Corrosion Inhibition of Low-Carbon Steel by Hydrophobic Organosilicon Dispersions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solutions
- −
- Organosilane: vinyltrimethoxysilane (VS) C5H12O3Si (WitcoCo);
- −
- Adsorption-type corrosion inhibitor: 1,2,3–benzotriazole (BTA) C6H5N3 (GP Chemicals);
- −
- Hydrophobic solvent: trichloromethane CHCl3 (LenReactiv);
- −
- Surfactant Strodex TH-100 (Strodex). The Strodex base is the sodium salt of an aliphatic ester of phosphonic acid (R1)(R2)P(OONa).
2.2. Steel Surface Treatment and Preparation of IDs
2.3. Electrochemical Studies
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Corrosion Tests
2.5.1. Tests at Room Temperature without Stirring the Solution
2.5.2. Tests at Elevated Temperature and Constant Stirring of the Solution
2.5.3. Salt Spray Test
3. Results
3.1. Electrochemical and Corrosion Studies of IDs in an Aqueous Chloride-Containing Electrolyte
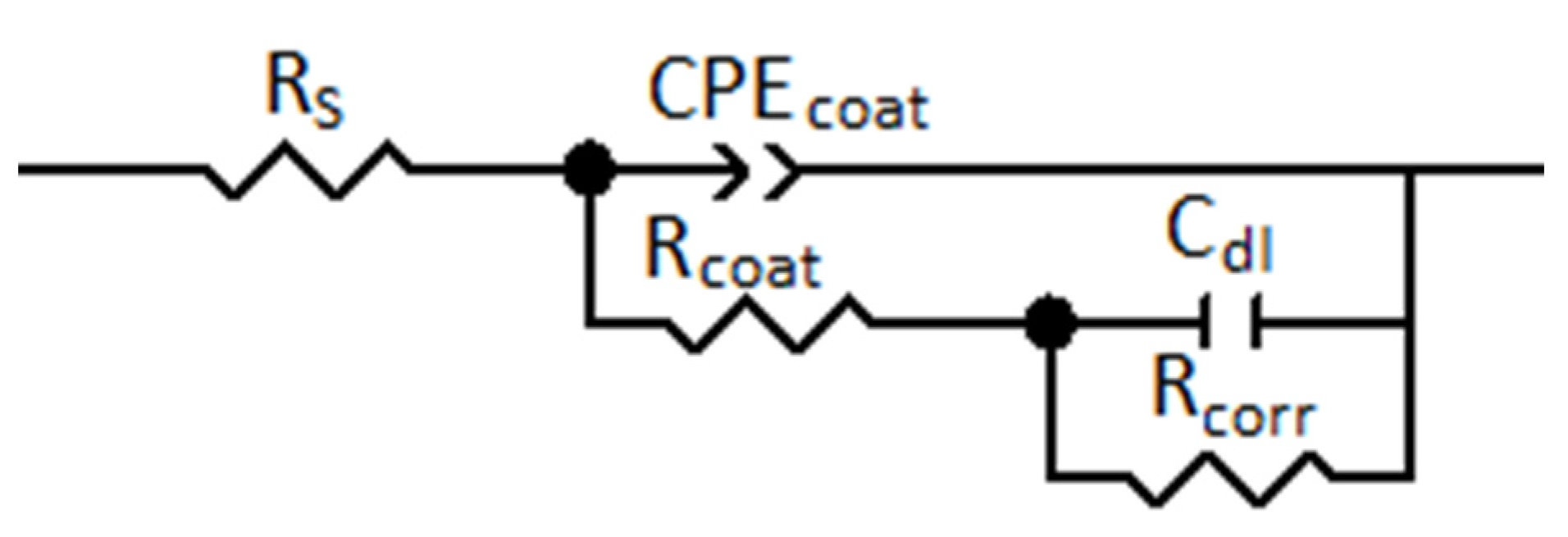
3.2. Investigation of the Physical and Chemical Properties of Protective Layers Using EIS
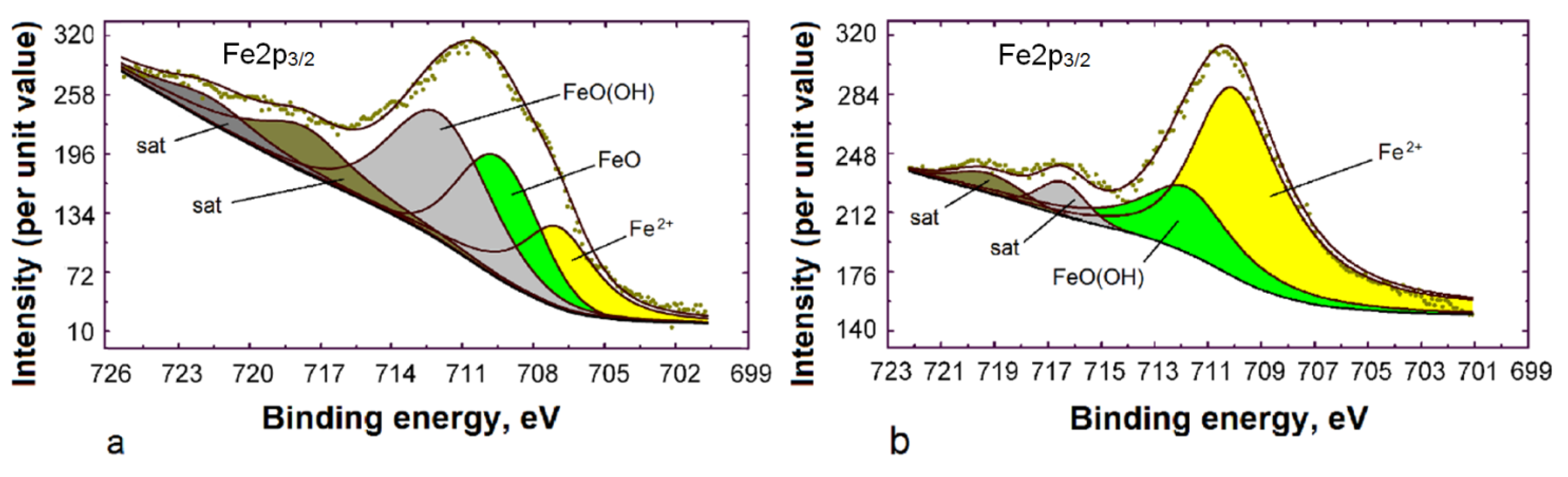
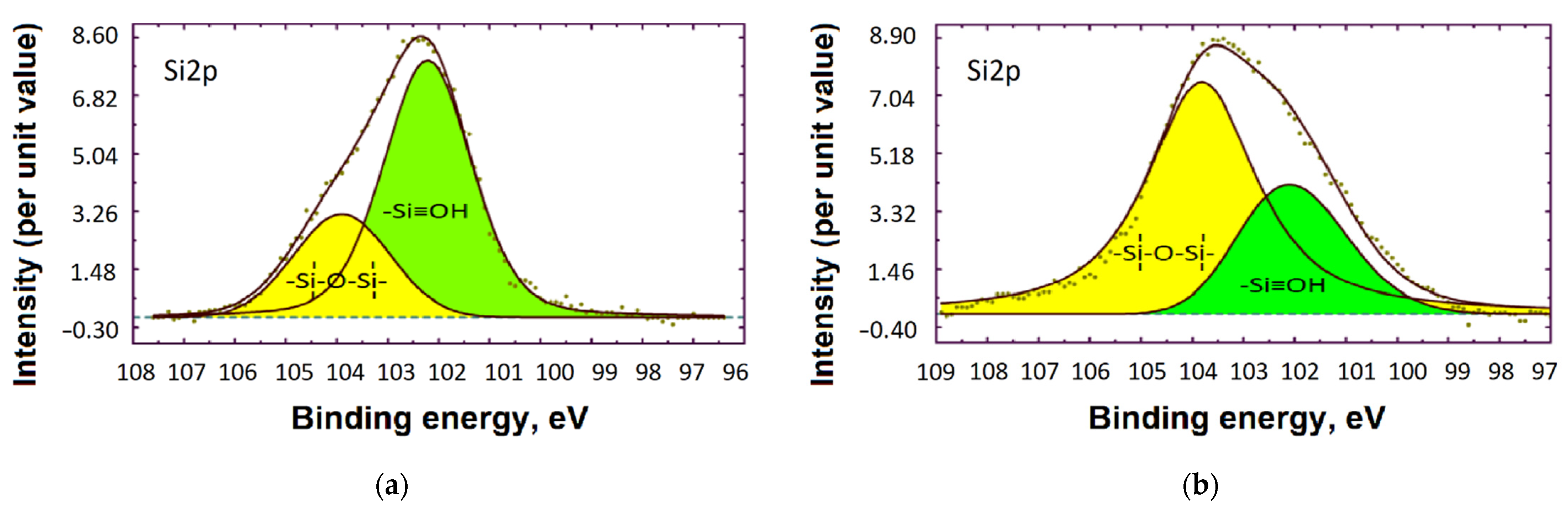
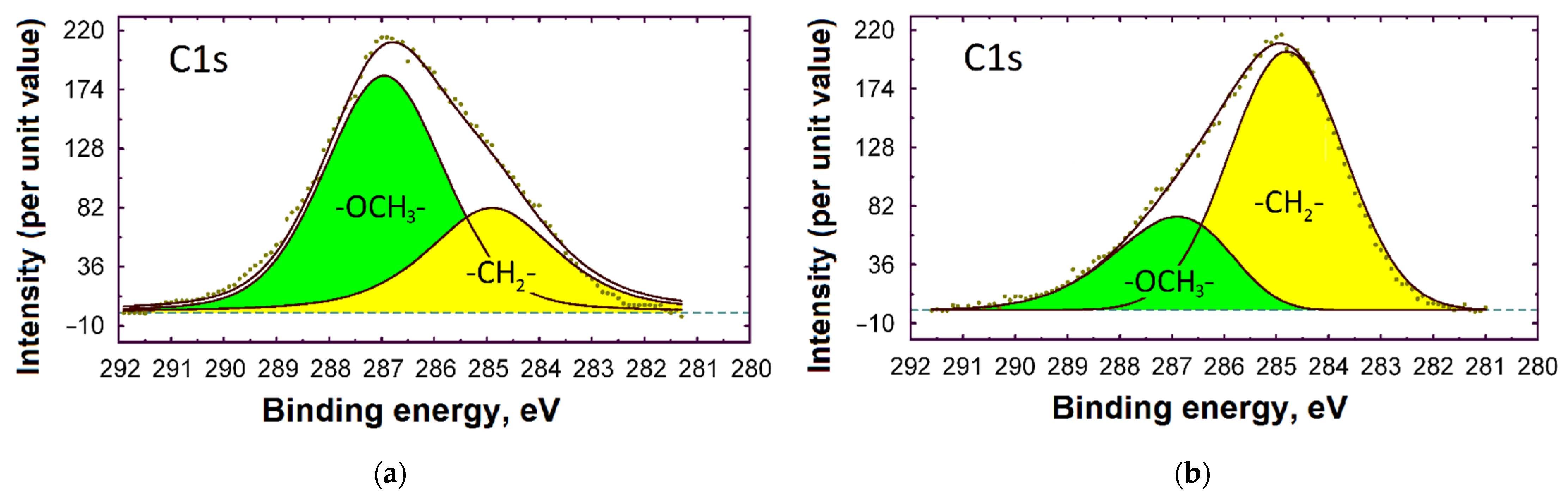
3.3. XPS Study
Adsorption of VS and BTA on the Steel Surface from ID
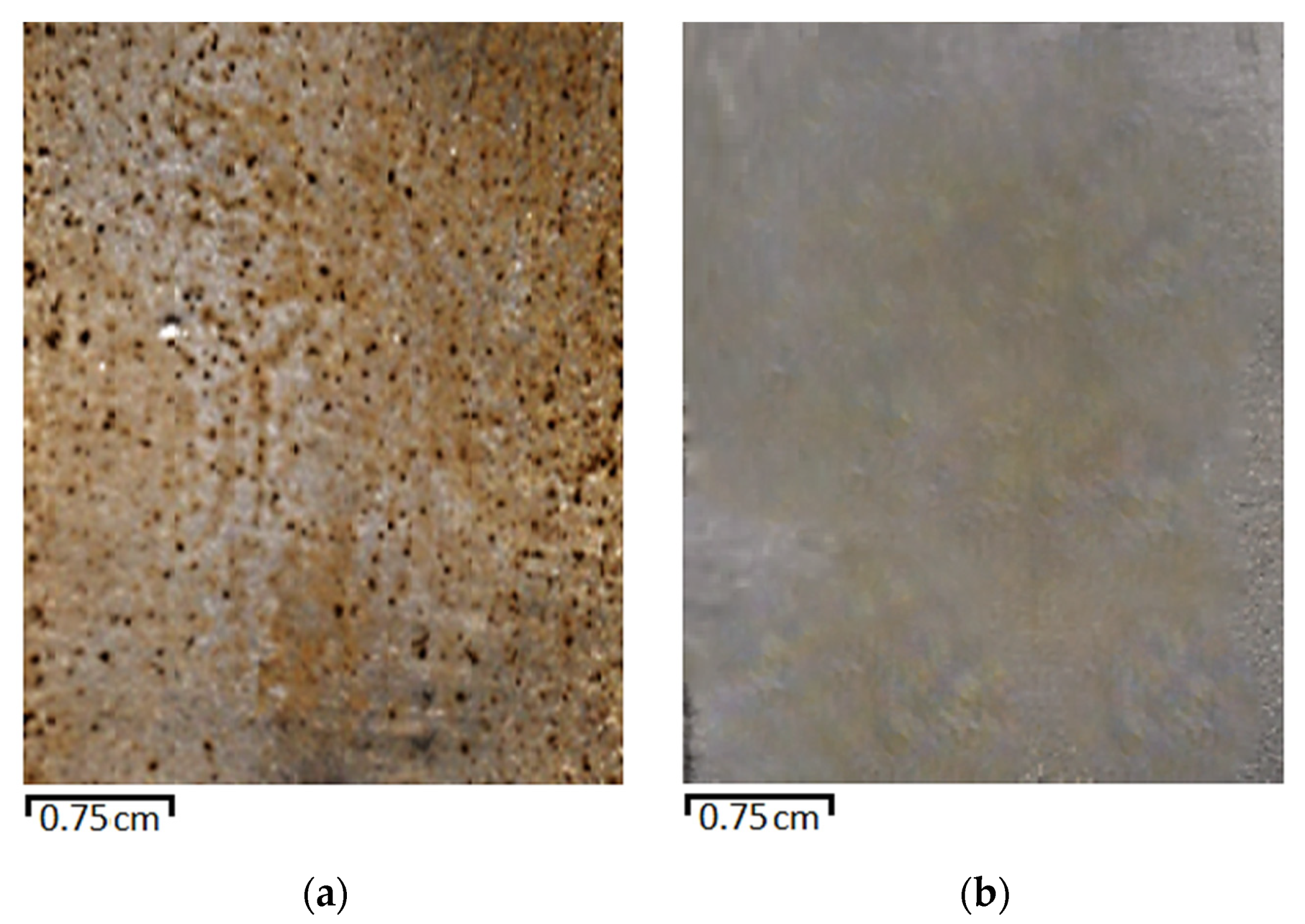
3.4. Corrosion Tests
3.4.1. Tests at Elevated Temperature and Constant Stirring of the Solution
3.4.2. Tests under Conditions of Anodic Polarization
3.4.3. Paint Coatings Salt Spray Test
4. Discussion of Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petrunin, M.A.; Maksaeva, L.B.; Yurasova, T.A.; Terekhova, E.V.; Maleeva, M.A.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. Formation of organosilicon self–organizing nanolayerson an iron surface from vapor phase and their effecton corrosion behavior of metal. J. Prot. Met. Phys. Chem. Surf. 2015, 51, 1010–1017. [Google Scholar] [CrossRef]
- Semiletov, A.M.; Chirkunov, A.A.; Kuznetsov, Y.I.; Andreeva, N.P. Passivation of steel with aqueous solutions of trialkoxysilanes. J. Prot. Met. Phys. Chem. Surf. 2015, 51, 1154–1159. [Google Scholar]
- Aramaki, K. Protection of iron corrosion by ultrathin two-dimensionalpolymer films of an alkanethiolmonolayer modified with alkylethoxysilanes. Corros. Sci. 1999, 41, 1715–1730. [Google Scholar] [CrossRef]
- Aramaki, K. Prevention of iron corrosion at scratched surfaces in NaCl solutions by thin organosiloxane polymer films containing octylthiopropionate. Corros. Sci. 2000, 42, 2023–2036. [Google Scholar] [CrossRef]
- Maleeva, M.A.; Ignatenko, V.E.; Shapagin, A.V.; Sherbina, A.A.; Maksaeva, L.B.; Marshakov, A.I.; Petrunin, M.A. Modification of bituminous coatings to prevent stress corrosion cracking of carbon steel. Int. J. Corros. Scale Inhib. 2015, 4, 226–234. [Google Scholar] [CrossRef]
- Zhu, D.; Van Ooij, W.J. Corrosion protection of metals by water-based silane mixturesofbis-[trimethoxysilylpropyl]amine and vinyltriacetoxysilane. J. Prog. Org. Coat. 2004, 49, 42–53. [Google Scholar] [CrossRef]
- Palanivel, V.; Zhu, D.G.; Van Ooij, W.J. Nanoparticle-filled silane films as chromatereplacements for aluminum alloys. J. Prog. Org. Coat. 2003, 47, 384–392. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Yurasova, T.A.; Terekhova, E.V.; Maleeva, M.A.; Shcherbina, A.A.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. The effect of self-organizing vinyl siloxane nanolayerson the corrosion behavior of aluminumin neutral chloride containing solutions. J. Prot. Met. Phys. Chem. Surf. 2014, 50, 784–791. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Yurasova, T.A.; Terekhova, E.V.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. The directional formation and protective effect of selfassembling vinyl siloxane nanolayers on copper surface. J. Prot. Met. Phys. Chem. Surf. 2012, 48, 656–664. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Marshakov, A.I. The effect of surface siloxane layers on the penetration of hydrogen into iron. J. Prot. Met. 2001, 37, 139–145. [Google Scholar]
- Nazarov, A.P.; Petrunin, M.A.; Mikhailovsky, Y.N. Formation mechanism and anticorrosive properties of thin siloxane films on metal surfaces. J. Prot. Met. 1996, 143, 251–257. [Google Scholar]
- Gladkikh, N.A.; Makarychev, Y.B.; Maleeva, M.A.; Petrunin, M.A.; Maksaeva, L.B.; Rybkina, A.; Marshakov, A.I.; Kuznetsov, Y.I. Synthesis of thin organic layers containing silane coupling agents and azole on the surface of mild steel. Synergism of inhibitors for corrosion protection of underground pipelines. J. Prog. Org. Coat. 2019, 132, 481–489. [Google Scholar] [CrossRef]
- Gladkikh, N.A.; Makarychev, Y.B.; Chirkunov, A.A.; Shapagin, A.; Petrunin, M.A.; Maksaeva, L.B.; Maleeva, M.A.; Yurasova, T.A.; Marshakov, A.I. Formation of polymer-like anticorrosive films based on organosilanes with benzotriazole, carboxylic and phosphonic acids. Protection of copper and steel against atmospheric corrosion. J. Prog. Org. Coat. 2020, 141, 105544. [Google Scholar] [CrossRef]
- Kazanskii, L.P.; Selyaninov, I.A. XPES of 1,2,3-benzotriazole nanolayers formed on iron surface. J. Prot. Met. Phys. Chem. Surf. 2010, 46, 797–804. [Google Scholar] [CrossRef]
- Khadom, A.A. Protection of steel corrosion reaction by benzotriazoles: A historical background. J. Fail. Anal. Prev. 2015, 15, 794–802. [Google Scholar] [CrossRef]
- Rammelt, U.; Koehler, S.; Reinhard, G. Synergistic effect of benzoate and benzotriazole on passivation of mild steel. Corros. Sci. 2008, 50, 1659–1663. [Google Scholar] [CrossRef]
- Matheswaran, P.; Ramasamy, A.K. Influence of benzotriazole on corrosion inhibition of mild steel in citric acid medium. J. Chem. 2010, 7, 1090–1094. [Google Scholar] [CrossRef] [Green Version]
- Solehudin, A. Study of benzotriazole as corrosion inhibitors of carbon steel in chloride solution containing hydrogen sulfide using electrochemical impedance spectroscopy (EIS). AIP Conf. Proc. 2014, 1589, 164. [Google Scholar]
- Finšgar, M.; Petovar, B.; Xhanari, K.; Maver, U. The corrosion inhibition of certain azoles on steel in chloride media: Electrochemistry and surface analysis. Corros. Sci. 2016, 111, 370–381. [Google Scholar] [CrossRef]
- Yao, J.L.; Ren, B.; Huang, Z.F.; Cao, P.G.; Tian, Z. Extending surface Raman spectroscopy to transition metals for practical applications IV. A study on corrosion inhibition of benzotriazole on bare Fe electrodes. J. Electrochim. Acta 2003, 48, 1263–1271. [Google Scholar] [CrossRef]
- Liu, S.; Xu, N.; Duan, J.; Zeng, Z.; Feng, Z.; Xiao, R. Corrosion inhibition of carbon steel in tetra-n-butylammonium bromide aqueous solution by benzotriazole and Na3PO4. Corros. Sci. 2009, 51, 1356–1363. [Google Scholar] [CrossRef]
- Popova, A.; Christov, M.; Zwetanova, A. Effect of the molecular structure on the inhibitor properties of azoles on mild steel corrosion in 1 M hydrochloric acid. Corros. Sci. 2007, 49, 2131–2143. [Google Scholar] [CrossRef]
- Silva, D.K.; Ribas, G.C.B.; da Cunha, M.T.; Agostinho, S.M.L.; Rodrigues, P.R.P. Benzotriazole and tolytriazole as corrosion inhibitors of carbon steel 1008 in sulfuric acid. Electrochim. Acta 2006, 24, 323–335. [Google Scholar] [CrossRef]
- Satpati, A.K.; Ravindran, P.V. Electrochemical study of the inhibition of corrosion of stainless steel by 1,2,3-benzotriazole in acidic media. J. Mater. Chem. Phys. 2008, 109, 352–359. [Google Scholar] [CrossRef]
- Gomma, G.K. Corrosion inhibition of steel by benzotriazole in sulphuric acid. J. Mater. Chem. Phys. 1998, 55, 235–240. [Google Scholar] [CrossRef]
- Eldakar, N.; Nobe, K. Effect of substituted benzotriazoles on the anodic dissolution of iron in H2SO4. J. Corros. 1981, 37, 271–278. [Google Scholar] [CrossRef]
- Eldakar, N.; Nobe, K. Electrochemical and corrosion behavior of iron in presence of substituted benzotriazoles. J. Corros. 1977, 33, 128–130. [Google Scholar] [CrossRef]
- Chin, R.J.; Altura, D.; Nobe, K. Corrosion inhibition of iron and copper in chloride solutions by benzotriazole. J. Corros. 1973, 29, 185–187. [Google Scholar] [CrossRef]
- Selvi, S.T.; Raman, V.; Rajendran, N. Corrosion inhibition of mild steel by benzotriazole derivatives in acidic medium. J. Appl. Electrochem. 2003, 33, 1175–1182. [Google Scholar] [CrossRef]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. J. Surf. Interface Anal. 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Plueddenmann, E.P. Silane Coupling Agents, 2nd ed.; Plenum Press: New York, NY, USA, 1991; pp. 79–152. [Google Scholar]
- Rybkina, A.; Gladkikh, N.; Marshakov, A.; Petrunin, M.; Nazarov, A. Effect of sign-alternating cyclic polarisation and hydrogen uptake on the localised corrosion of x70 pipeline steel in near-neutral solutions. Metals 2020, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- ASTM D 610-08. Standard Test Method for Evaluating Degree of Rusting on Painted Steel Surfaces; ASTM International: West Conshohocken, PA, USA, 2019; pp. 1–7. [Google Scholar]
- Gladkikh, N.A.; Makarychev, Y.B.; Maleeva, M.A.; Petrunin, M.A.; Maksaeva, L.B.; Marshakov, A.I. Synergistic effect of silanes and azole for enhanced corrosion protection of carbon steel by polymeric coatings. J. Prog. Org. Coat. 2020, 138, 105386. [Google Scholar] [CrossRef]
- Petrunin, M.; Maksaeva, L.; Gladkikh, N.; Makarychev, Y.; Maleeva, M.; Yurasova, T.; Nazarov, A. Thin benzotriazole films for inhibition of carbon steel corrosion in neutral electrolytes. Coatings 2020, 10, 362. [Google Scholar] [CrossRef] [Green Version]
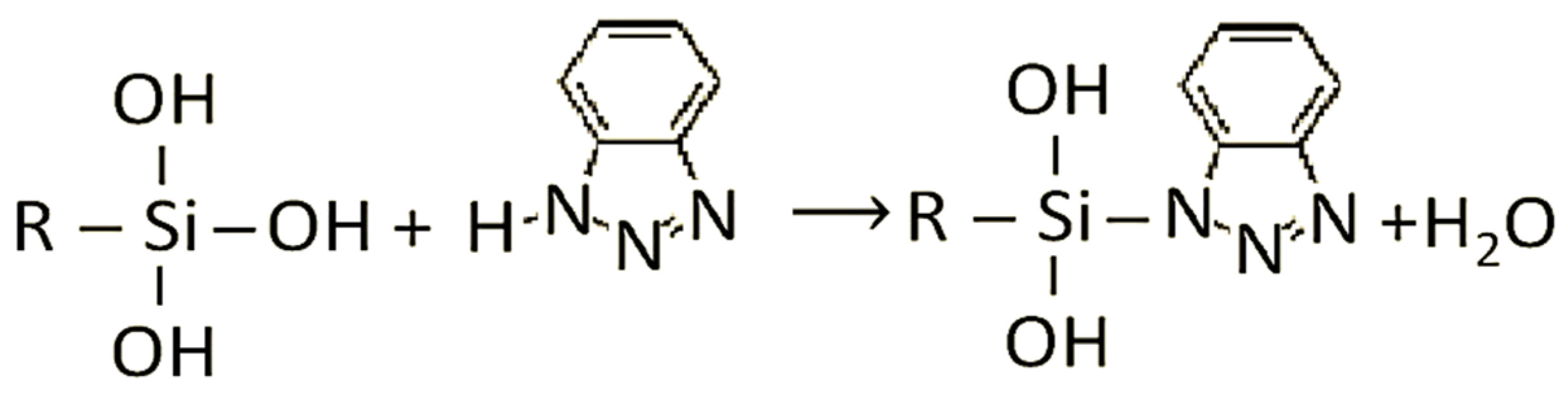
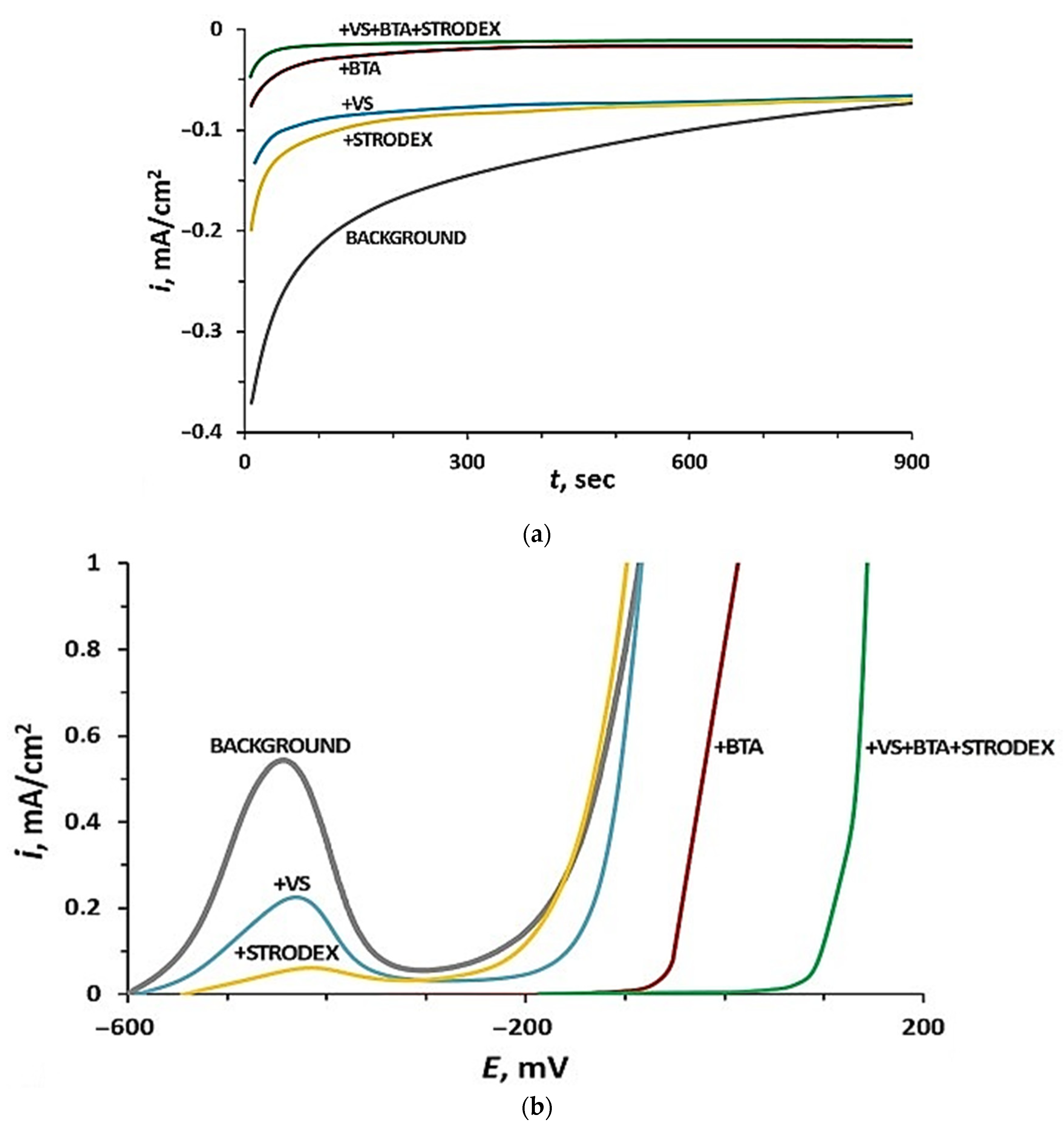
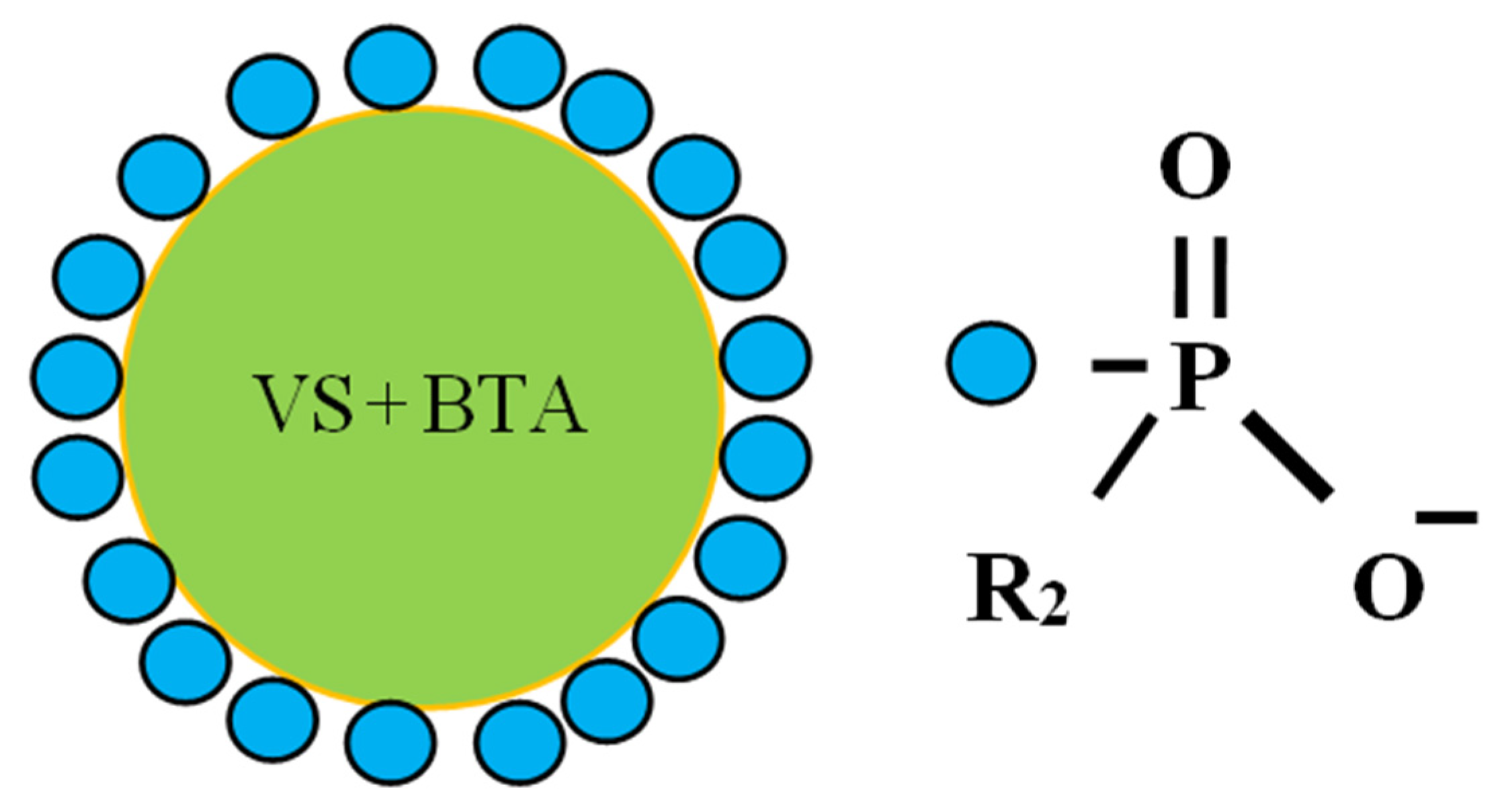





| Material | Concentration of Elements | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Si | Mn | P | S | Cr | Ni | Cu | |
| St3 | 98.775 | 0.12 | 0.03 | 0.5 | 0.035 | 0.04 | 0.1 | 0.3 | 0.1 |
| Media | Ecor, mV | Epit, mV |
|---|---|---|
| Background solution | −600.0 | −265.0 |
| +1% Strodex | −543.1 | −203.9 |
| +0.01 M VS | −582.3 | −173.7 |
| +0.01 M BTA | −378.6 | −91.8 |
| +0.01 M (VS + BTA) + 1% Strodex | −195.4 | 97.1 |
| Media | Vcor, mg/dm2/day | Corrosion Inhibition Efficiency Z, % |
|---|---|---|
| Background solution | 14.2 | – |
| +1% Strodex | 9.6 | 32.4 |
| +0.01 M VS | 8.3 | 41.5 |
| +0.01 M BTA | 6.4 | 54.9 |
| +0.01 M (VS + BTA) + 1% Strodex | 2.1 | 85.2 |
| Treatment Mode | Rcoat (KOhm × cm−2) | Rcorr (KOhm × cm−2) | Cdl (μF × cm−2) | CPEcoat-1T (Ω−1 × sn × cm−2) | CPEcoat-1P |
|---|---|---|---|---|---|
| Background | 1.1 | 3.1 | 31.4 | 18.7 | 0.94 |
| T = 25 °C | 4.8 | 5.2 | 24.7 | 5.4 | 0.91 |
| Stirring T = 25 °C | 6.3 | 6.7 | 18.2 | 2.1 | 0.87 |
| Stirring T = 60 °C | 8.1 | 7.4 | 9.5 | 0.83 | 0.84 |
| Exposure Time | Fe | Si | N | O | C | P | Thickness, nm |
|---|---|---|---|---|---|---|---|
| 10 min | 6.6 | 10.4 | 6.2 | 36.2 | 38.3 | 2.3 | 6 ± 0.5 |
| 60 min | 1.5 | 14.2 | 1.2 | 41.4 | 40.1 | 1.6 | 15 ± 0.5 |
| Solution | Corrosion Damage Rate, % |
|---|---|
| 3% NaCl | 37 |
| 3 % NaCl + 0.01 M (VS + BTA) + 1% Strodex | 3 |
| Sample | Test Conditions | Corrosion Damage Rate, % |
|---|---|---|
| HomaCryl | 24 h salt spray test | 16 |
| HomaCryl + 0.01 M (VS + BTA)+ 1% Strodex | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarychev, Y.; Gladkikh, N.; Arkhipushkin, I.; Kuznetsov, Y. Corrosion Inhibition of Low-Carbon Steel by Hydrophobic Organosilicon Dispersions. Metals 2021, 11, 1269. https://doi.org/10.3390/met11081269
Makarychev Y, Gladkikh N, Arkhipushkin I, Kuznetsov Y. Corrosion Inhibition of Low-Carbon Steel by Hydrophobic Organosilicon Dispersions. Metals. 2021; 11(8):1269. https://doi.org/10.3390/met11081269
Chicago/Turabian StyleMakarychev, Yuri, Natalia Gladkikh, Ivan Arkhipushkin, and Yuri Kuznetsov. 2021. "Corrosion Inhibition of Low-Carbon Steel by Hydrophobic Organosilicon Dispersions" Metals 11, no. 8: 1269. https://doi.org/10.3390/met11081269
APA StyleMakarychev, Y., Gladkikh, N., Arkhipushkin, I., & Kuznetsov, Y. (2021). Corrosion Inhibition of Low-Carbon Steel by Hydrophobic Organosilicon Dispersions. Metals, 11(8), 1269. https://doi.org/10.3390/met11081269







