Analysis of Hydrogen Embrittlement on Aluminum Alloys for Vehicle-Mounted Hydrogen Storage Tanks: A Review
Abstract
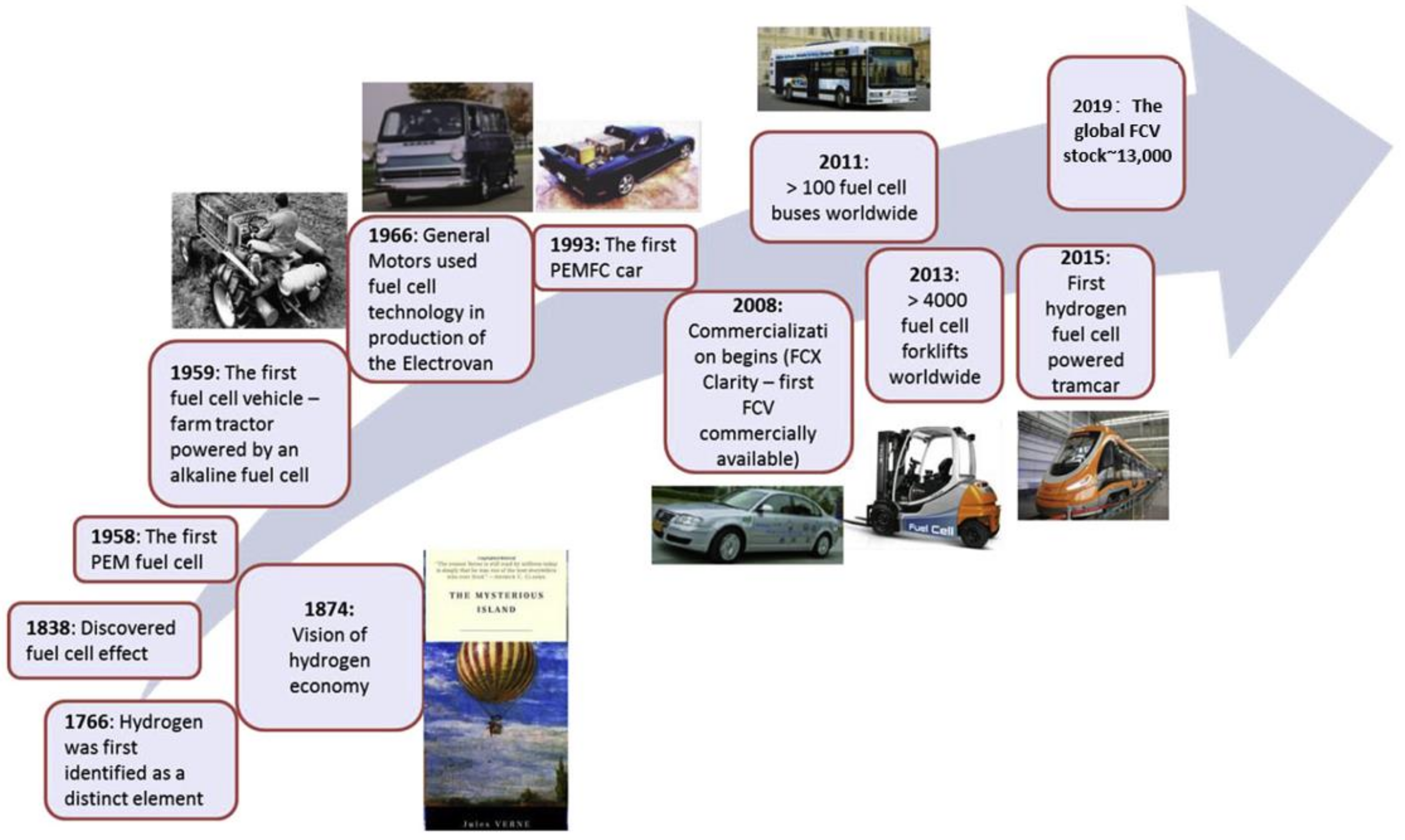
:1. Introduction
2. Mechanism of Hydrogen Embrittlement
2.1. Hydrogen Enhanced Local Plasticity Model (HELP)
2.2. Hydrogen Enhanced Decohesion Mechanism (HEDE)
2.3. Hydrogen Pressure Theory
2.4. Adsorption-Induced Dislocation Emission (AIDE)
2.5. Hydrogen-Enhanced Strain-Induced Vacancy Generation (HESIV)
2.6. The Influential Factors
2.6.1. Solution Environment
2.6.2. Alloy Composition
2.6.3. The Depth of Hydrogen Penetration
3. Detection Methods of Hydrogen Embrittlement
3.1. Methods to Prepare the Material
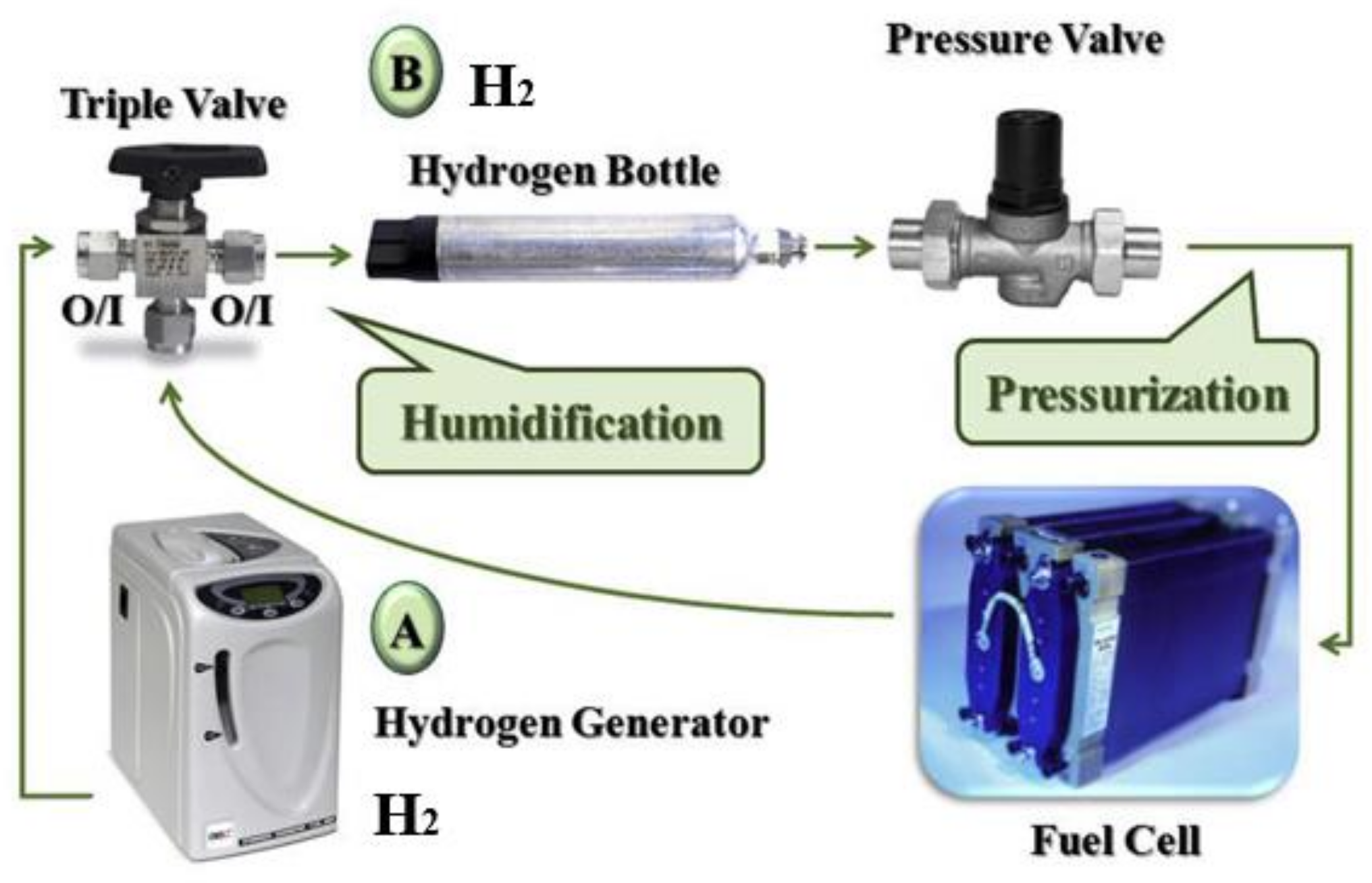
3.1.1. Electrochemical Cathode Hydrogen Permeation Method
3.1.2. Corrosive Environment Simulation Test
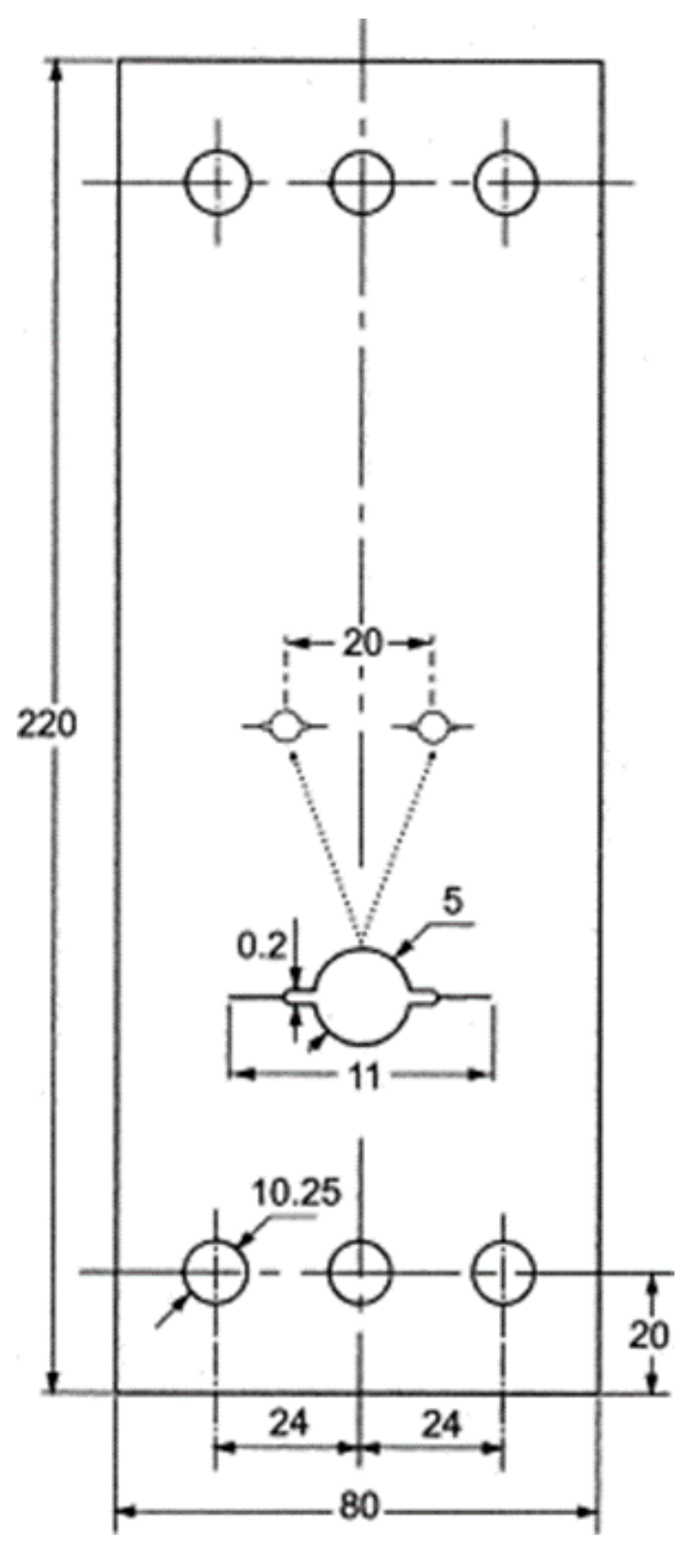
3.2. Tensile Test Method
3.2.1. Slow Strain Rate Test (SSRT)
3.2.2. Linearly Increasing Stress Test (LIST)
3.3. Tools to Measure Hydrogen Content
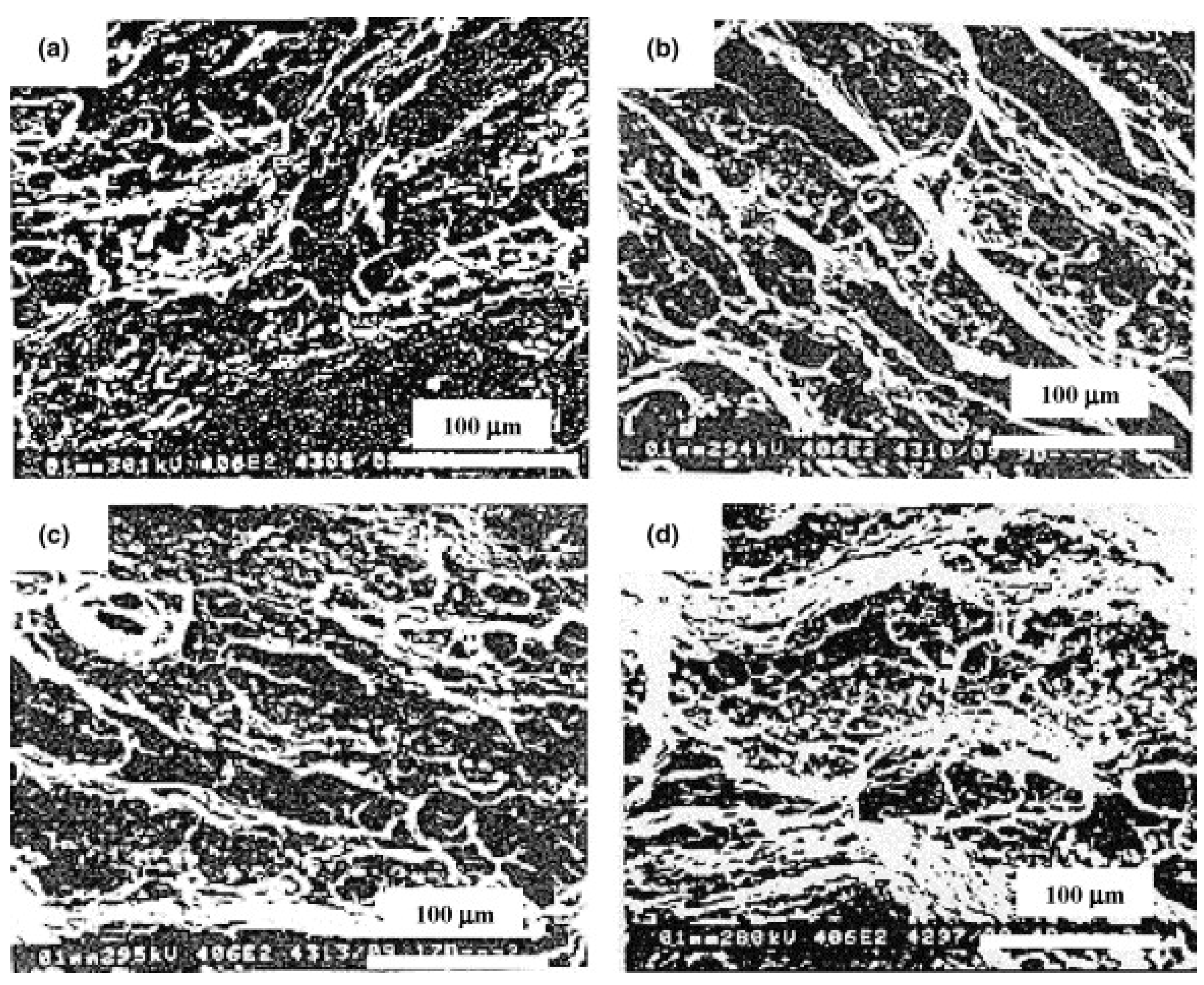
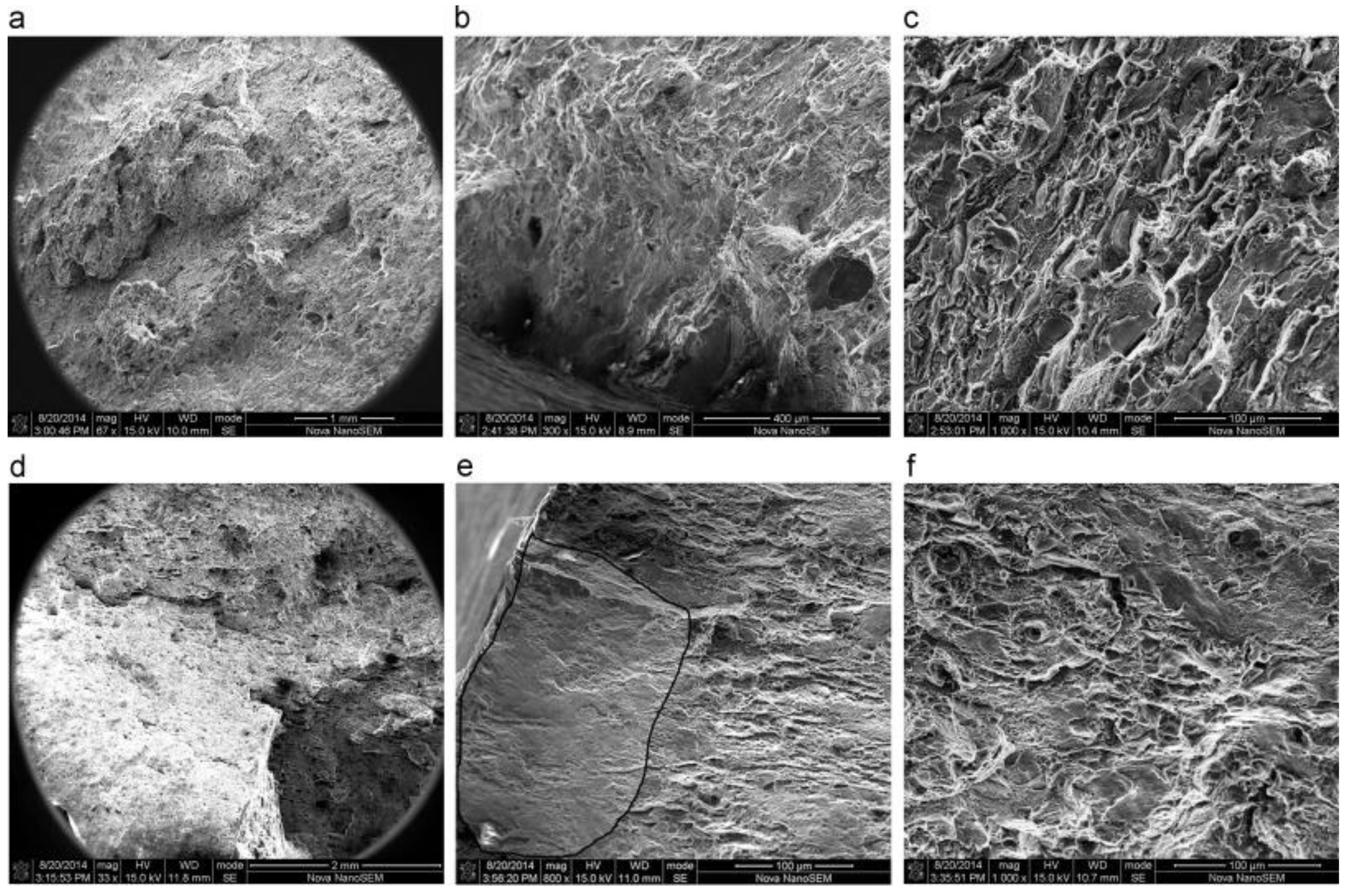
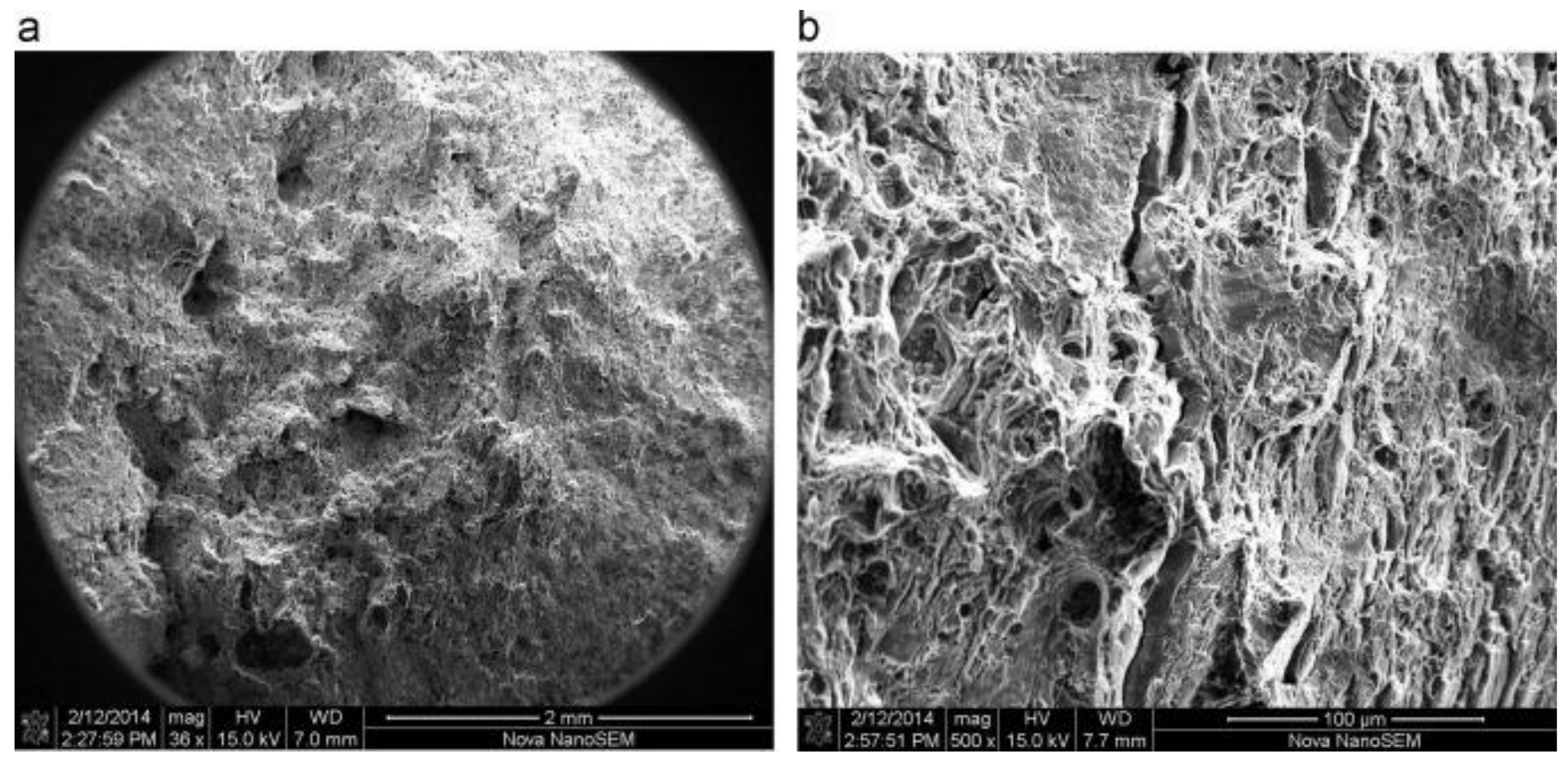
3.4. Microstructural Analysis
3.5. Hydrogen Microprint Technique (HMT)
3.6. In-Situ SP Test Method
4. Prevention of Hydrogen Embrittlement
4.1. Inhibition of Film Formation on Aluminum Alloy Surface
4.1.1. Film Forming Method in an Alkaline Environment
4.1.2. Acid Radical Suppression
4.1.3. Sodium Silicate Solution Inhibition Method
4.2. Coatings
4.2.1. Cadmium and Nickel Coatings
4.2.2. Other Metal Coatings
4.2.3. Metal Oxide for Coating
4.2.4. Nitride as Coating
4.3. Heat Treatment of Materials
4.4. Grain Refining
5. Discussion
6. Conclusions
- (1)
- The HE mechanisms of aluminum alloy mainly include the hydrogen enhanced local plasticity model (HELP), the hydrogen enhanced decohesion mechanism (HEDE) and the hydrogen pressure theory. The HELP is widely accepted. This theory holds that through stress-induced diffusion, hydrogen atoms are concentrated to the crack tip, leading to fracture of materials.
- (2)
- The HE detection methods mainly includes slow strain rate test (SSRT), linearly increasing stress test (LIST) and tools to measure hydrogen content (TDS). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are also used for microstructure analysis. Among the methods to conduct the tensile experiments, LIST has the advantages of simple operation and easy process.
- (3)
- The HE prevention methods mainly involve surface film formation suppression methods, coating prevention methods, heat treatment methods and grain refining methods. Among these, the sodium silicate solution inhibition method effectively prevents hydrogen from penetrating into microscopic cracks during the production of the hydrogen storage tanks. It is also believed that further prevention measures will be developed based on the integration of multiple prevention methods.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambituuni, A.; Amezaga, J.M.; Werner, D. Risk assessment of petroleum product transportation by road: A framework for regulatory improvement. Saf. Sci. 2015, 79, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Fleming, C.H.; Spencer, M.; Thomas, J.; Leveson, N.; Wilkinson, C. Safety assurance in NextGen and complex transportation systems. Saf. Sci. 2013, 55, 173–187. [Google Scholar] [CrossRef]
- Ji, M.; You, X.; Lan, J.; Yang, S. The impact of risk tolerance, risk perception and hazardous attitude on safety operation among airline pilots in China. Saf. Sci. 2011, 49, 1412–1420. [Google Scholar] [CrossRef]
- Greene, D.L.; Ogden, J.M.; Lin, Z. Challenges in the designing, planning and deployment of hydrogen refueling infrastructure for fuel cell electric vehicles. eTransportation 2020, 6, 100086. [Google Scholar] [CrossRef]
- Michler, T.; Wackermann, K.; Schweizer, F. Review and Assessment of the Effect of Hydrogen Gas Pressure on the Embrittlement of Steels in Gaseous Hydrogen Environment. Metals 2021, 11, 637. [Google Scholar] [CrossRef]
- Kansara, S.; Gupta, S.K.; Sonvane, Y.; Gajjar, P.N. Ultrathin Pd and Pt nanowires for potential applications as hydrogen economy. Mater. Today Commun. 2021, 26, 101761. [Google Scholar] [CrossRef]
- Schaupp, T.; Schroeder, N.; Schroepfer, D.; Kannengiesser, T. Hydrogen-Assisted Cracking in GMA Welding of High-Strength Structural Steel—A New Look into This Issue at Narrow Groove. Metals 2021, 11, 904. [Google Scholar] [CrossRef]
- Ajanovic, A.; Haas, R. Prospects and impediments for hydrogen and fuel cell vehicles in the transport sector. Int. J. Hydrogen Energy 2020, 46, 10049–10058. [Google Scholar] [CrossRef]
- Gong, L.; Li, Z.; Jin, K.; Gao, Y.; Duan, Q.; Zhang, Y.; Sun, J. Numerical study on the mechanism of spontaneous ignition of high-pressure hydrogen during its sudden release into a tube. Saf. Sci. 2020, 129, 104807. [Google Scholar] [CrossRef]
- Wang, Y. Research on the Hydrogen Supply System of 70 MPa Hydrogen Storage Cylinder for Vehicles and the Fast Filling Process; Dalian University of Technology: Dalian, China, 2019. [Google Scholar]
- Zhang, M.; Lv, H.; Kang, H.; Zhou, W.; Zhang, C. A literature review of failure prediction and analysis methods for composite high-pressure hydrogen storage tanks. Int. J. Hydrogen Energy 2019, 44, 25777–25799. [Google Scholar] [CrossRef]
- Allam, T.; Guo, X.; Lipińska-Chwałek, M.; Hamada, A.; Ahmed, E.; Bleck, W. Impact of precipitates on the hydrogen embrittlement behavior of a V-alloyed medium-manganese austenitic stainless steel. J. Mater. Res. Technol. 2020, 9, 13524–13538. [Google Scholar] [CrossRef]
- Venezuela, J.; Zhou, Q.; Liu, Q.; Li, H.; Zhang, M.; Dargusch, M.S.; Atrens, A. The influence of microstructure on the hydrogen embrittlement susceptibility of martensitic advanced high strength steels. Mater. Today Commun. 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Talbot, D.E.J. Effects of Hydrogen in Aluminium, Magnesium, Copper, and Their Alloys. Int. Metall. Rev. 1975, 20, 166–184. [Google Scholar] [CrossRef]
- Wang, H.; Deng, W.; Zhang, T.; Yao, J.; Wang, S. Development of Elastoplastic-Damage Model of AlFeSi Phase for Aluminum Alloy 6061. Metals 2021, 11, 954. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, Y.; Huang, J.; Li, B.; Wang, B.; Liu, S.; Fu, L. Effects of quenching agents, two-step aging and microalloying on tensile properties and stress corrosion cracking of Al-Zn-Mg-Cu alloys. J. Mater. Res. Technol. 2020, 9, 10198–10208. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.; Yoon, S.; Vucko, F.; Lee, C.; Theirry, D.; Kim, S. Diffusible hydrogen behavior and delayed fracture of cold rolled martensitic steel in consideration of automotive manufacturing process and vehicle service environment. J. Mater. Res. Technol. 2020, 9, 13483–13501. [Google Scholar] [CrossRef]
- Li, L.X.; Sun, M.H.; Fan, M.C.; Yang, T.S.; Du, F.S. New Rapid prototyping technology for the prevention of hydrogen embrittlement of metal strips. Corros. Sci. 2020, 164, 108341. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Safyari, M.; Kuramoto, S.; Hojo, T. Unraveling the effect of dislocations and deformation-induced boundaries on environmental hydrogen embrittlement behavior of a cold-rolled Al–Zn–Mg–Cu alloy. Int. J. Hydrogen Energy 2021, 46, 8285–8299. [Google Scholar] [CrossRef]
- Sajjad, U.; Abbas, A.; Sadeghianjahromi, A.; Abbas, N.; Liaw, J.; Wang, C. Enhancing corrosion resistance of Al 5050 alloy based on surface roughness and its fabrication methods; an experimental investigation. J. Mater. Res. Technol. 2021, 11, 1859–1867. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Yao, J.; Ye, D.; Lang, Z.; Glowacz, A. Mapping the knowledge domains of new energy vehicle safety: Informetrics analysis-based studies. J. Energy Storage 2021, 35, 102275. [Google Scholar] [CrossRef]
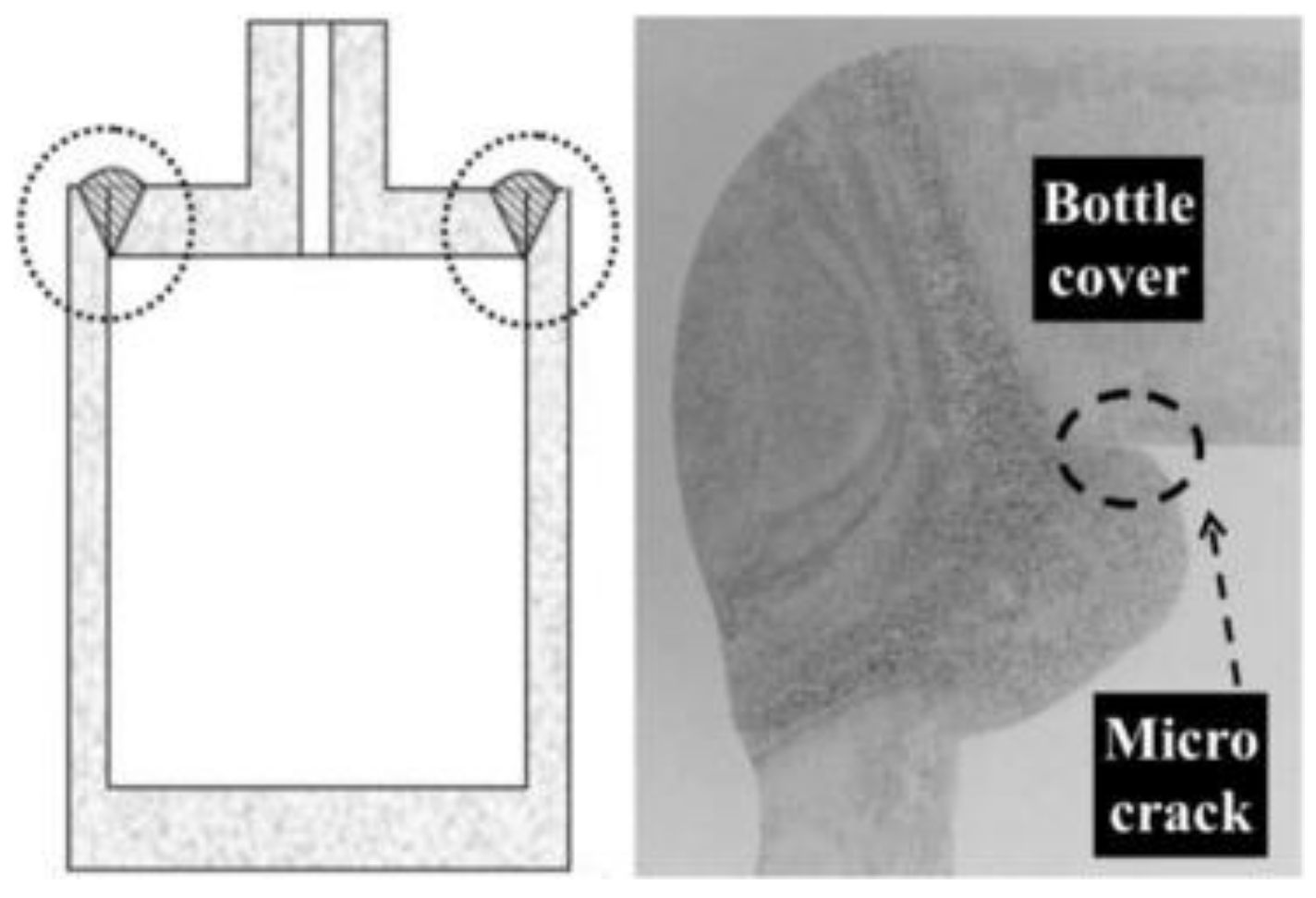
- Wu, S.; Cheng, W.; Yang, J.H.C.; Yang, C.; Liao, S. The corrosion protection study on inner surface from welding of aluminum alloy 7075-T6 hydrogen storage bottle. Int. J. Hydrogen Energy 2016, 41, 570–596. [Google Scholar] [CrossRef]
- Luo, T.; Peng, Y.; Guo, Y.; Cao, J. Influence of interactions between hydrogen and (101¯2) twin boundary on hydrogen embrittlement in α-Ti. Mater. Today Commun. 2021, 26, 101802. [Google Scholar] [CrossRef]
- Song, R.G.; Blawert, C.; Dietzel, W.; Atrens, A. A study on stress corrosion cracking and hydrogen embrittlement of AZ31 magnesium alloy. Mater. Sci. Eng. A 2005, 399, 308–317. [Google Scholar] [CrossRef]
- Borrego, L.P.; Costa, J.M.; Antunes, F.V.; Ferreira, J.M. Fatigue crack growth in heat-treated aluminium alloys. Eng. Fail. Anal. 2010, 17, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Metalnikov, P.; Eliezer, D.; Ben-Hamu, G.; Tal-Gutelmacher, E.; Gelbstein, Y.; Munteanu, C. Hydrogen embrittlement of electron beam melted Ti–6Al–4V. J. Mater. Res. Technol. 2020, 9, 16126–16134. [Google Scholar] [CrossRef]
- Wang, J.; Ma, L.; Han, M.; Jia, J. Molecular and dissociated adsorption of hydrogen on TiC6H6. Int. J. Hydrogen Energy 2019, 44, 25800–25808. [Google Scholar] [CrossRef]
- Mayer, T.; Semmel, M.; Guerrero Morales, M.A.; Schmidt, K.M. Techno-economic evaluation of hydrogen refueling stations with liquid or gaseous stored hydrogen. Int. J. Hydrogen Energy 2019, 44, 25809–25833. [Google Scholar] [CrossRef]
- Zhao, L.; Li, F.; Li, Z.; Zhang, L.; He, G. Thermodynamic analysis of the emptying process of compressed hydrogen tanks. Int. J. Hydrogen Energy 2019, 44, 3993–4005. [Google Scholar] [CrossRef]
- Bakare, A.K.; Shaikh, A.A.; Kale, S.S. Corrosion behaviour of stop-hole and notch instead of crack in thin aluminium alloy sheet repaired with bonded patch exposed to saline environment under constant load. Eng. Fail. Anal. 2021, 120, 105067. [Google Scholar] [CrossRef]
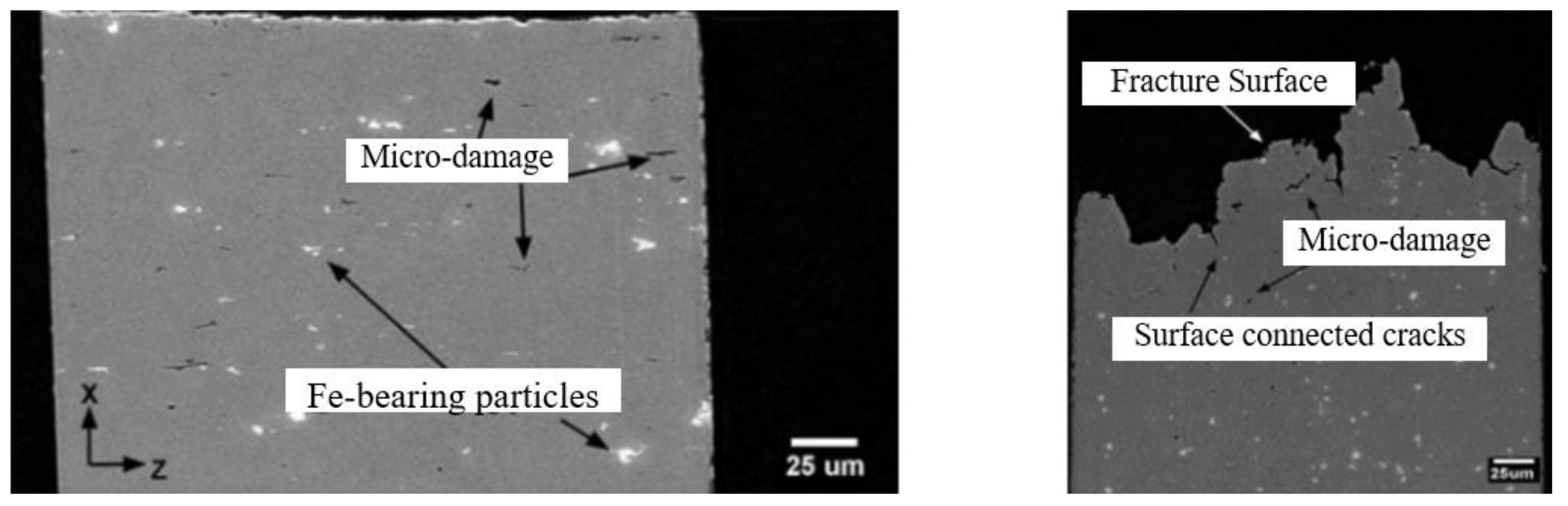
- Gupta, C.; Toda, H.; Fujioka, T.; Kobayashi, M.; Hoshino, H.; Uesugi, K.; Takeuchi, A.; Suzuki, Y. State of 3-D micro-damage in hydrogen redistributed regions of precharged high strength aluminium alloy. Corros. Sci. 2016, 111, 26–38. [Google Scholar] [CrossRef]
- Qi, W.J. A Study on Hydrogen-Induced Cracking Mechanism of 7050 High Strength Aluminum Alloy; Changzhou University: Changzhou, China, 2015. [Google Scholar]
- Xie, Z.; Wang, Y.; Lu, C.; Dai, L. Sluggish hydrogen diffusion and hydrogen decreasing stacking fault energy in a high-entropy alloy. Mater. Today Commun. 2021, 26, 101902. [Google Scholar] [CrossRef]
- Verners, O.; Psofogiannakis, G. Comparative molecular dynamics study of fcc-Al hydrogen embrittlement. Corros. Sci. 2015, 98, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Rao, A.C.U.; Vasu, V.; Govindaraju, M. Stress corrosion cracking behavior of 7xxx aluminum alloys: A literature review. Trans. Nonferrous Met. Soc. China 2016, 26, 1447–1471. [Google Scholar] [CrossRef]
- Liang, S.; Huang, M.S.; Zhao, L.; Zhu, Y.X.; Li, Z.H. Effect of multiple hydrogen embrittlement mechanisms on crack propagation behavior of FCC metals: Competition vs. synergy. Int. J. Plast. 2021, 143, 103023. [Google Scholar] [CrossRef]
- Fukai, Y. Formation of superabundant vacancies in M-H alloys and some of its consequences: A review. J. Alloys Compd. 2003, 356–357, 263–269. [Google Scholar] [CrossRef]
- Panagopoulos, C.; Papapanayiotou, P. The influence of cathodic hydrogen charging on the mechanical behavior of Al-Zn-Mg alloy. J. Mater. Sci. 1995, 30, 3449–3456. [Google Scholar] [CrossRef]
- Rin, T. Hydrogen generation from the hydrolysis of aluminum promoted by NieLieB catalyst. Int. J. Hydrogen Energy 2021, 46, 28450–28461. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, H.; Zuo, Z.; Li, R.; Liu, F. A thermodynamic and kinetic study of catalyzed hydrolysis of aluminum nitride in secondary aluminum dross. J. Mater. Res. Technol. 2020, 9, 9735–9745. [Google Scholar] [CrossRef]
- Song, R.G.; Dietzel, W.; Zhang, B.J.; Liu, W.J.; Tseng, M.K.; Atrens, A. Stress corrosion cracking and hydrogen embrittlement of an Al–Zn–Mg–Cu alloy. Acta Mater 2004, 52, 4727–4743. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Zhang, Y.G.; Wang, M.M.; Zhao, B.L.; Xie, Z.M. Hydrogen embrittlement of bulk W-0.5 wt% ZrC alloy induced by annealing in hydrogen atmosphere. J. Nucl. Mater. 2021, 556, 153177. [Google Scholar] [CrossRef]
- Dey, S.; Chattoraj, I. Interaction of strain rate and hydrogen input on the embrittlement of 7075 T6 Aluminum alloy. Mater. Sci. Eng. A 2016, 661, 168–178. [Google Scholar] [CrossRef]
- Álvarez, G.; Zafra, A.; Belzunce, F.J.; Rodríguez, C. Hydrogen embrittlement testing procedure for the analysis of structural steels with Small Punch Tests using notched specimens. Eng. Fract. Mech. 2021, 253, 107906. [Google Scholar] [CrossRef]
- Petroyiannis, P.V.; Kermanidis, A.T.; Papanikos, P.; Pantelakis, S.G. Corrosion-induced hydrogen embrittlement of 2024 and 6013 aluminum alloys. Trans. Nonferrous Met. Soc. China 2004, 41, 173–183. [Google Scholar] [CrossRef]
- ASTM E8m-94a. Standard Test Methods for Tension Testing of Metallic Materials; American Society for Testing Material: West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM E561-94. Standard Test Method for Kr Curve Determination; American Society for Testing Material: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM G34-90. Adjunct to G34 Test Method for Exfoliation Corrosion Susceptibility in 2Xxx and 7Xxx Series Aluminum Alloys (Exco Test); American Society for Testing Material: West Conshohocken, PA, USA, 2010. [Google Scholar]
- ASTM G129-2000(R2013). Standard Practice for Slow Strain Rate Testing to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking; American Society for Testing Material: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM A370. Standard Test Methods and Definitions for Mechanical Testing of Steel Products; American Society for Testing Material: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Zhang, J.; Jiang, P.; Chen, Q.; Zhou, J.; Meng, Y. Tensile properties and strain hardening mechanism of Cr-Mn-Si-Ni alloyed ultra-strength steel at different temperatures and strain rates. J. Alloys Compd. 2020, 842, 155856. [Google Scholar] [CrossRef]
- Venezuela, J.; Liu, Q.; Zhang, M.; Zhou, Q.; Atrens, A. The influence of hydrogen on the mechanical and fracture properties of some martensitic advanced high strength steels studied using the linearly increasing stress test. Corros. Sci. 2015, 99, 98–117. [Google Scholar] [CrossRef] [Green Version]
- Villalba, E.; Atrens, A. Metallurgical aspects of rock bolt stress corrosion cracking. Mater. Sci. Eng. A 2008, 491, 8–18. [Google Scholar] [CrossRef]
- Irwanto, B. Influence of hydrogen on the mechanical properties of some medium-strength Ni–Cr–Mo steels. Mater. Sci. Eng. A 2014, 617, 200–210. [Google Scholar]
- Liu, Q. The influence of hydrogen on 3.5NiCrMoV steel studied using the linearly increasing stress test. Corros. Sci. J. Environ. Degrad. Mater. Control. 2013, 67, 193–203. [Google Scholar] [CrossRef]
- Pedergnana, A.; Ollé, A.; Evans, A.A. A new combined approach using confocal and scanning electron microscopy to image surface modifications on quartzite. J. Archaeol. Sci. Rep. 2020, 30, 102237. [Google Scholar] [CrossRef]
- Li, H.; Hu, Z.; Chen, Y.; Sun, Q. Modeling mechanical properties and plastic strain for hot forming-quenching AA6061 aluminum alloy parts. Int. J. Lightweight Mater. Manuf. 2020, 3, 66–72. [Google Scholar] [CrossRef]
- Chanyathunyaroj, K.; Phetchcrai, S.; Laungsopapun, G.; Rengsomboon, A. Fatigue characteristics of 6061 aluminum alloy subject to 3.5% NaCl environment. Int. J. Fatigue 2020, 133, 105420. [Google Scholar] [CrossRef]
- Matsuda, S.; Ichitani, K.; Kanno, M. Visualization of hydrogen diffusion path by a high sensitivity hydrogen microprint technique. In Environment Induced Cracking Materials; Elsevier: Amsterdam, The Netherlands, 2008; pp. 239–248. [Google Scholar]
- Mohtadi-Bonab, M.A.; Szpunar, J.A.; Razavi-Tousi, S.S. A comparative study of hydrogen induced cracking behavior in API 5L X60 and X70 pipeline steels. Eng. Fail. Anal. 2013, 33, 163e75. [Google Scholar] [CrossRef]
- Nakatani, M.; Fujihara, H.; Sakihara, M.; Minoshima, K. Influence of irreversible hydrogen and stress cycle frequency on the fatigue crack growth property in high-strength steel and hydrogen visualization. Procedia Eng. 2011, 10, 2381e6. [Google Scholar] [CrossRef] [Green Version]
- Kyung-Oh, B.; Hyung-Seop, S.; Un-Bong, B. Quantitative evaluation of hydrogen embrittlement susceptibility in various steels for energy use using an in-situ small punch test. Int. J. Hydrogen Energy 2021, 46, 20107–20118. [Google Scholar] [CrossRef]
- Liao, H.; Lin, J.; Lee, S. Effect of pre-immersion on the SCC of heat-treated AA7050 in an alkaline 3.5%NaCl. Corros. Sci. 2009, 51, 209–216. [Google Scholar] [CrossRef]
- Badawy, W.A.; Al-Kharafi, F.M.; El-Azab, A.S. Electrochemical behavior and corrosion inhibition of Al, Al-6061, and Al-Cu in neutral aqueous solutions. Corros. Sci. 1999, 4, 709–727. [Google Scholar] [CrossRef]
- Hillier, E.M.K.; Robinson, M.J. Hydrogen embrittlement of high strength steel electroplated with zinc–cobalt alloys. Corros. Sci. 2004, 46, 715–727. [Google Scholar] [CrossRef] [Green Version]
- Melendez, J.; de Nooijer, N.; Coenen, K.; Fernandez, E. Effect of Au addition on hydrogen permeation and the resistance to H2S on Pd-Ag alloy membranes. J. Membr. Sci. 2017, 542, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Zakorchemna, I.; Carmona, N.; Zakroczymski, T. Hydrogen permeation through sol-gel-coated iron during galvanostatic charging. Electrochim. Acta 2008, 53, 8154–8160. [Google Scholar] [CrossRef]
- Nemanič, V. Hydrogen permeation barriers: Basic requirements, materials selection, deposition methods, and quality evaluation. Nucl. Mater. Energy 2019, 19, 451–457. [Google Scholar] [CrossRef]
- Checchetto, R.; Chayahara, A.; Horino, H.; Miotello, A.; Fujii, K. A study of deuterium permeation through thin BN films. Thin Solid Film. 1997, 299, 5–9. [Google Scholar] [CrossRef]
- Bazzanella, N.; Checchetto, R.; Miotello, A.; Patton, B.; Kale, A.N.; Kothari, D.C. High temperature efficient deuterium permeation and oxidation (Al,Ti)N barriers deposited on stainless steel. Appl. Phys. Lett. 2002, 81, 3762–3764. [Google Scholar] [CrossRef]
- Wu, K.; Lu, X.; Zhou, P.; Li, W.; Jin, X. Improved resistance to hydrogen embrittlement by tailoring the stability of retained austenite. Mater. Sci. Technol. 2017, 33, 1497–1504. [Google Scholar] [CrossRef]
- Wei, L.; Han, B.; Ye, F.; Ditta, A.; Li, L.; Xu, Y.; Wu, S. Influencing mechanisms of heat treatments on microstructure and comprehensive properties of Al–Zn–Mg–Cu alloy formed by spray forming. J. Mater. Res. Technol. 2020, 9, 6850–6858. [Google Scholar] [CrossRef]
- Yang, J.; Huang, F.; Guo, Z.; Rong, Y.; Chen, N. Effect of retained austenite on the hydrogen embrittlement of a medium carbon quenching and partitioning steel with refined microstructure. Mater. Sci. Eng. A 2016, 665, 76–85. [Google Scholar] [CrossRef]
- Fasching, A.A.; Edwards, G.R.; David, S.A. Grain refinement and hydrogen embrittlement in iron aluminide alloy FA129. Sci. Technol. Weld. Join. 1997, 2, 167–173. [Google Scholar] [CrossRef]
- Tsay, L.W.; Lu, H.L.; Chen, C. The effect of grain size and aging on hydrogen embrittlement of a maraging steel. Corros. Sci. 2008, 50, 2506–2511. [Google Scholar] [CrossRef]
- Park, I.; Lee, S.; Jeon, H.; Lee, Y. The advantage of grain refinement in the hydrogen embrittlement of Fe–18Mn–0.6C twinning-induced plasticity steel. Corros. Sci. 2015, 93, 63–69. [Google Scholar] [CrossRef]
- Kristensen, P.K.; Niordson, C.F.; Martínez-Pañeda, E. A phase field model for elastic-gradient-plastic solids undergoing hydrogen embrittlement. J. Mech. Phys. Solids 2020, 143, 104093. [Google Scholar] [CrossRef]
- Martínez-Pañeda, E.; Niordson, C.F.; Gangloff, R.P. Strain gradient plasticity-based modeling of hydrogen environment assisted cracking. Acta Mater. 2016, 117, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zheng, W.G. Performance Study for Ultra-high-strength Aluminum Cold Spray Layer. Hot Work. Technol. 2013, 42, 132–133. [Google Scholar]
- Lu, K. Gradient nanostructured materials. Acta Metall. Sin. 2015, 51, 1–10. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhao, S.; Ma, H.; Wang, H.; Hua, L.; Fu, S. Analysis of Hydrogen Embrittlement on Aluminum Alloys for Vehicle-Mounted Hydrogen Storage Tanks: A Review. Metals 2021, 11, 1303. https://doi.org/10.3390/met11081303
Chen Y, Zhao S, Ma H, Wang H, Hua L, Fu S. Analysis of Hydrogen Embrittlement on Aluminum Alloys for Vehicle-Mounted Hydrogen Storage Tanks: A Review. Metals. 2021; 11(8):1303. https://doi.org/10.3390/met11081303
Chicago/Turabian StyleChen, Yizhe, Shilong Zhao, Huijuan Ma, Hui Wang, Lin Hua, and Shuang Fu. 2021. "Analysis of Hydrogen Embrittlement on Aluminum Alloys for Vehicle-Mounted Hydrogen Storage Tanks: A Review" Metals 11, no. 8: 1303. https://doi.org/10.3390/met11081303
APA StyleChen, Y., Zhao, S., Ma, H., Wang, H., Hua, L., & Fu, S. (2021). Analysis of Hydrogen Embrittlement on Aluminum Alloys for Vehicle-Mounted Hydrogen Storage Tanks: A Review. Metals, 11(8), 1303. https://doi.org/10.3390/met11081303







