Study on Roasting for Selective Lithium Leaching of Cathode Active Materials from Spent Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Materials
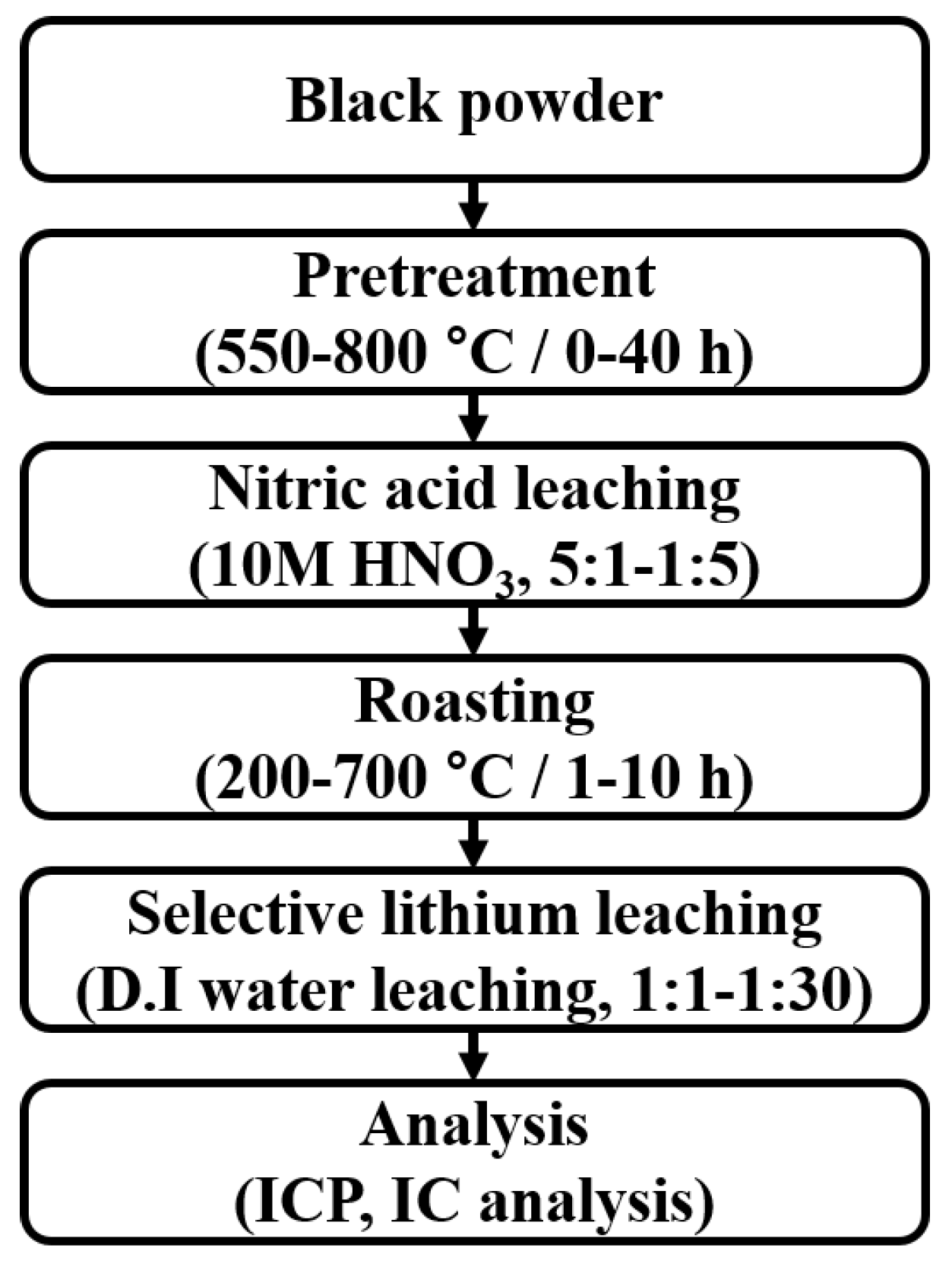
2.2. Experimental Procedure
2.2.1. Pretreatment
2.2.2. Nitric Acid Leaching
2.2.3. Roasting
2.2.4. Selective Lithium Leaching in DI Water
3. Results and Discussion
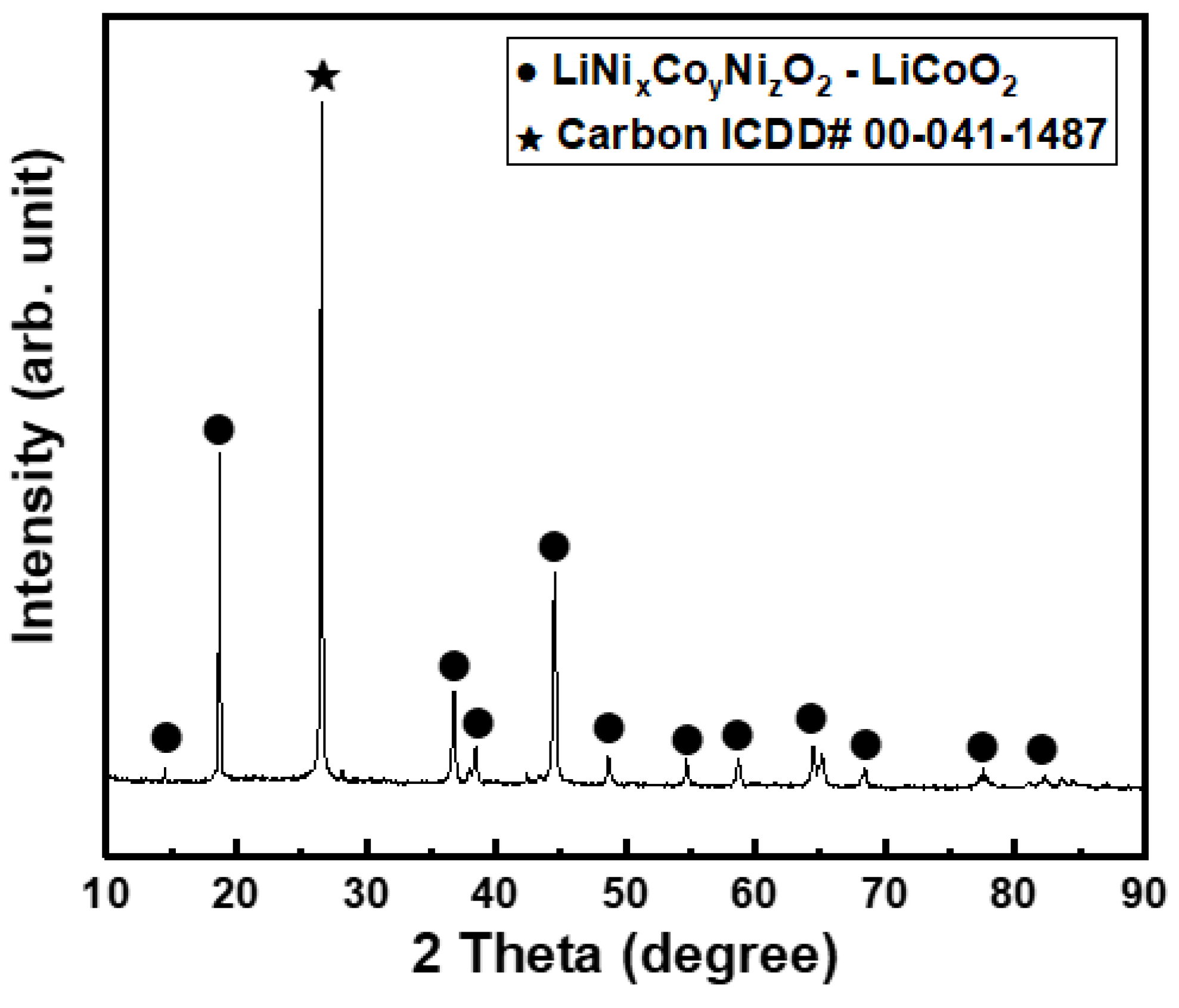
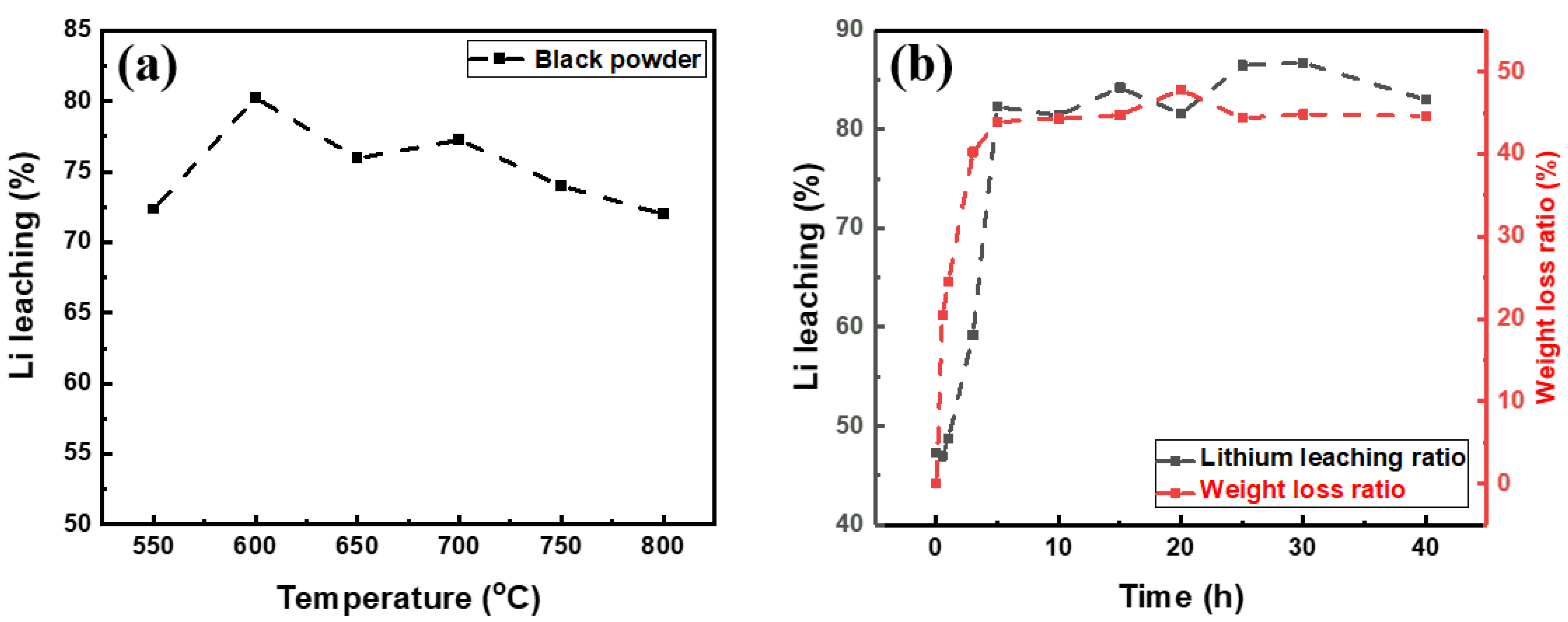
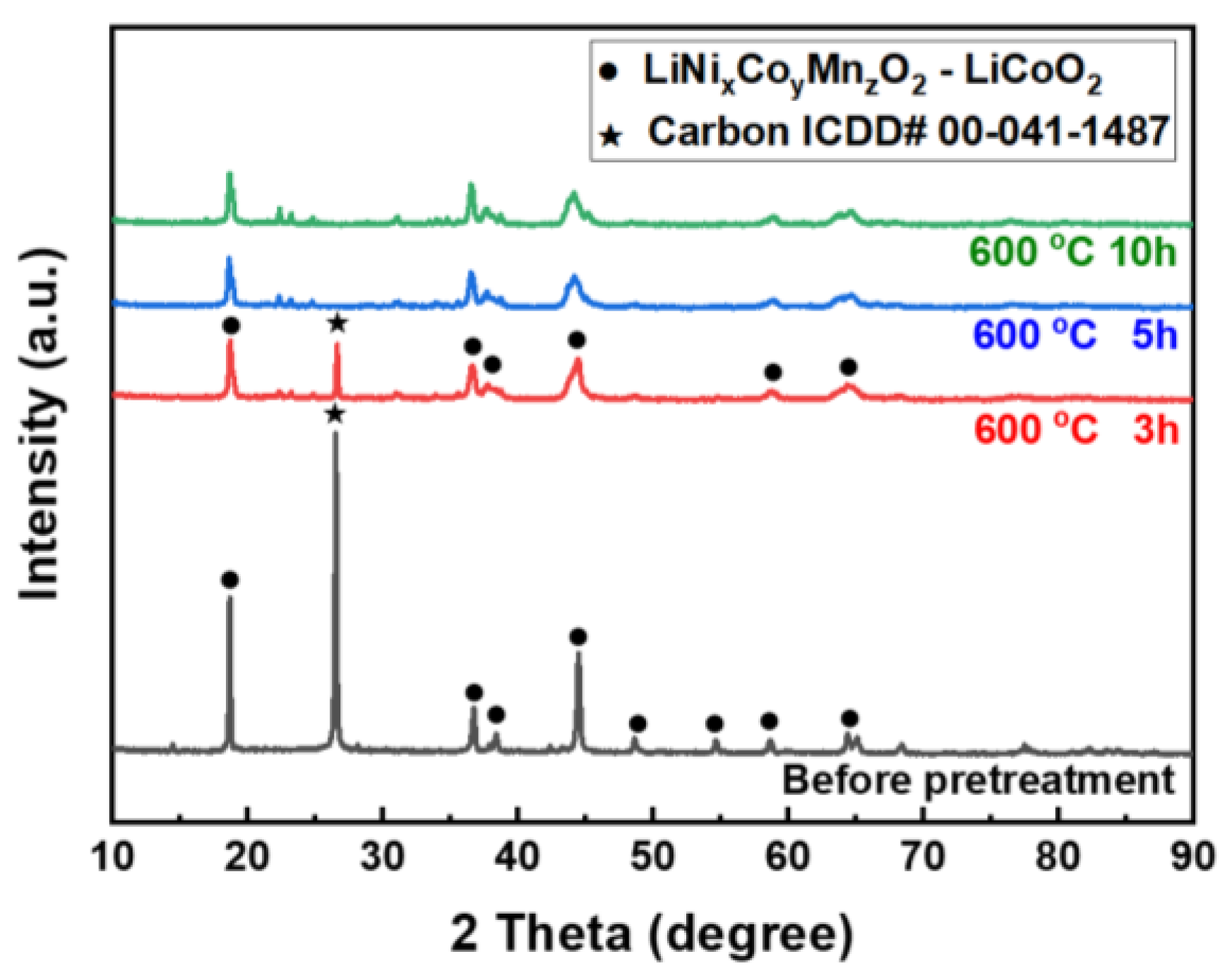
3.1. Effect of Pretreatment
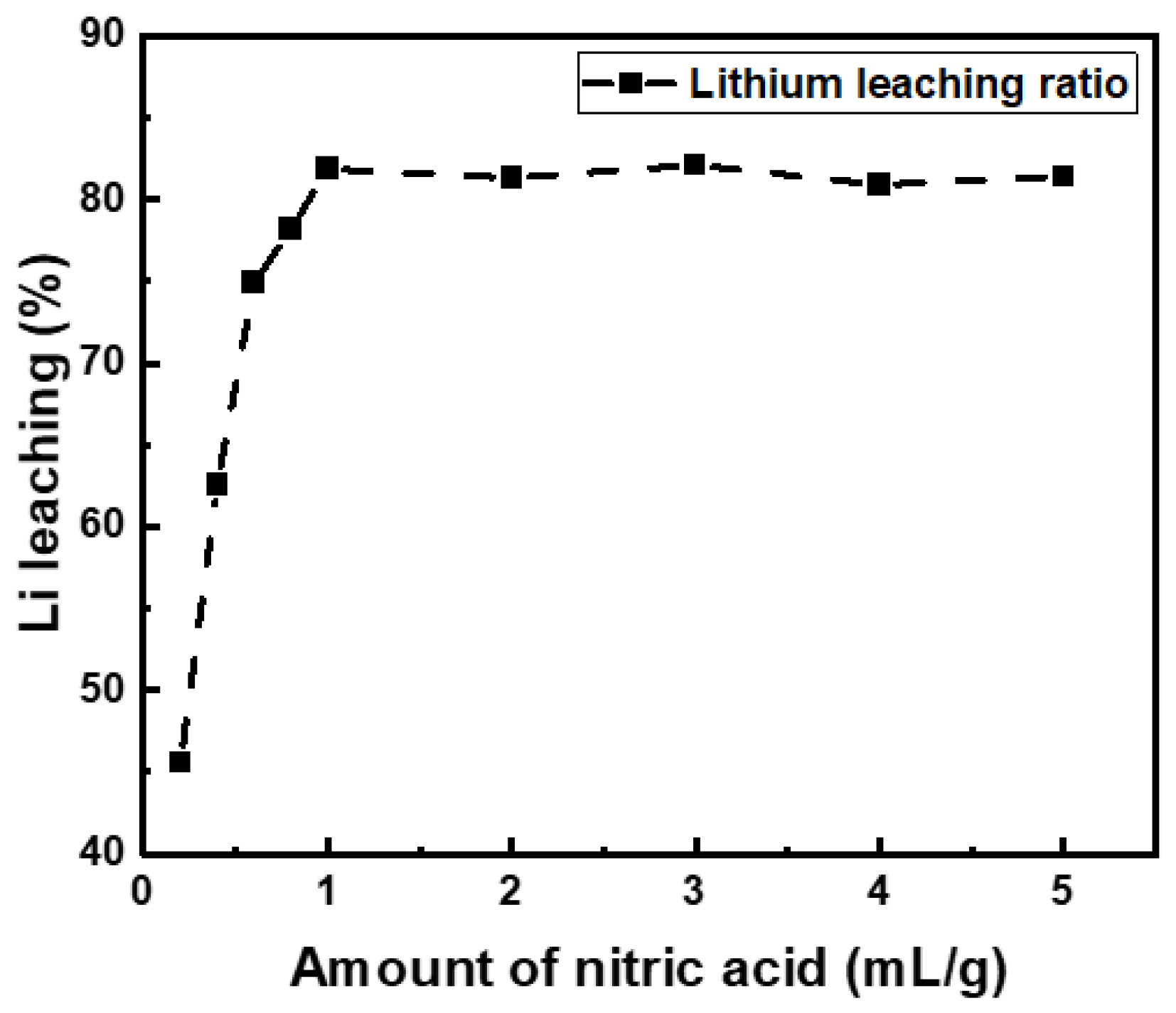
3.2. Effect of Nitric Acid Leaching
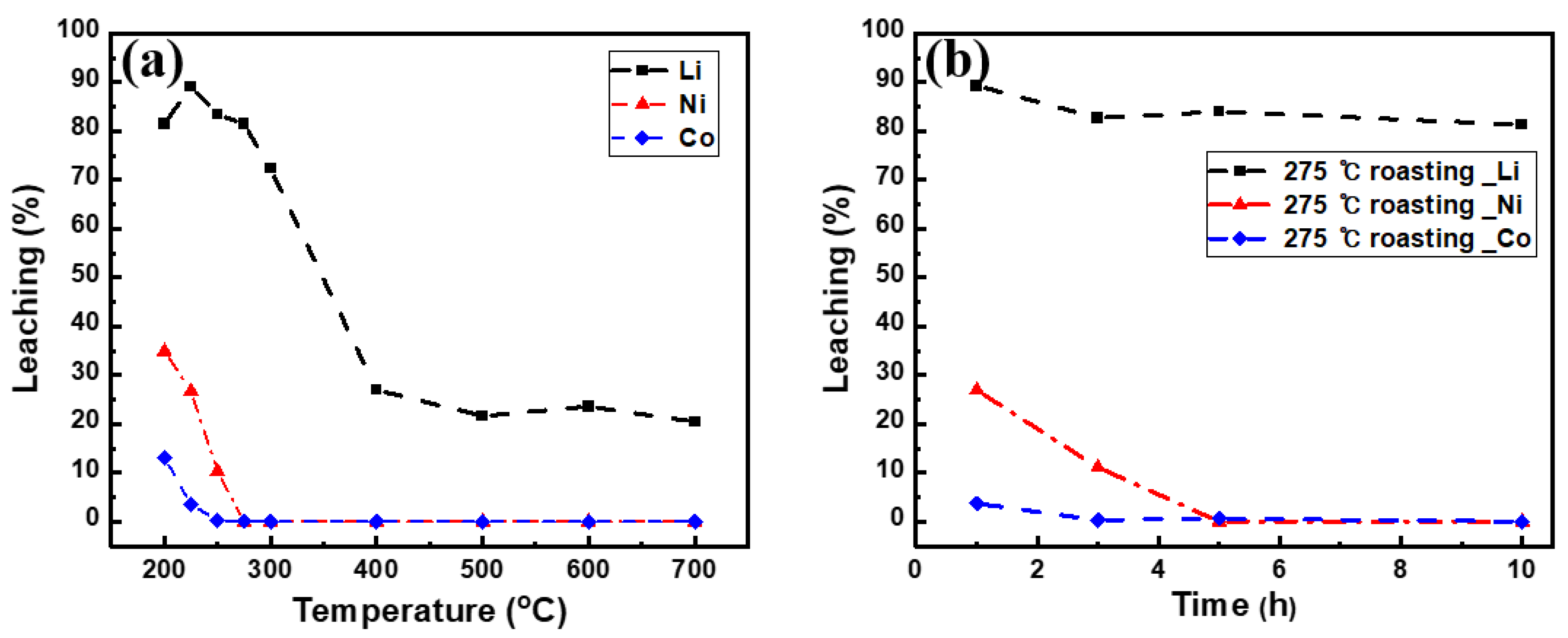
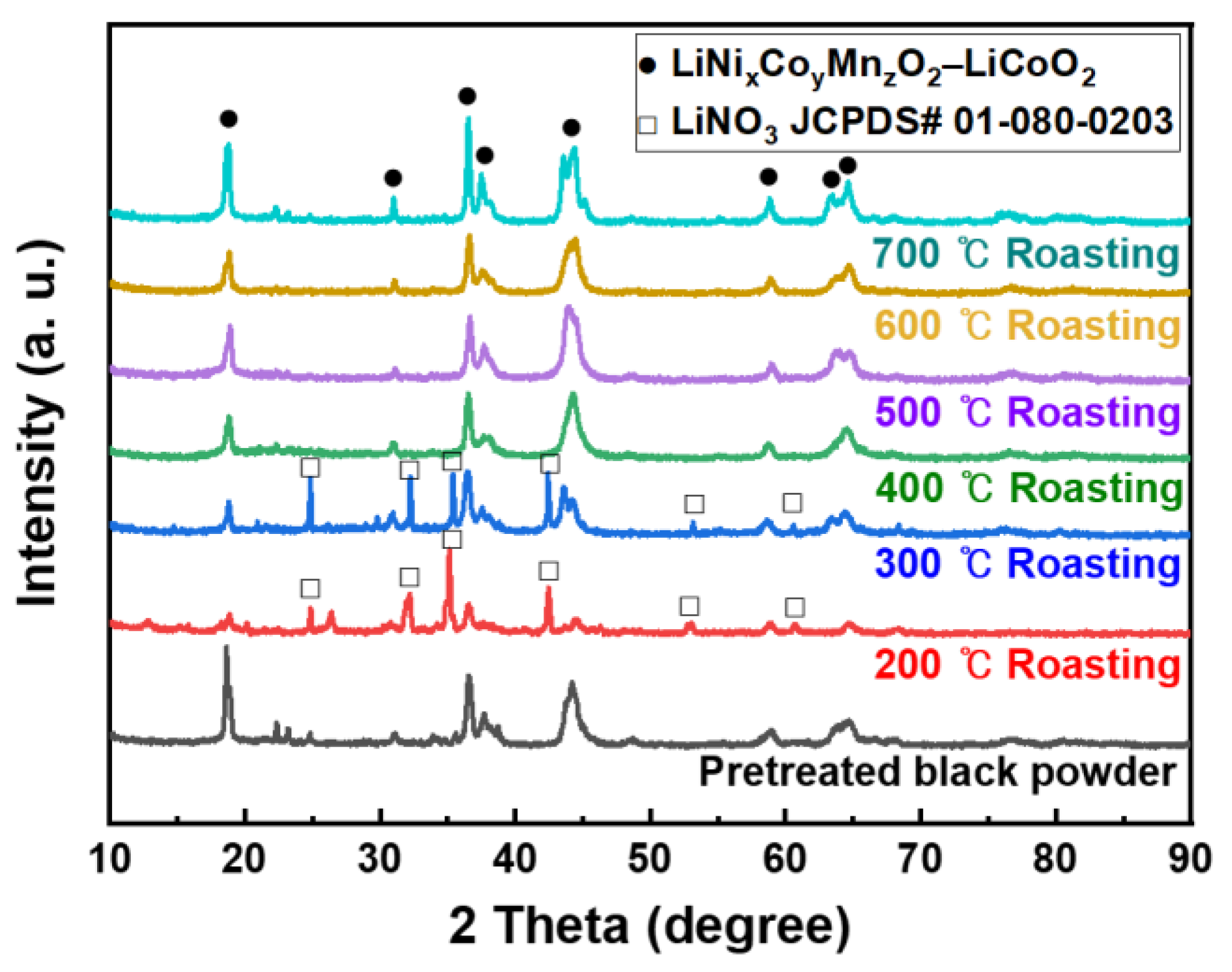
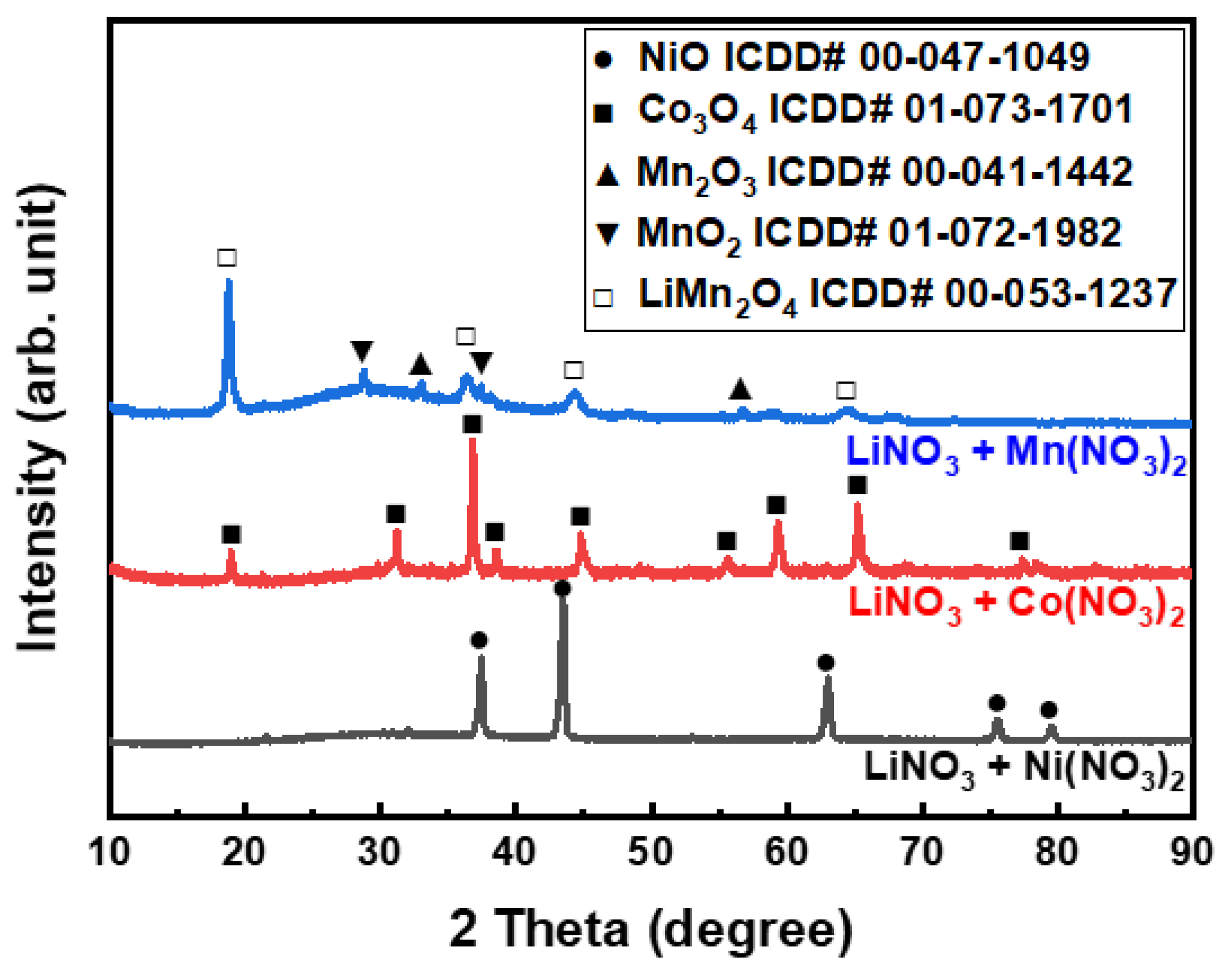
3.3. Effect of Roasting
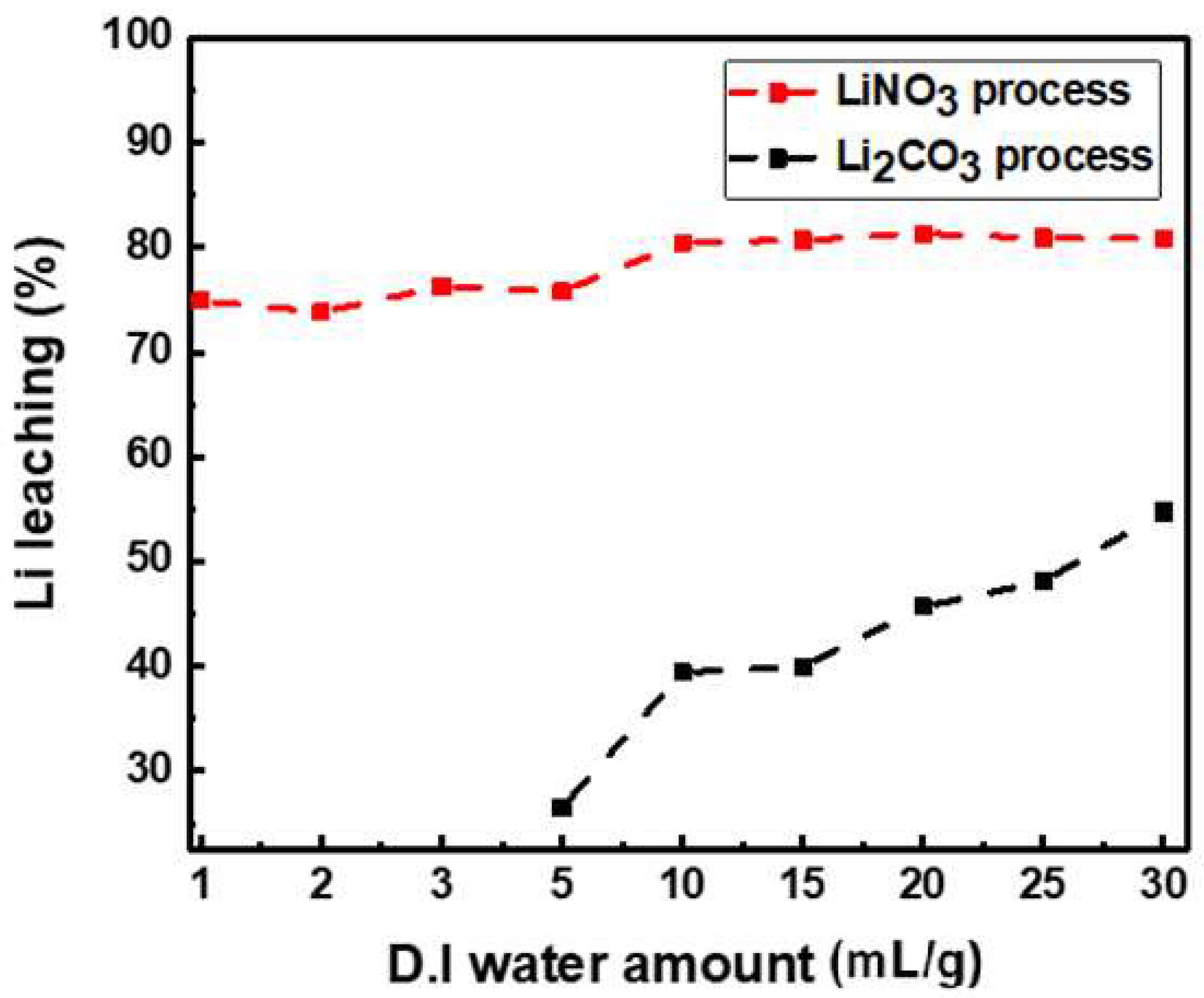
3.4. Effect of Solid–Liquid Ratio
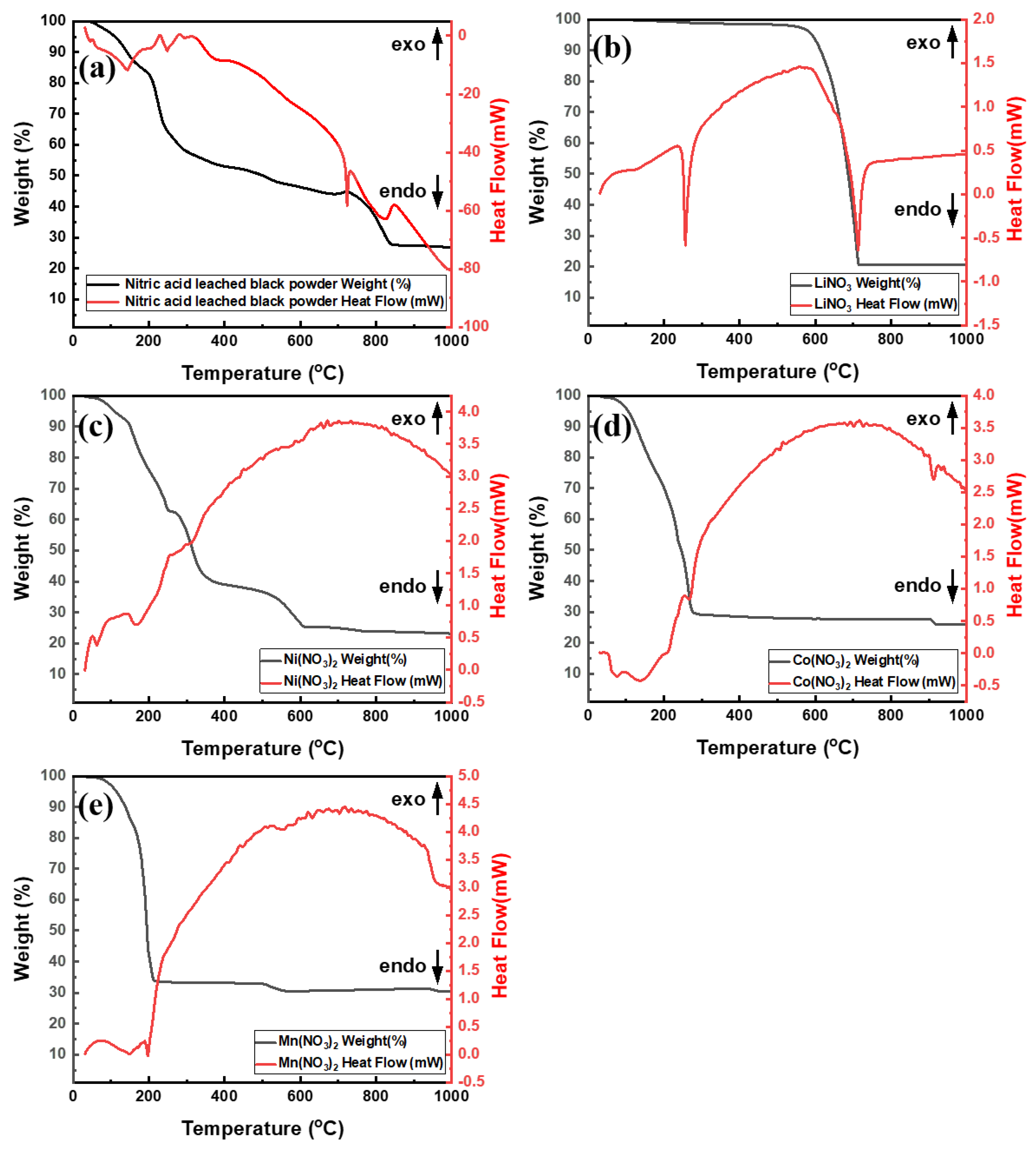
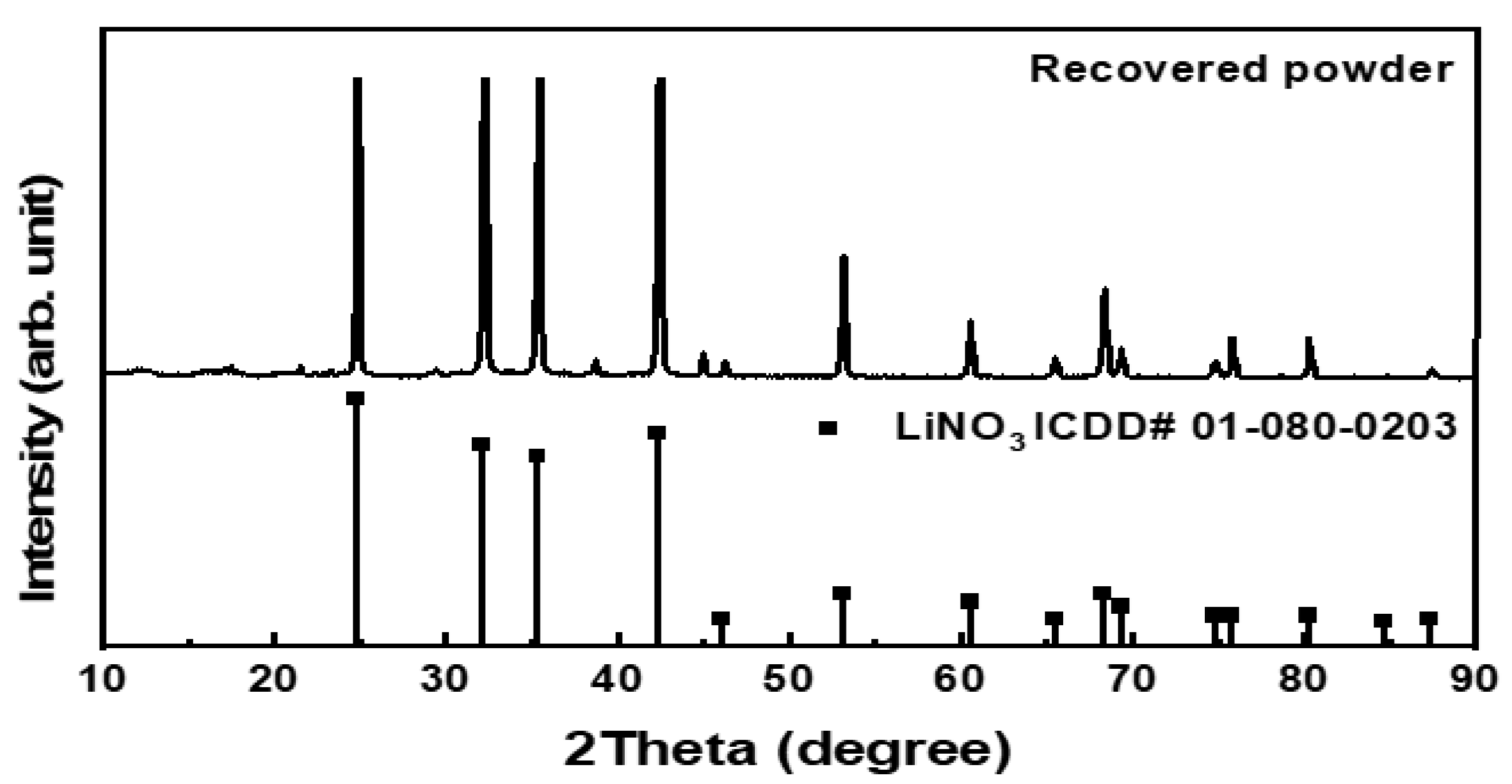
3.5. Recovered Powder Analysis
4. Conclusions
- The organic matter in the black power led to a decrease in the lithium leaching rate. Thus, organic substances, including carbon, should be removed through pretreatment. To obtain optimal results, pretreatment should be conducted for more than 5 h at 600 °C.
- LiNixCoyMnzO2 and LiCoO2 in the black powder were converted into nitrate compounds such as LiNO3, Co(NO3)3, Ni(NO3)2, and Mn(NO3)2 by nitric acid leaching with more than 1 mL/g of 10 M nitric acid. The residual nitric acid that was not involved in the reaction was removed during the roasting process.
- With the exception of LiNO3, the nitrate compounds were converted into oxides through roasting, allowing selective lithium leaching. Roasting at 275 °C for a minimum of 5 h was found to be adequate for selective lithium leaching. When leaching was performed after roasting above 400 °C, the leaching rate decreased significantly owing to the formation of LiMn2O4, which could not be leached in DI water.
- When the sample was leached with DI water (10 mL/g) after roasting, over 80% of the lithium was leached. This indicates that more than twice the amount of lithium can be recovered compared with the lithium carbonate recovery method by employing carbon reduction at the same solid–liquid ratio.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lévay, P.Z.; Drossinos, Y.; Thiel, C. The effect of fiscal incentives on market penetration of electric vehicles: A pairwise comparison of total cost of ownership. Energy Policy 2017, 105, 524–533. [Google Scholar] [CrossRef]
- Global EV Outlook 2018: Towards Cross-Modal Electrification; International Energy Agency: Paris, France, 2018. [CrossRef]
- Yanghwa, K.; Jaewan, L.; Taeck, L.O. Electric vehicle market and battery related technology research trends. Trans. Korean Hydrog. New Energy Soc. 2019, 30, 362–368. [Google Scholar] [CrossRef]
- Whittingham, M.S. History, evolution, and future status of energy storage. Proc. IEEE. 2012, 100, 1518–1534. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef]
- Juntao, H.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Chao, P.; Liu, F.; Wang, Z.; Wilson, B.P.; Lundström, M. Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. J. Power Sources 2019, 415, 179–188. [Google Scholar] [CrossRef]
- Orlova, V.T. IUPAC-NIST solubility data series. 89. Alkali metal nitrates. Part 1. Lithium nitrate. J. Phys. Chem. Ref. Data 2010, 39, 033104. [Google Scholar] [CrossRef]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Johnston, H.S.; Foering, L.; Tao, Y.S.; Messerly, G.H. The kinetics of the thermal decomposition of nitric acid vapor. J. Am. Chem. Soc. 1951, 73, 2319–2321. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Fan-Yuan, L.; Tsong-Huei, C.; Chuin-Tih, Y. Thermal decomposition of metal nitrates in air and hydrogen environments. J. Phys. Chem. B 2003, 107, 1044–1047. [Google Scholar] [CrossRef]
- Jung, Y.J.; Son, S.H.; Park, S.C.; Kim, Y.H.; Yoo, B.Y.; Lee, M.S. Study on selective lithium leaching effect on roasting conditions of the waste electric vehicle cell powder. J. Kor. Inst. Resour. Recycl. 2019, 28, 79–86. [Google Scholar] [CrossRef]

| ICP Analysis | Component (Wt. %) | ||||||
|---|---|---|---|---|---|---|---|
| Ni | Co | Mn | Li | Al | Cu | Fe | |
| Black powder | 15.63 | 8.37 | 7.64 | 3.92 | 3.20 | 1.15 | 0.38 |
| IC Analysis | Component (mg/kg) | ||||
|---|---|---|---|---|---|
| Li | NO3 | F | SO4 | Na | |
| Recovered LiNO3 | 98,009 | 864,826 | 1982 | 2530 | 2472 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.; Yoo, B.; Park, S.; Kim, Y.; Son, S. Study on Roasting for Selective Lithium Leaching of Cathode Active Materials from Spent Lithium-Ion Batteries. Metals 2021, 11, 1336. https://doi.org/10.3390/met11091336
Jung Y, Yoo B, Park S, Kim Y, Son S. Study on Roasting for Selective Lithium Leaching of Cathode Active Materials from Spent Lithium-Ion Batteries. Metals. 2021; 11(9):1336. https://doi.org/10.3390/met11091336
Chicago/Turabian StyleJung, Yeonjae, Bongyoung Yoo, Sungcheol Park, Yonghwan Kim, and Seongho Son. 2021. "Study on Roasting for Selective Lithium Leaching of Cathode Active Materials from Spent Lithium-Ion Batteries" Metals 11, no. 9: 1336. https://doi.org/10.3390/met11091336
APA StyleJung, Y., Yoo, B., Park, S., Kim, Y., & Son, S. (2021). Study on Roasting for Selective Lithium Leaching of Cathode Active Materials from Spent Lithium-Ion Batteries. Metals, 11(9), 1336. https://doi.org/10.3390/met11091336







