data Representation
3. Results and Discussion
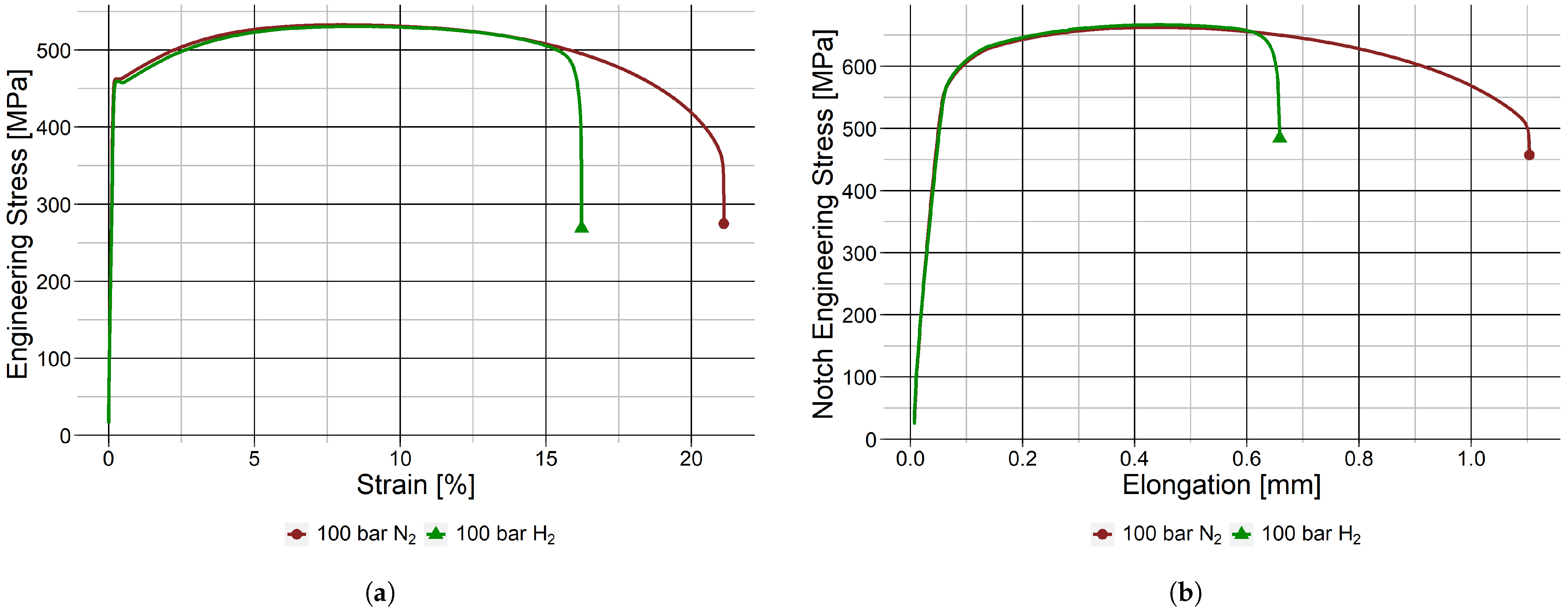
3.1. HE in the Base Metal
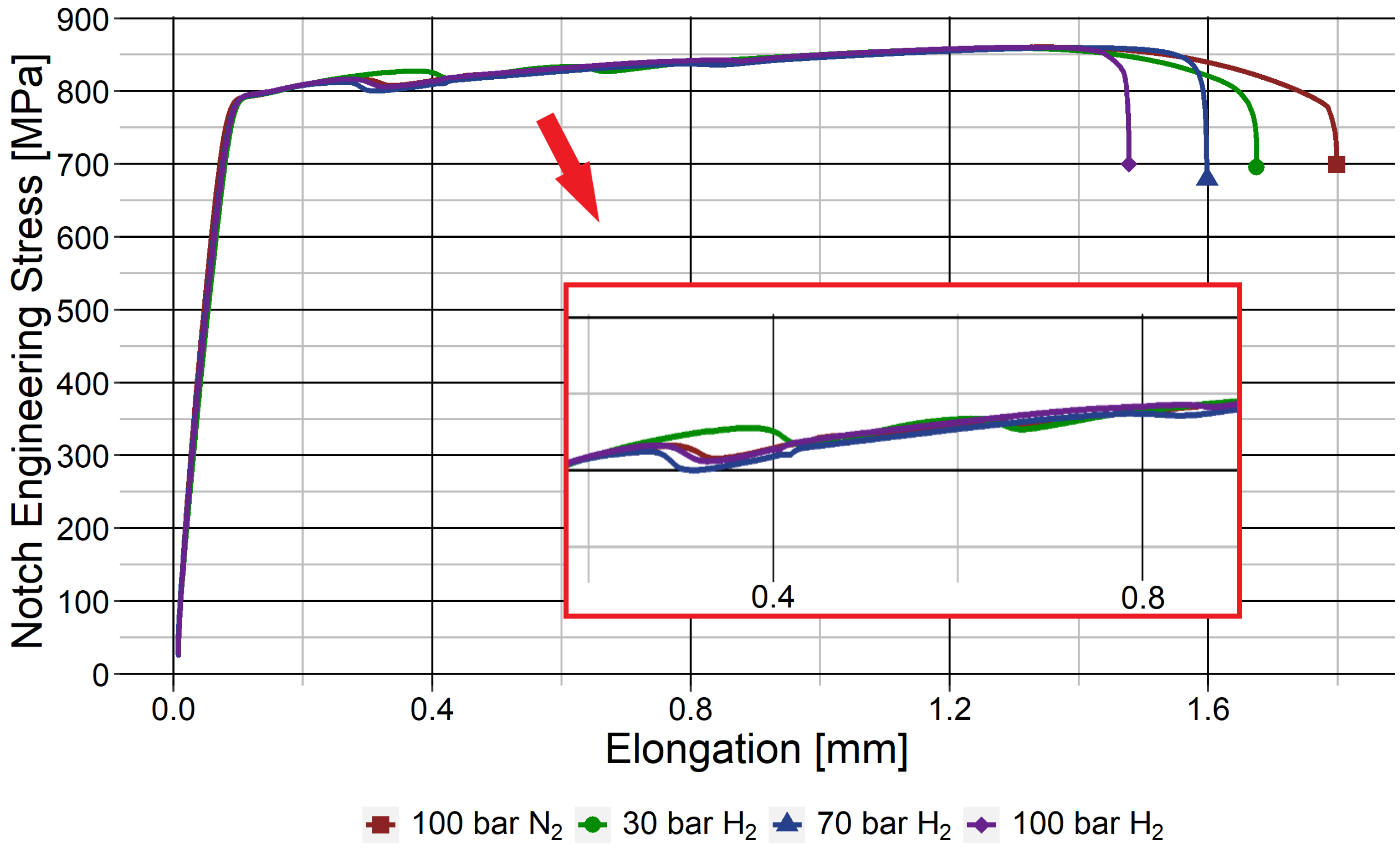
3.2. HE in the Weld Metal
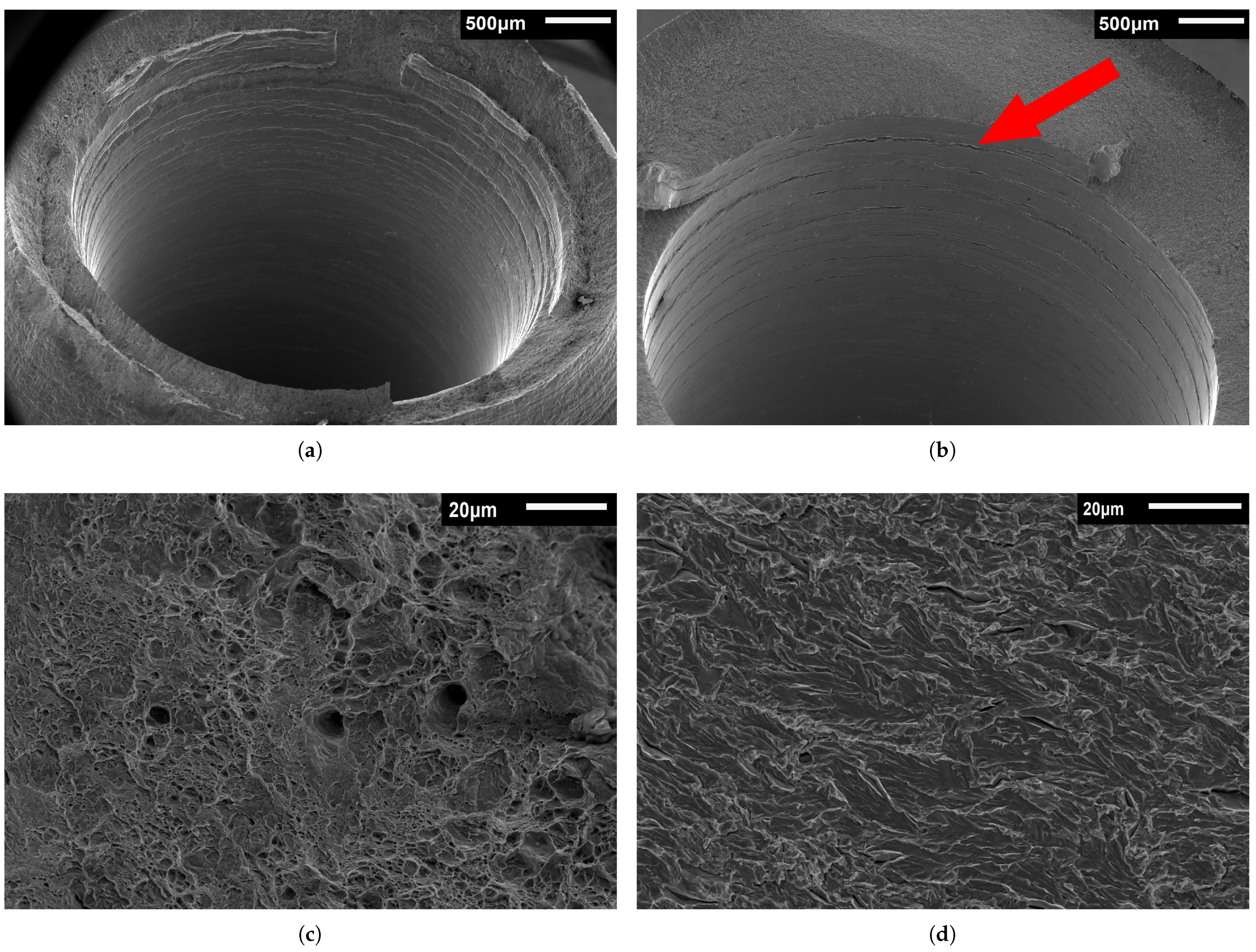
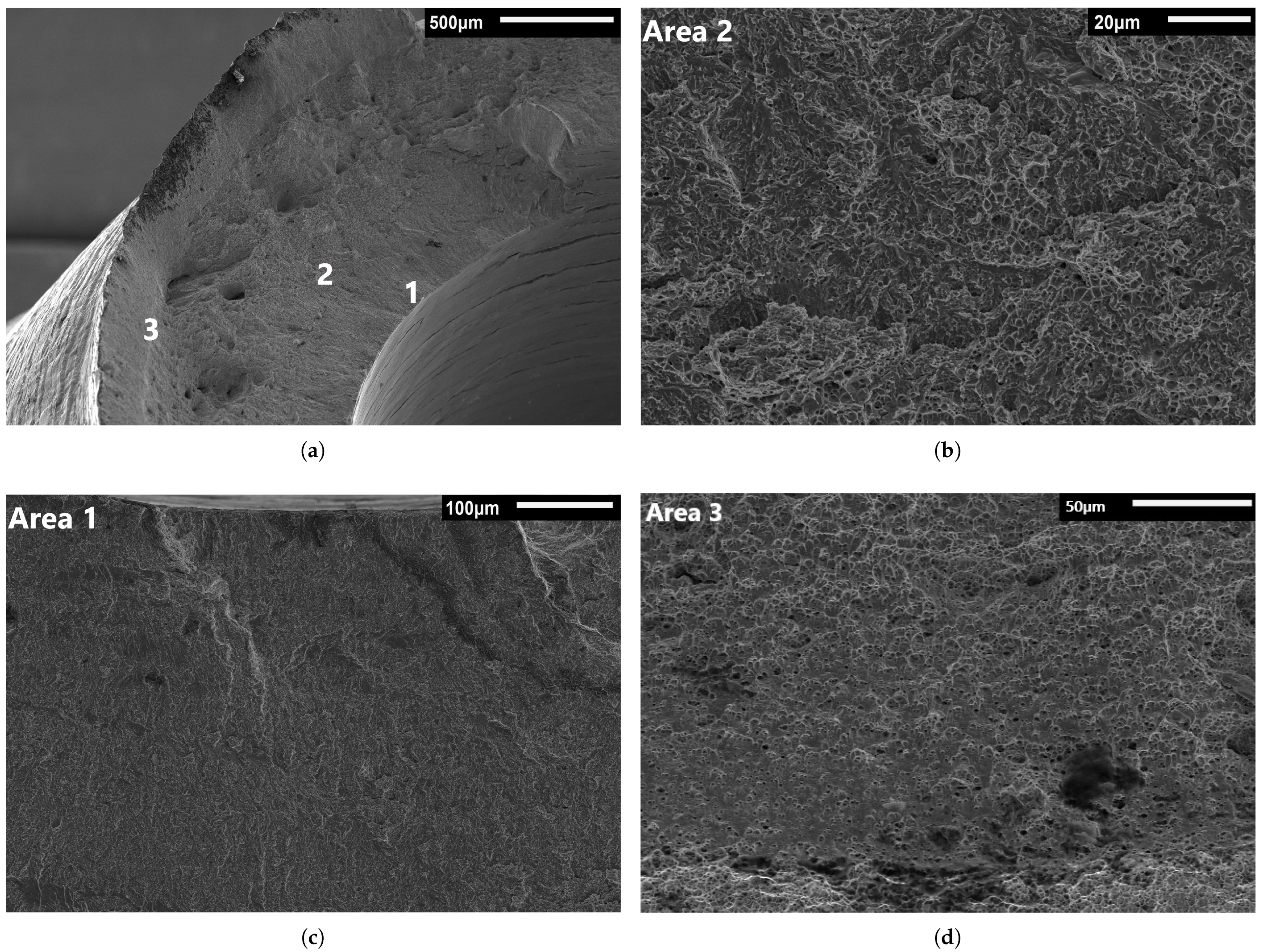
3.3. Fractography
3.3.1. Base Metal Fractography
3.3.2. Weld Metal Fractography
3.4. Comparison between Base Metal and Weld Metal Susceptibility
4. Recommendations
5. Conclusions
- 1.
- The test setup is capable of loading steels with hydrogen by exposing them to a constant pressure of hydrogen gas for an extended duration. By releasing just 6 ml of pressurised gas upon fracture of the sample, steel specimens can be safely laboratory tested until failure.
- 2.
- The test setup allows for characterisation of the HE susceptibility of specific regions of a material by selective notching, which was demonstrated here with the weld zone.
- 3.
- Both API 5L X60 pipeline steel and its girth weld were found to be susceptible to HE at 100 barg of H2 pressure and plastic strains exceeding 15%. This was supported by fractographic analysis showing the emergence of the quasi-cleavage (QC) fracture mode which is characteristic for steels embrittled by hydrogen. The strength of all specimens remained unaffected.
- 4.
- The WM was less susceptible to HE because of its refined microstructure and resulting high grain boundary density, showing an embrittlement index I of 11% compared to 28% for the BM. The extent of embrittlement in the WM was unaffected by a change in H2 pressure from 30 to 100 barg.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Maroufmashat, A.; Fowler, M. Transition of future energy system infrastructure; through power-to-gas pathways. Energies 2017, 10, 1089. [Google Scholar] [CrossRef]
- Song, E.J.; Baek, S.W.; Nahm, S.H.; Baek, U.B. Notched-tensile properties under high-pressure gaseous hydrogen: Comparison of pipeline steel X70 and austenitic stainless type 304 L, 316 L steels. Int. J. Hydrog. Energy 2017, 42, 8075–8082. [Google Scholar] [CrossRef]
- Nanninga, N.; Levy, Y.; Drexler, E.; Condon, R.; Stevenson, A.; Slifka, A. Comparison of hydrogen embrittlement in three pipeline steels in high pressure gaseous hydrogen environments. Corros. Sci. 2012, 59, 1–9. [Google Scholar] [CrossRef]
- Briottet, L.; Batisse, R.; de Dinechin, G.; Langlois, P.; Thiers, L. Recommendations on X80 steel for the design of hydrogen gas transmission pipelines. Int. J. Hydrog. Energy 2012, 37, 9423–9430. [Google Scholar] [CrossRef]
- Depover, T.; Vercruysse, F.; Elmahdy, A.; Verleysen, P.; Verbeken, K. Evaluation of the hydrogen embrittlement susceptibility in DP steel under static and dynamic tensile conditions. Int. J. Impact Eng. 2019, 123, 118–125. [Google Scholar] [CrossRef]
- Djukic, M.B.; Sijacki Zeravcic, V.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Djukic, M.; Zeravcic, V.S.; Bakic, G.; Sedmak, A.; Rajicic, B. Hydrogen Embrittlement of Low Carbon Structural Steel. Procedia Mater. Sci. 2014, 3, 1167–1172. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. Hydrogen embrittlement of industrial components: Prediction, prevention, and models. Corrosion 2016, 72, 943–961. [Google Scholar] [CrossRef]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Crosshead speed dependence of the notch tensile strength of a high strength steel in the presence of hydrogen. Scr. Mater. 2005, 53, 713–718. [Google Scholar] [CrossRef]
- Moro, I.; Briottet, L.; Lemoine, P.; Andrieu, E.; Blanc, C.; Odemer, G. Hydrogen embrittlement susceptibility of a high strength steel X80. Mater. Sci. Eng. A 2010, 527, 7252–7260. [Google Scholar] [CrossRef]
- Zhao, Y.; Seok, M.Y.; Choi, I.C.; Lee, Y.H.; Park, S.J.; Ramamurty, U.; Suh, J.Y.; Jang, J.I. The role of hydrogen in hardening/softening steel: Influence of the charging process. Scr. Mater. 2015, 107, 46–49. [Google Scholar] [CrossRef]
- Rahman, K.M.; Mohtadi-Bonab, M.A.; Ouellet, R.; Szpunar, J. A Comparative Study of the Role of Hydrogen on Degradation of the Mechanical Properties of API X60, X60SS, and X70 Pipeline Steels. Steel Res. Int. 2019, 90, 1900078. [Google Scholar] [CrossRef]
- Liang, P.; Li, X.; Du, C.; Chen, X. Stress corrosion cracking of X80 pipeline steel in simulated alkaline soil solution. Mater. Des. 2009, 30, 1712–1717. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.; Szpunar, J.; Razavi-Tousi, S. A comparative study of hydrogen induced cracking behavior in API 5L X60 and X70 pipeline steels. Eng. Fail. Anal. 2013, 33, 163–175. [Google Scholar] [CrossRef]
- Huang, F.; Liu, J.; Deng, Z.J.; Cheng, J.H.; Lu, Z.H.; Li, X.G. Effect of microstructure and inclusions on hydrogen induced cracking susceptibility and hydrogen trapping efficiency of X120 pipeline steel. Mater. Sci. Eng. A 2010, 527, 6997–7001. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Szpunar, J.A.; Collins, L.; Stankievech, R. Evaluation of hydrogen induced cracking behavior of API X70 pipeline steel at different heat treatments. Int. J. Hydrog. Energy 2014, 39, 6076–6088. [Google Scholar] [CrossRef]
- Sieverts, A. Die aufnahme von gasen durch metalle. Z. Met. 1929, 21, 37–46. [Google Scholar]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Metall. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Pérez Escobar, D.; Wallaert, E.; Duprez, L.; Atrens, A.; Verbeken, K. Thermal desorption spectroscopy study of the interaction of hydrogen with TiC precipitates. Met. Mater. Int. 2013, 19, 741–748. [Google Scholar] [CrossRef]
- Park, G.T.; Koh, S.U.; Jung, H.G.; Kim, K.Y. Effect of microstructure on the hydrogen trapping efficiency and hydrogen induced cracking of linepipe steel. Corros. Sci. 2008, 50, 1865–1871. [Google Scholar] [CrossRef]
- ASTM. ASTM G142-98 Standard Test Method for Determination of Susceptibility of Metals to Embrittlement in Hydrogen Containing Environments at High Pressure, High Temperature, or Both; ASTM International: West Conshohocken, PA, USA, 2016; Volume 03.02, pp. 1–8. [Google Scholar]
- ASTM. ASTM G129-00 Standard Practice for Slow Strain Rate Testing to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking 1; ASTM International: West Conshohocken, PA, USA, 2013; Volume 03.02, pp. 1–7. [Google Scholar]
- ASTM. ASTM F1624-12 Standard Test Method for Measurement of Hydrogen Embrittlement Threshold in Steel by the Incremental Step Loading Technique; ASTM International: West Conshohocken, PA, USA, 2018; Volume 15.02, pp. 1–12. [Google Scholar]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen on the fracture behavior of high strength steel during slow strain rate test. Corros. Sci. 2007, 49, 4081–4097. [Google Scholar] [CrossRef]
- Drexler, A.; Bergmann, C.; Manke, G.; Kokotin, V.; Mraczek, K.; Pohl, M.; Ecker, W. On the local evaluation of the hydrogen susceptibility of cold-formed and heat treated advanced high strength steel (AHSS) sheets. Mater. Sci. Eng. A 2021, 800, 140276. [Google Scholar] [CrossRef]
- Michler, T.; Naumann, J.; Sattler, E. Influence of high pressure gaseous hydrogen on S–N fatigue in two austenitic stainless steels. Int. J. Fatigue 2013, 51, 1–7. [Google Scholar] [CrossRef]
- Novak, P.; Yuan, R.; Somerday, B.; Sofronis, P.; Ritchie, R. A statistical, physical-based, micro-mechanical model of hydrogen-induced intergranular fracture in steel. J. Mech. Phys. Solids 2010, 58, 206–226. [Google Scholar] [CrossRef]
- Ronevich, J.A.; Somerday, B.P. Assessing gaseous hydrogen assisted fatigue crack growth susceptibility of pipeline steel weld fusion zones and heat affected zones. Mater. Perform. Charact. 2016, 5, 290–304. [Google Scholar] [CrossRef]
- Capelle, J.; Gilgert, J.; Dmytrakh, I.; Pluvinage, G. Sensitivity of pipelines with steel API X52 to hydrogen embrittlement. Int. J. Hydrog. Energy 2008, 33, 7630–7641. [Google Scholar] [CrossRef]
- Chandler, W.; Walter, R. Testing to determine the effect of high-pressure hydrogen environments on the mechanical properties of metals. In Hydrogen Embrittlement Testing; ASTM International: West Conshohocken, PA, USA, 1974; pp. 178–179. [Google Scholar]
- Ogata, T. Evaluation of hydrogen embrittlement by internal high-pressure hydrogen environment in specimen. J. Jpn. Inst. Met. 2008, 72, 125–131. [Google Scholar] [CrossRef]
- Ogata, T. Hydrogen environment embrittlement evaluation in fatigue properties of stainless steel SUS304L at cryogenic temperatures. In Proceedings of the AIP Conference Proceedings, Tucson, AZ, USA, 28 June–2 July 2009; American Institute of Physics: Danvers, MA, USA, 2010; Volume 1219, pp. 25–32. [Google Scholar]
- Ogata, T. Influence of high pressure hydrogen environment on tensile and fatigue properties of stainless steels at low temperatures. In Proceedings of the AIP Conference Proceedings, Spokane, WA, USA, 13–17 June 2011; American Institute of Physics: Danvers, MA, USA, 2012; Volume 1435, pp. 39–46. [Google Scholar]
- Ogata, T. Influence of 70 MPa Hydrogen Gas on SUS 630 From 77 K to 373 K by Simple Testing Method. In Proceedings of the Pressure Vessels and Piping Conference, Volume 6B: Materials and Fabrication, Prague, Czech Republic, 15–20 July; American Society of Mechanical Engineers: New York, NY, USA, 2018. [Google Scholar]
- Chen, W.; Spätig, P.; Seifert, H.P. Fatigue behavior of 316L austenitic stainless steel in air and LWR environment with and without mean stress. Matec Web Conf. 2018, 165, 03012. [Google Scholar] [CrossRef][Green Version]
- Kogut, N.; Pan’ko, I. Use of a tubular sample for determination of the crack resistance of the material of pressure vessels and weld joints. Sov. Mater. Sci. 1984, 20, 55–59. [Google Scholar] [CrossRef]
- European Commission. Pressure Energy Directive. DIRECTIVE 2014/68/EU of the EUropean Parliament and of the Council of 15 May 2014 on the Harmonisation of the Laws of the Member States Relating to the Making Available on the Market of Pressure Equipment; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Boot, T.; Riemslag, T.; Reinton, E.; Liu, P.; Walters, C.L.; Popovich, V. Assessing the susceptibility of existing pipelines to Hydrogen Embrittlement. In Proceedings of the Minerals, Metals & Materials Society (eds) TMS 2021 150th Annual Meeting & Exhibition, Orlando, FL, USA, 15–18 March; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Lufrano, J.; Sofronis, P. Enhanced hydrogen concentrations ahead of rounded notches and cracks—Competition between plastic strain and hydrostatic stress. Acta Mater. 1998, 46, 1519–1526. [Google Scholar] [CrossRef]
- Najam, H.; Koyama, M.; Bal, B.; Akiyama, E.; Tsuzaki, K. Strain rate and hydrogen effects on crack growth from a notch in a Fe-high-Mn steel containing 1.1 wt% solute carbon. Int. J. Hydrog. Energy 2020, 45, 1125–1139. [Google Scholar] [CrossRef]
- Panico, M.; Baker, D.; Nawathe, S.; Bahrami, A.; Fairchild, D. Effect of testing variables on fracture toughness in sour environment. Proc. Int. Offshore Polar Eng. Conf. 2015, 25, 134–139. [Google Scholar]
- Nagumo, M. Hydrogen related failure of steels—A new aspect. Mater. Sci. Technol. 2004, 20, 940–950. [Google Scholar] [CrossRef]
- McLellan, R.B.; Xu, Z.R. Hydrogen-induced vacancies in the iron lattice. Scr. Mater. 1997, 36. [Google Scholar] [CrossRef]
- Martin, M.L.; Fenske, J.A.; Liu, G.S.; Sofronis, P.; Robertson, I.M. On the formation and nature of quasi-cleavage fracture surfaces in hydrogen embrittled steels. Acta Mater. 2011, 59, 1601–1606. [Google Scholar] [CrossRef]
- Merson, E.D.; Myagkikh, P.N.; Poluyanov, V.A.; Merson, D.L.; Vinogradov, A. Quasi-cleavage hydrogen-assisted cracking path investigation by fractographic and side surface observations. Eng. Fract. Mech. 2019, 214, 177–193. [Google Scholar] [CrossRef]
- Liu, H.; Hamada, S.; Koyama, M.; Noguchi, H. Distinguishing geometric and metallurgic hydrogen-embrittlement susceptibilities in pre-cracked structures made of interstitial-free steel under monotonic tension. Theor. Appl. Fract. Mech. 2020, 108, 102574. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Q.; Venezuela, J.; Zhang, M.; Atrens, A. Hydrogen influence on some advanced high-strength steels. Corros. Sci. 2017, 125, 114–138. [Google Scholar] [CrossRef]
- Maroef, I.; Olson, D.L.; Eberhart, M.; Edwards, G.R. Hydrogen trapping in ferritic steel weld metal. Int. Mater. Rev. 2002, 47, 191–223. [Google Scholar] [CrossRef]
- Rivera, P.C.; Ramunni, V.; Bruzzoni, P. Hydrogen trapping in an API 5L X60 steel. Corros. Sci. 2012, 54, 106–118. [Google Scholar] [CrossRef]
- Drexler, E.S.; Slifka, A.J.; Amaro, R.L.; Barbosa, N.; Lauria, D.S.; Hayden, L.E.; Stalheim, D.G. Fatigue crack growth rates of API X70 pipeline steel in a pressurized hydrogen gas environment. Fatigue Fract. Eng. Mater. Struct. 2014, 37, 517–525. [Google Scholar] [CrossRef]
- Briottet, L.; Moro, I.; Escot, M.; Furtado, J.; Bortot, P.; Tamponi, G.M.; Solin, J.; Odemer, G.; Blanc, C.; Andrieu, E. Fatigue crack initiation and growth in a CrMo steel under hydrogen pressure. Int. J. Hydrog. Energy 2015, 40, 17021–17030. [Google Scholar] [CrossRef]
- An, T.; Peng, H.; Bai, P.; Zheng, S.; Wen, X.; Zhang, L. Influence of hydrogen pressure on fatigue properties of X80 pipeline steel. Int. J. Hydrog. Energy 2017, 42, 15669–15678. [Google Scholar] [CrossRef]
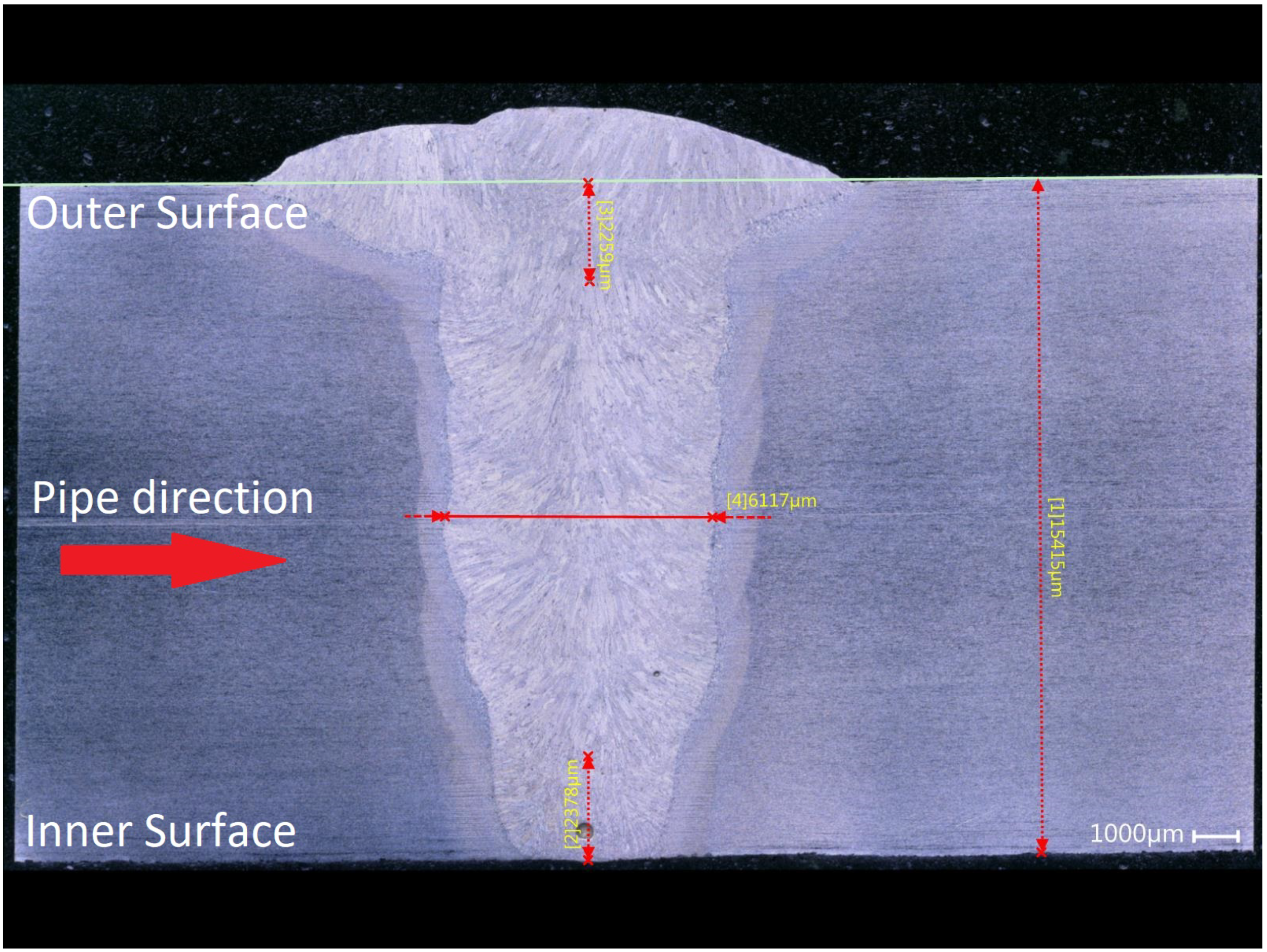
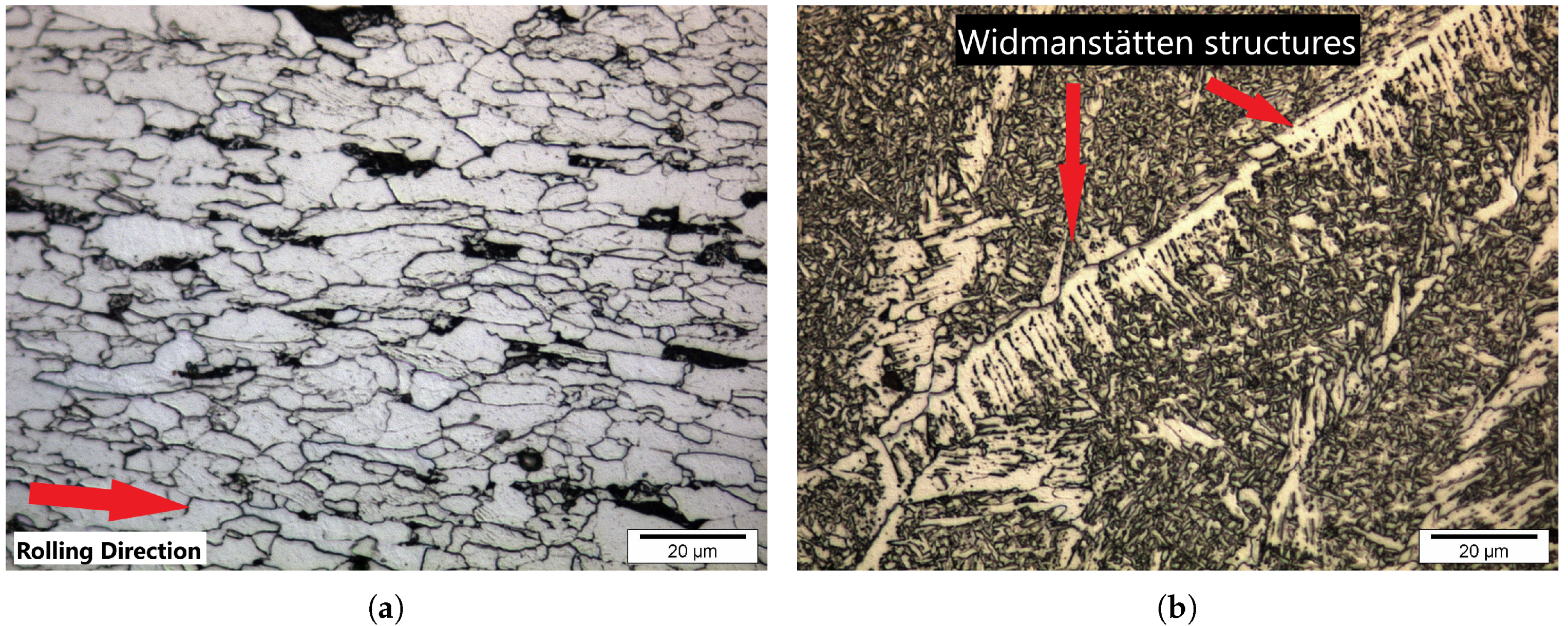
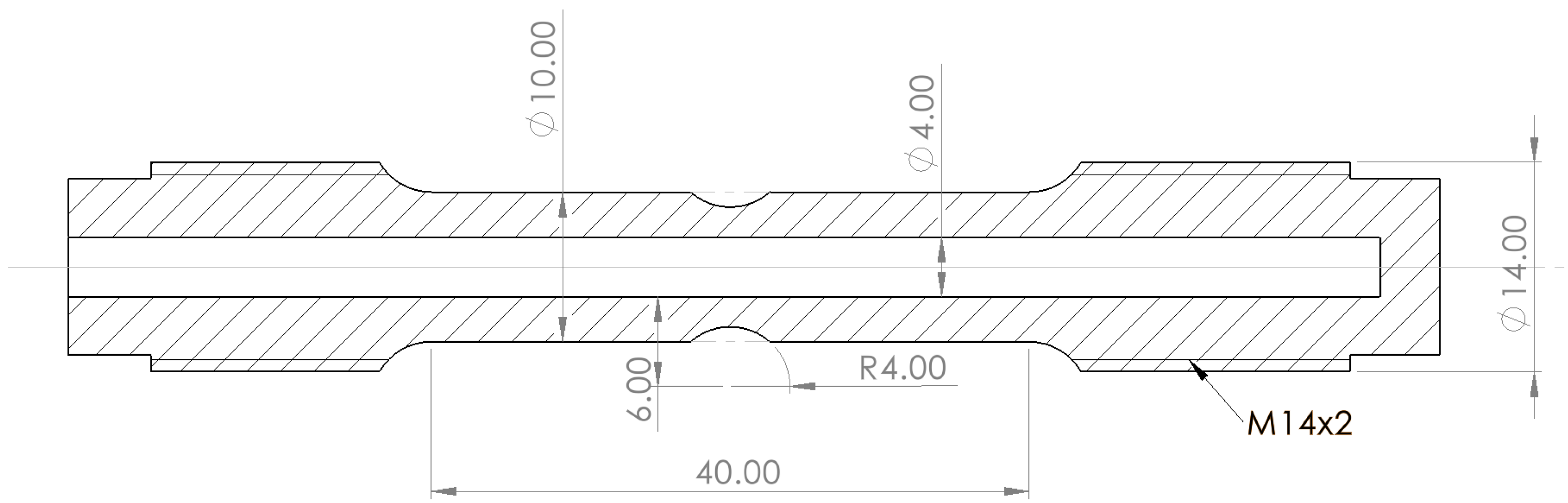
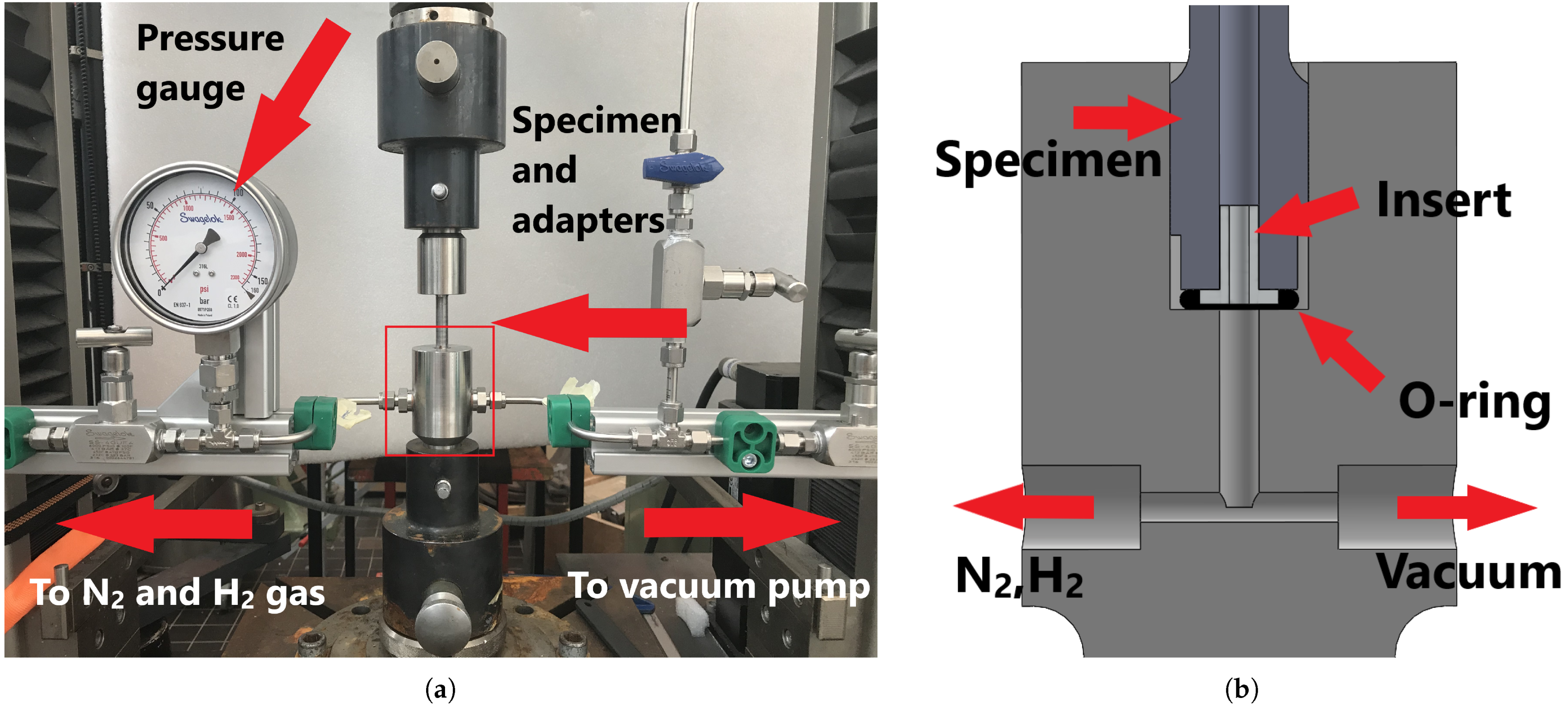

| Element | C | Mn | Si | Cr | Nb | Al | P | S | Bal |
|---|---|---|---|---|---|---|---|---|---|
| Base Metal | 0.06 | 1.66 | 0.26 | 0.06 | 0.04 | 0.04 | <0.01 | <0.01 | 97.88 |
| Weld Metal | 0.07 | 1.45 | 0.58 | 0.05 | 0.01 | 0.01 | 0.01 | 0.01 | 97.81 |
| Gas | Smooth BM | Notched BM | Notched WM |
|---|---|---|---|
| Air | 0 barg | Not tested | Not tested |
| N2 | 100 barg | 100 barg | 100 barg |
| H2 | 100 barg | 100 barg | 30, 70, 100 barg |
| Test Matrix and Results | |||||
|---|---|---|---|---|---|
| Material | Geometry | Pressure | Gas | %RA | Embrittlement Index I [%] |
| Base metal | Smooth | 0 barg | Air | 75.5 ± 0.6 | 0 |
| 100 barg | N2 | 76.6 ± 0.9 | −1.5 ± 2.1 | ||
| 100 barg | H2 | 55.2 ± 2.6 | 27.9 ± 4.6 | ||
| Notched | 100 barg | N2 | 72.1 ± 1.0 | 0 | |
| 100 barg | H2 | 52.0 ± 2.0 | 28.0 ± 4.7 | ||
| Weld metal | Notched | 100 barg | N2 | 48.2 ± 1.5 | 0 |
| 30 barg | H2 | 42.9 ± 0.6 | 11.0 ± 4.6 | ||
| 70 barg | H2 | 43.0 ± 0.8 | 10.9 ± 5.1 | ||
| 100 barg | H2 | 43.0 ± 1.8 | 10.8 ± 9.8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boot, T.; Riemslag, T.; Reinton, E.; Liu, P.; Walters, C.L.; Popovich, V. In-Situ Hollow Sample Setup Design for Mechanical Characterisation of Gaseous Hydrogen Embrittlement of Pipeline Steels and Welds. Metals 2021, 11, 1242. https://doi.org/10.3390/met11081242
Boot T, Riemslag T, Reinton E, Liu P, Walters CL, Popovich V. In-Situ Hollow Sample Setup Design for Mechanical Characterisation of Gaseous Hydrogen Embrittlement of Pipeline Steels and Welds. Metals. 2021; 11(8):1242. https://doi.org/10.3390/met11081242
Chicago/Turabian StyleBoot, Tim, Ton (A. C.) Riemslag, Elise (T. E.) Reinton, Ping Liu, Carey L. Walters, and Vera Popovich. 2021. "In-Situ Hollow Sample Setup Design for Mechanical Characterisation of Gaseous Hydrogen Embrittlement of Pipeline Steels and Welds" Metals 11, no. 8: 1242. https://doi.org/10.3390/met11081242
APA StyleBoot, T., Riemslag, T., Reinton, E., Liu, P., Walters, C. L., & Popovich, V. (2021). In-Situ Hollow Sample Setup Design for Mechanical Characterisation of Gaseous Hydrogen Embrittlement of Pipeline Steels and Welds. Metals, 11(8), 1242. https://doi.org/10.3390/met11081242







