Thermal Spray Processes in Concentrating Solar Power Technology
Abstract
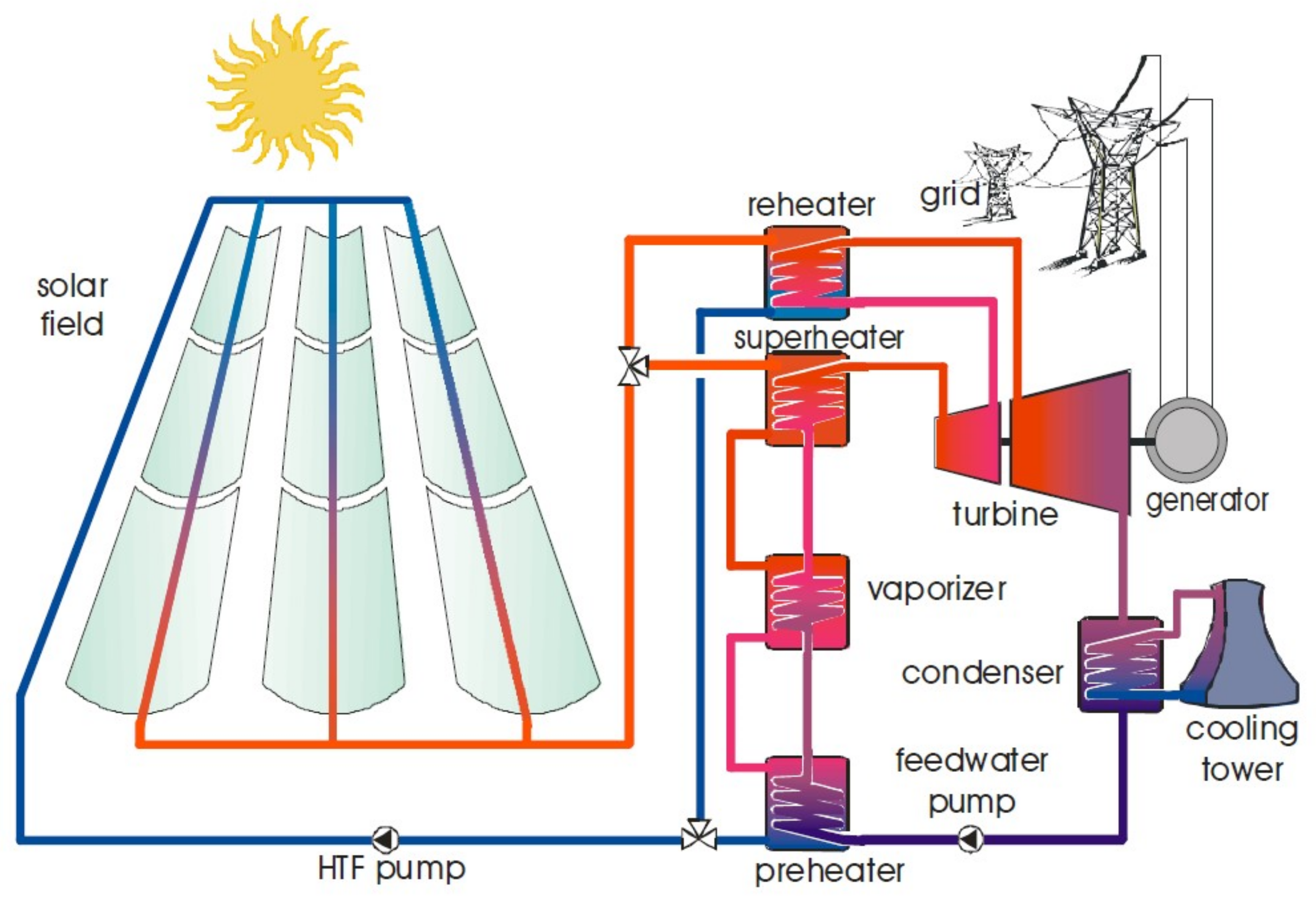
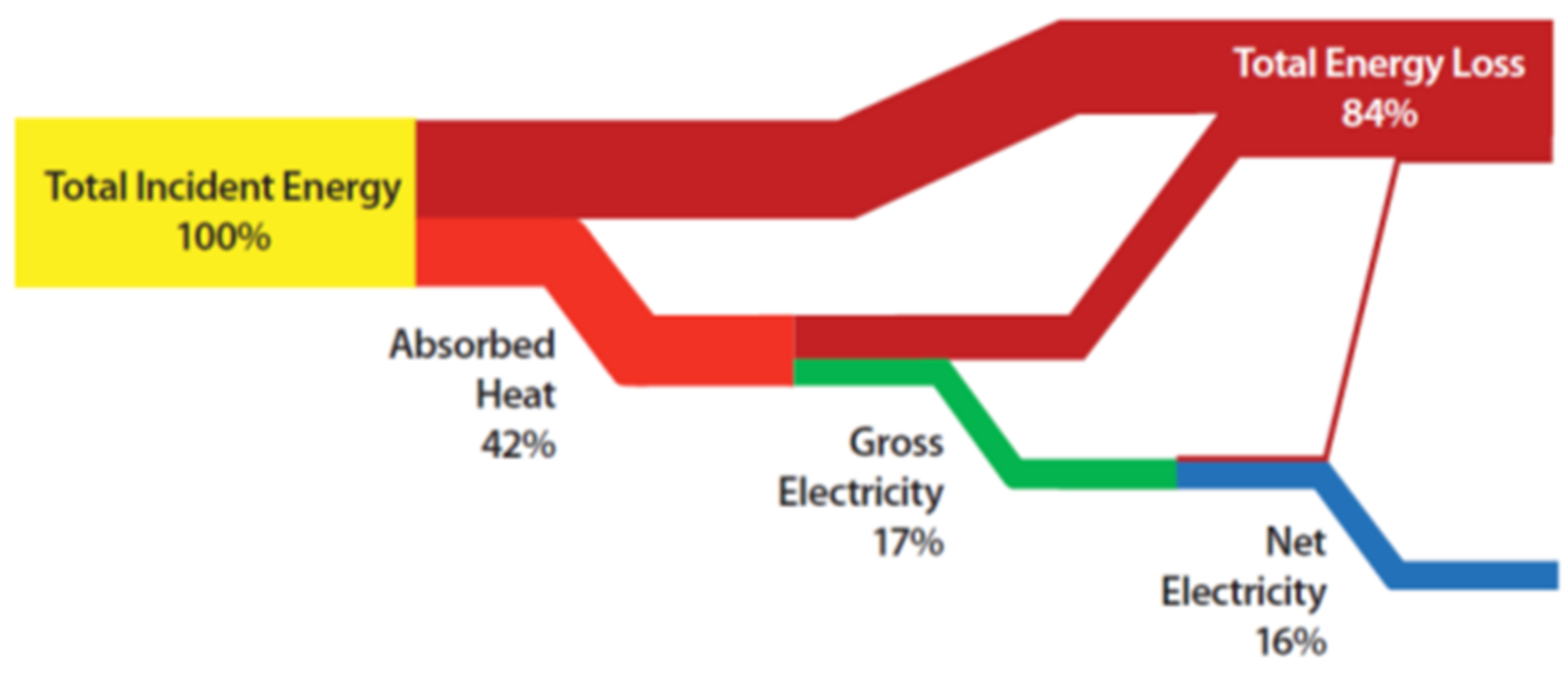
1. Introduction
2. Thermal Spray Processes for Manufacturing Solar Selective Coatings
2.1. Introduction
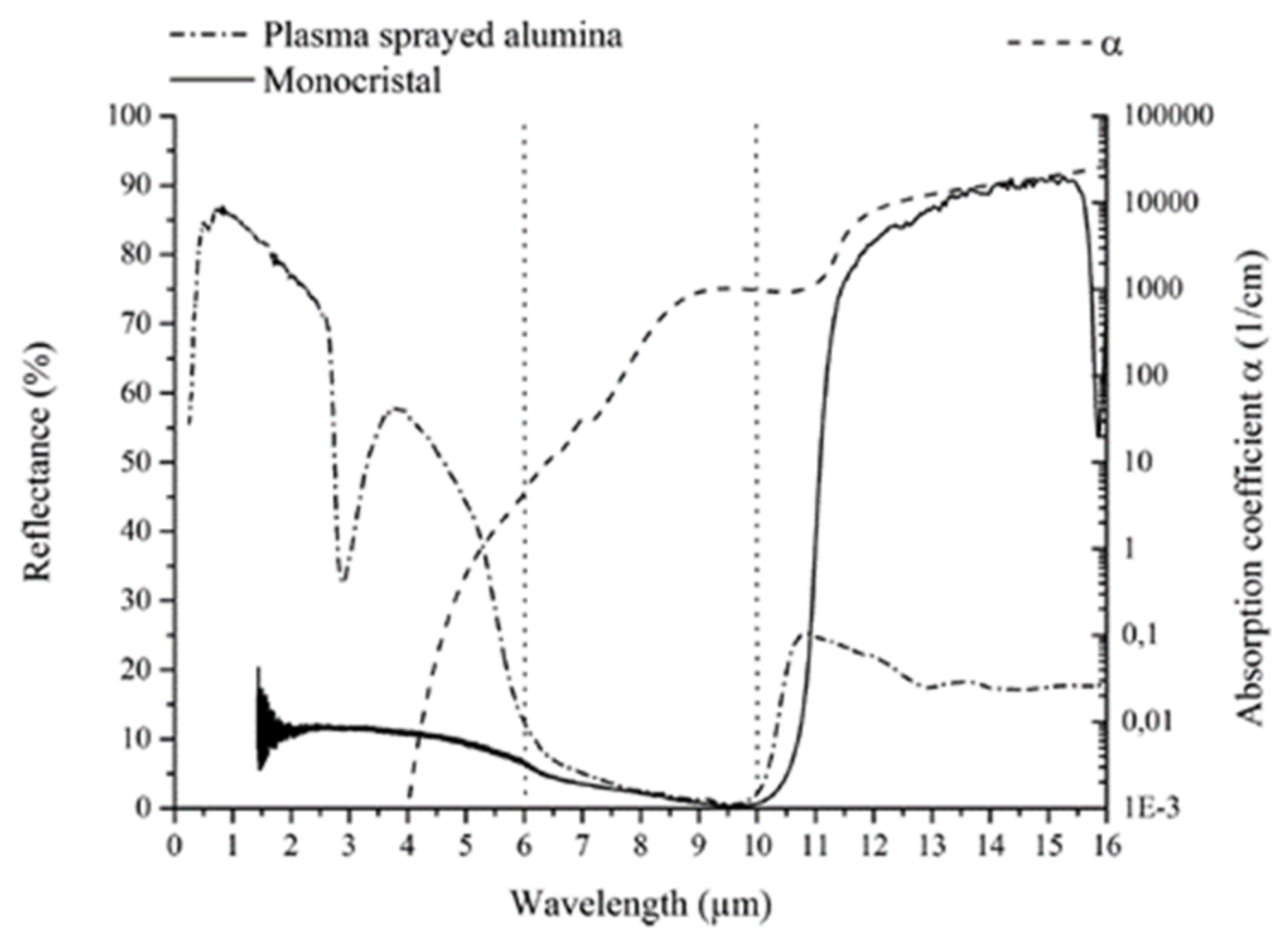
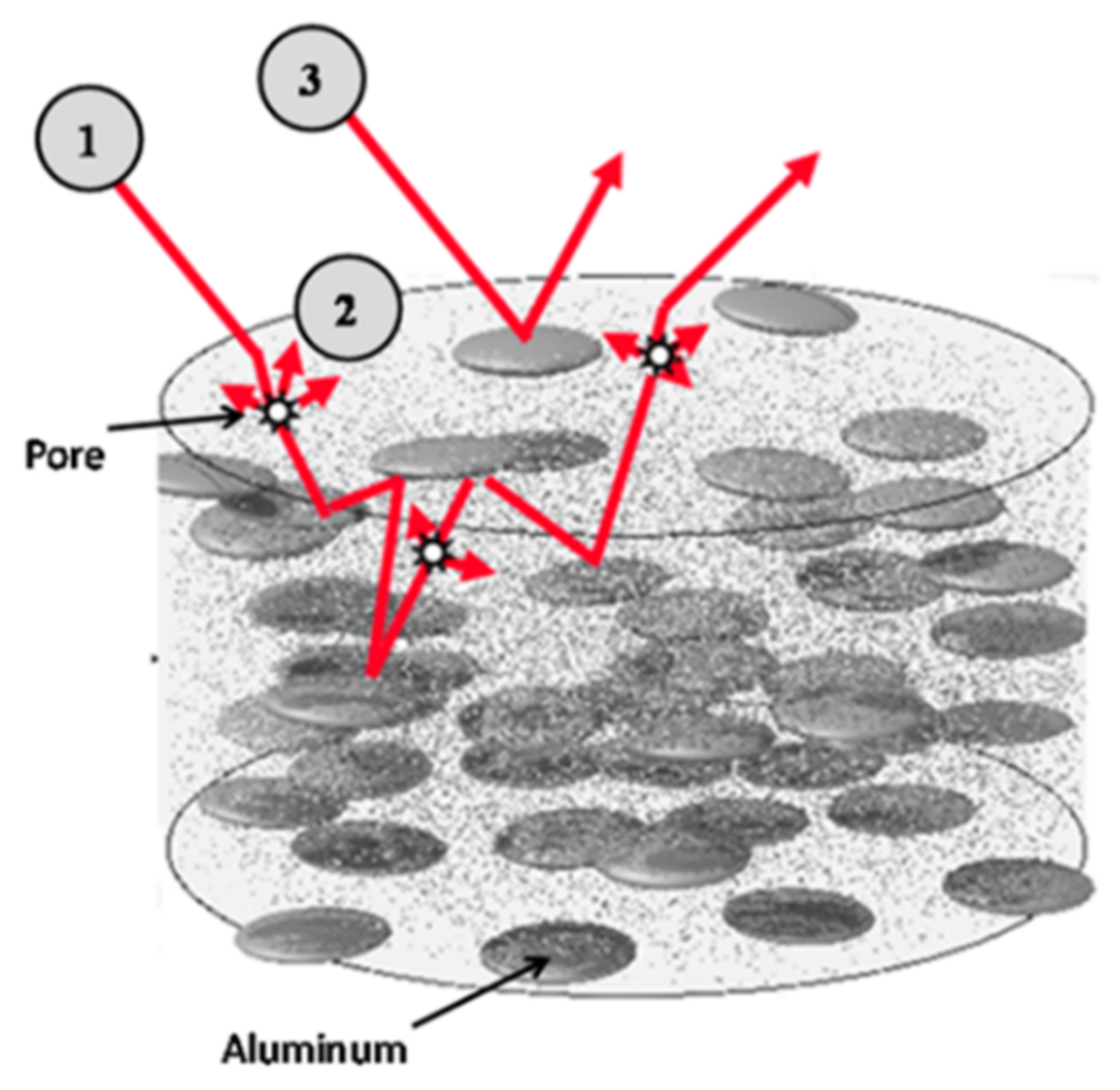
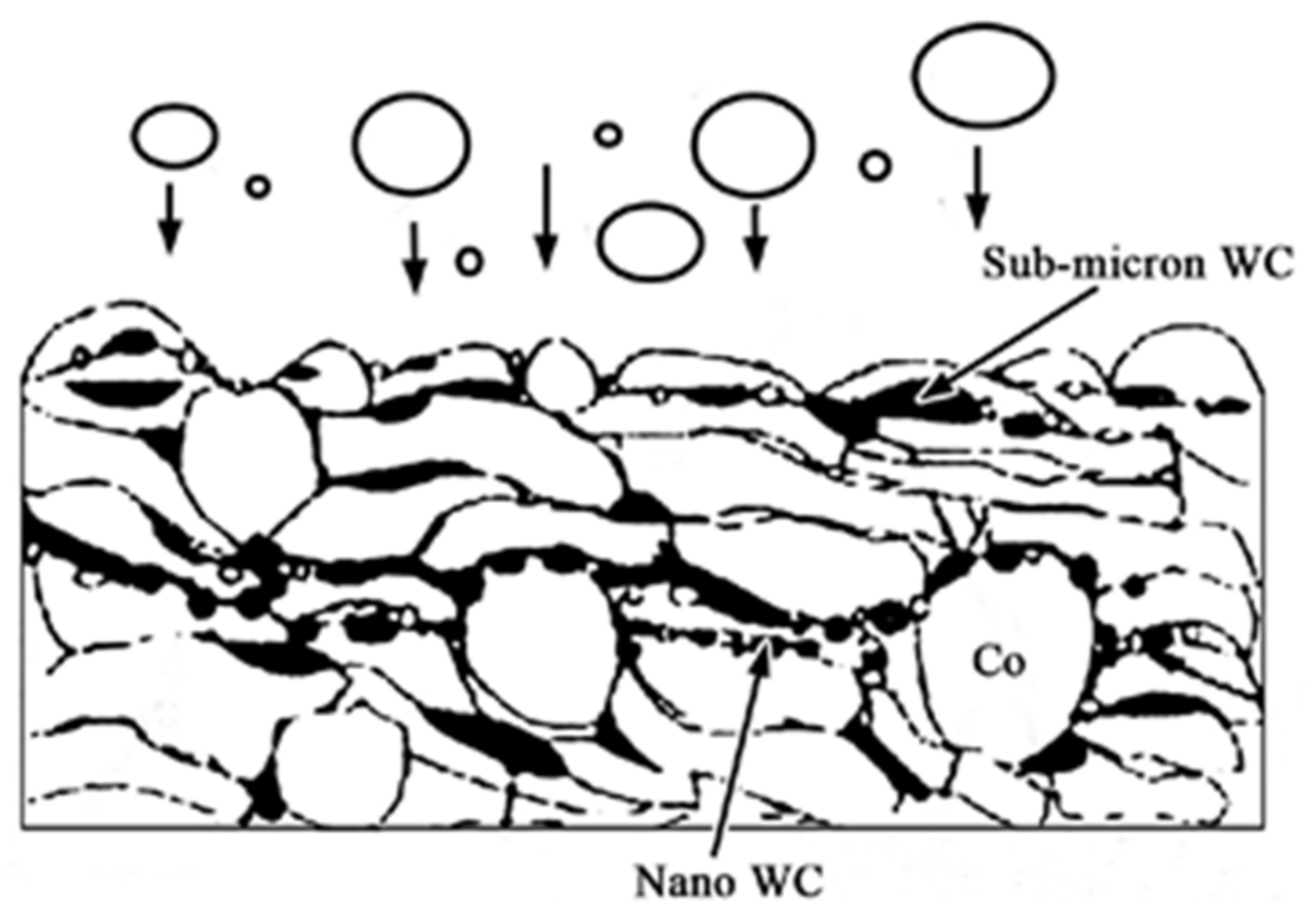
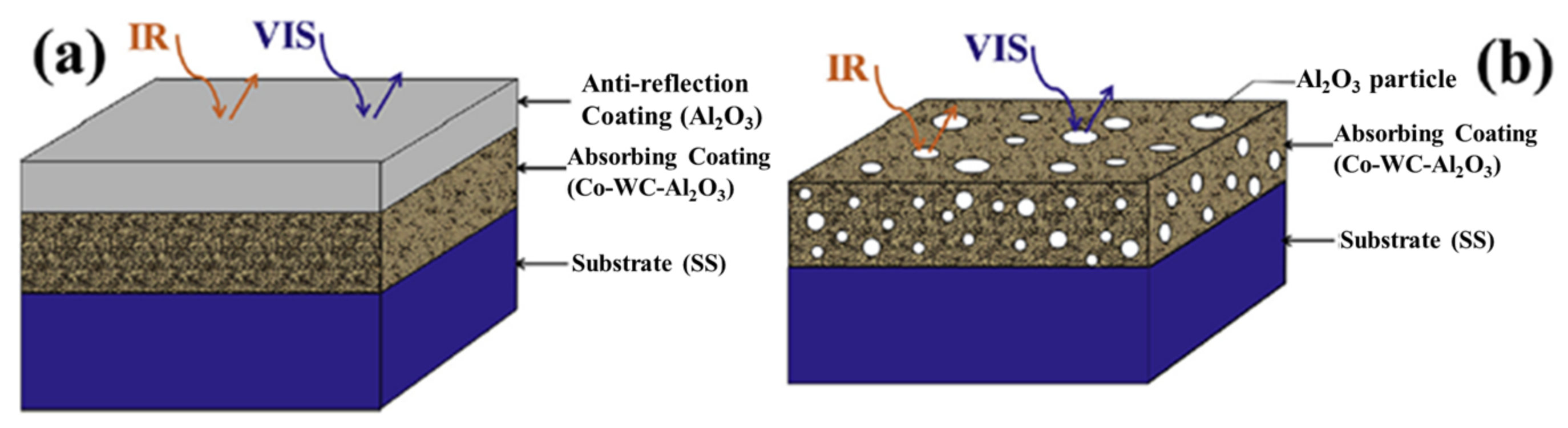
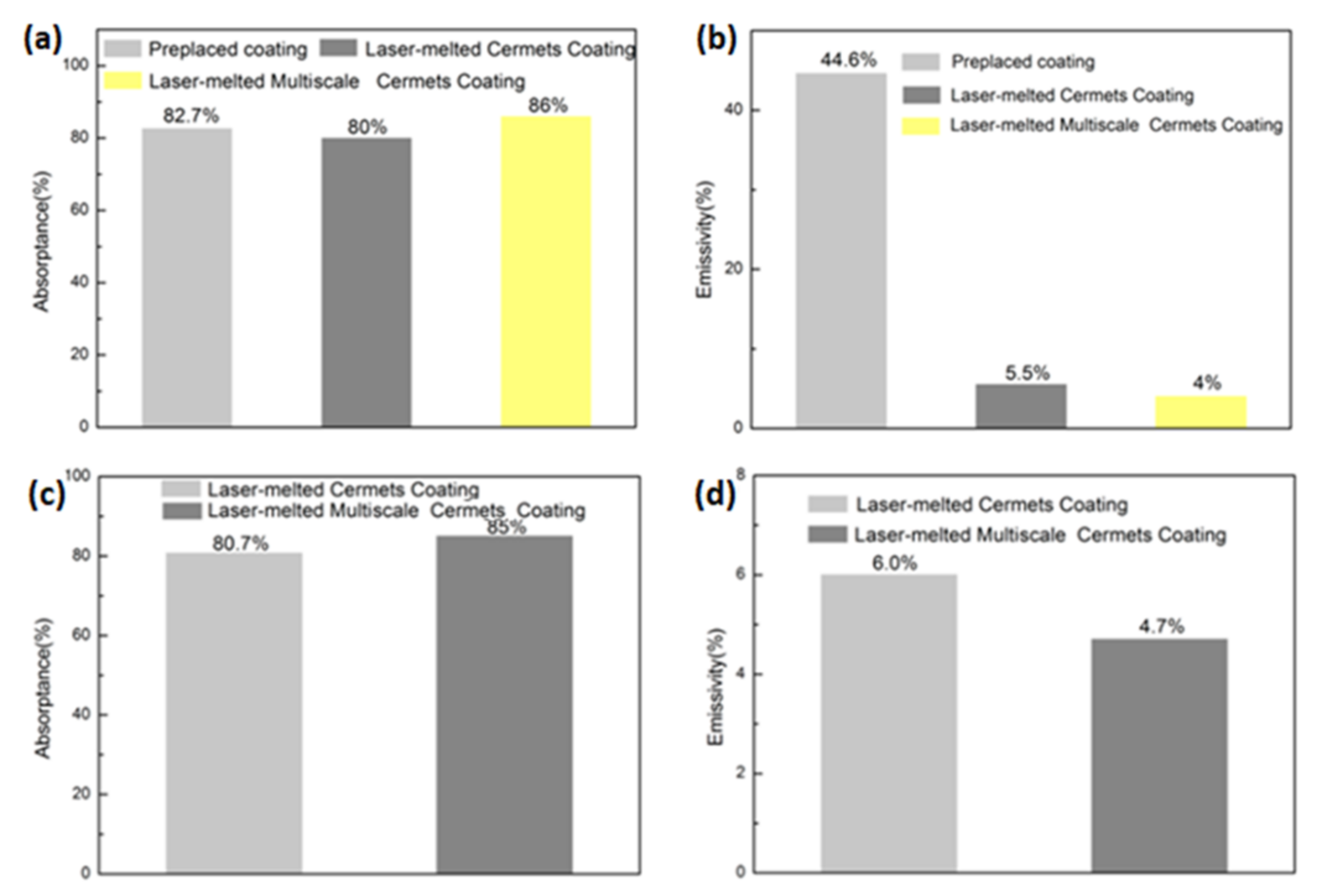
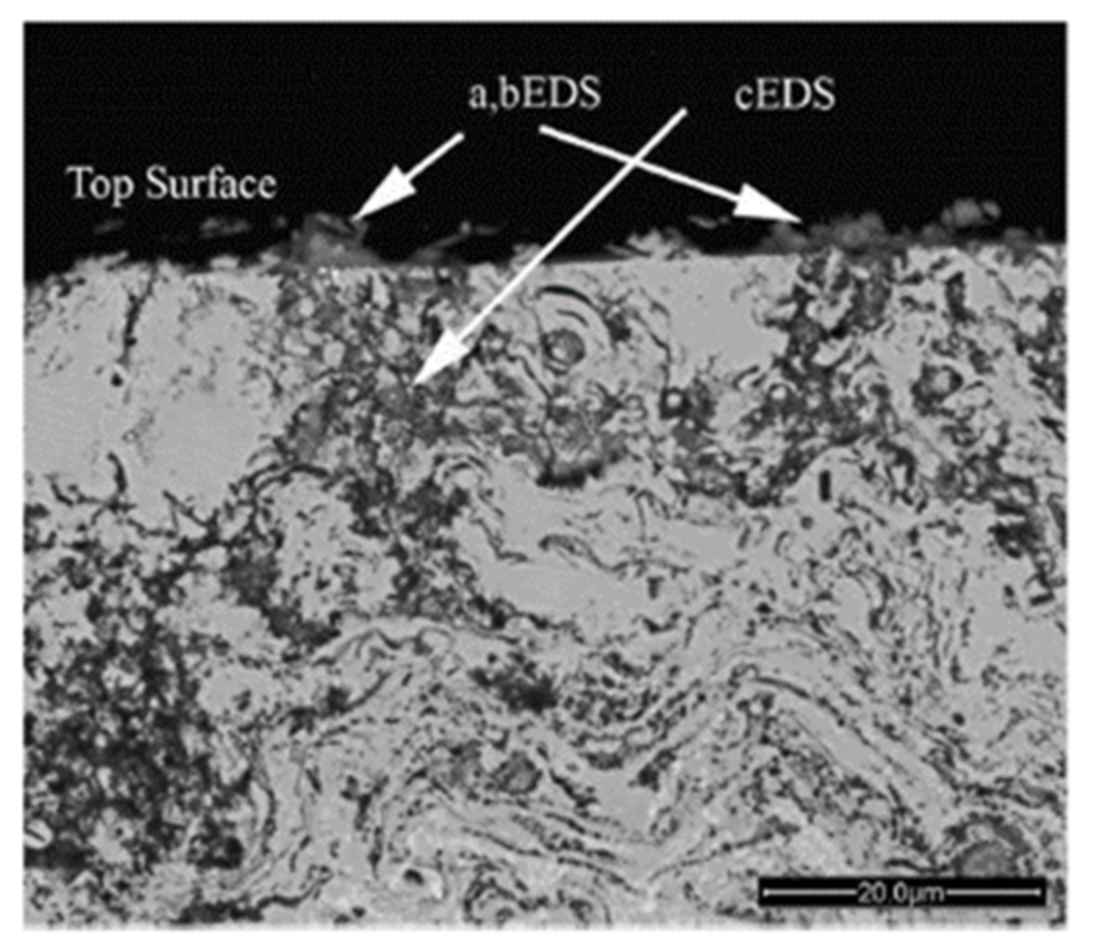
2.2. Thermal Spray Processes for Selective Absorber
3. Thermal Spray Coatings for Molten Salt Protection
3.1. Introduction
- Mitigation of the intermittency of the solar energy and better matching the electricity demand; in addition, the availability of storage allows the power cycle system to operate at a constant rate achieving the highest efficiency, increasing the overall efficiency of the CSP plant;
- Transform the solar source into a dispatchable generation source of electricity—the storage allows to generate and put electricity into the grid in the period of peaks in the demand and highest prices (usually the night hours);
- Increases the annual capacity factor of the CSP plant.
3.2. Corrosion Resistant Coatings in TES System
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments

Conflicts of Interest
Disclaimer
References
- Ho, C.K.; Iverson, B.D. Review of High-Temperature Central Receiver Designs for Concentrating Solar Power. Renew. Sustain. Energy Rev. 2014, 29, 835–846. [Google Scholar] [CrossRef]
- Epstein, M.; Liebermann, D.; Rosh, M.; Shor, A.J. Solar Testing of 2 MWth Water/Steam Receiver at the Weizmann Institute Solar Tower. Sol. Energy Mater. 1991, 24, 265–278. [Google Scholar] [CrossRef]
- Pacheco, J.E.; Bradshaw, R.W.; Dawson, D.B.; De la Rosa, W.; Gilbert, R.; Goods, S.H. Final Test and Evaluation Results from the Solar Two Project; SAND2002-0120; Sandia National Laboratories: Albuquerque, NM, USA, 2002; pp. 1–294. [Google Scholar]
- Forsberg, C.W.; Peterson, P.F.; Zhao, H. High-Temperature Liquid-Fluoride-Salt Closed-Brayton-Cycle Solar Power Towers. J. Sol. Energy Eng. 2007, 129, 141–146. [Google Scholar] [CrossRef]
- Schiel, W.J.C.; Geyer, M.A. Testing an External Sodium Receiver up to Heat Fluxes of 2.5 MW/M2: Results and Conclusions from the IEA-SSPS High Flux Experiment Conducted at the Central Receiver System of the Plataforma Solar de Almeria (Spain). Sol. Energy 1988, 41, 255–265. [Google Scholar] [CrossRef]
- Rovense, F.; Reyes-Belmonte, M.Á.; Romero, M.; González-Aguilar, J. Combined Heat/Cooling and Power Generation Using Hybrid Micro Gas Turbine in a CST Plant for a Residential off-Grid Application. AIP Conf. Proc. 2020, 2303, 080006. [Google Scholar] [CrossRef]
- Chen, R.; Romero, M.; González-Aguilar, J.; Rovense, F.; Rao, Z.; Liao, S. Design and Off-Design Performance Comparison of Supercritical Carbon Dioxide Brayton Cycles for Particle-Based High Temperature Concentrating Solar Power Plants. Energy Convers. Manag. 2021, 232, 113870. [Google Scholar] [CrossRef]
- Poullikkas, A.; Rouvas, C.; Hadjipaschalis, I.; Kourtis, G. Optimum Sizing of Steam Turbines for Concentrated Solar Power Plants. Int. J. Energy Environ. 2012, 3, 9–18. [Google Scholar]
- Rovense, F.; Reyes-Belmonte, M.A.; González-Aguilar, J.; Amelio, M.; Bova, S.; Romero, M. Application of Un-Fired Closed Brayton Cycle with Mass Flow Regulation and Particles-Based Thermal Energy Storage Systems for CSP. AIP Conf. Proc. 2019, 2126, 030047. [Google Scholar] [CrossRef]
- Rovense, F.; Reyes-Belmonte, M.A.; González-Aguilar, J.; Amelio, M.; Bova, S.; Romero, M. Flexible Electricity Dispatch for CSP Plant Using Un-Fired Closed Air Brayton Cycle with Particles Based Thermal Energy Storage System. Energy 2019, 173, 971–984. [Google Scholar] [CrossRef]
- Quaschning, V.; Geuder, N.; Richter, C.; Trieb, F. Contribution of Concentrated Solar Thermal Power For A Competitive Sustainable Energy Supply. Clean Air 2003, Lisbon. Available online: https://www.volker-quaschning.de/downloads/CleanAir2003.pdf (accessed on 11 June 2021).
- MIT Energy Initiative. The Future of Solar Energy; MIT Energy Initiative: Cambridge, MA, USA, 2015. [Google Scholar]
- Rovense, F.; Amelio, M.; Scornaienchi, N.M.; Ferraro, V. Performance Analysis of a Solar-Only Gas Micro Turbine, with Mass Flow Control. Energy Procedia 2017, 126, 675–682. [Google Scholar] [CrossRef]
- Islam, M.T.; Huda, N.; Abdullah, A.B.; Saidur, R. A Comprehensive Review of State-of-the-Art Concentrating Solar Power (CSP) Technologies: Current Status and Research Trends. Renew. Sustain. Energy Rev. 2018, 91, 987–1018. [Google Scholar] [CrossRef]
- Ambrosini, A.; Boubault, A.; Ho, C.K.; Banh, L.; Lewis, J.R. Influence of Application Parameters on Stability of Pyromark® 2500 Receiver Coatings. AIP Conf. Proc. 2019, 2126, 030002. [Google Scholar] [CrossRef]
- Ho, C.K.; Mahoney, A.R.; Ambrosini, A.; Bencomo, M.; Hall, A.; Lambert, T.N. Characterization of Pyromark 2500 Paint for High-Temperature Solar Receivers. J. Sol. Energy Eng. 2014, 136, 014502. [Google Scholar] [CrossRef]
- Bello, M.; Shanmugan, S. Achievements in Mid and High-Temperature Selective Absorber Coatings by Physical Vapor Deposition (PVD) for Solar Thermal Application-A Review. J. Alloys Compd. 2020, 839, 155510. [Google Scholar] [CrossRef]
- Kennedy, C.E. Review of Mid- to High-Temperature Solar Selective Absorber Materials; National Renewable Energy Laboratory, Cole Boulevard: Golden, CO, USA, 2002.
- Atkinson, C.; Sansom, C.L.; Almond, H.J.; Shaw, C.P. Coatings for Concentrating Solar Systems—A Review. Renew. Sustain. Energy Rev. 2015, 45, 113–122. [Google Scholar] [CrossRef]
- Xu, K.; Du, M.; Hao, L.; Mi, J.; Yu, Q.; Li, S. A Review of High-Temperature Selective Absorbing Coatings for Solar Thermal Applications. J. Mater. 2020, 6, 167–182. [Google Scholar] [CrossRef]
- Granqvist, C.G. Preparation of Thin Films and Nanostructured Coatings for Clean Tech Applications: A Primer. Sol. Energy Mater. Sol. Cells 2012, 99, 166–175. [Google Scholar] [CrossRef]
- Price, H.; Lüpfert, E.; Kearney, D.; Zarza, E.; Cohen, G.; Gee, R.; Mahoney, R. Advances in Parabolic Trough Solar Power Technology. J. Sol. Energy Eng. 2002, 124, 109–125. [Google Scholar] [CrossRef]
- Mwema, F.M.; Akinlabi, E.T.; Oladijo, O.P. Sustainability Issues in Sputtering Deposition Technology. In Proceedings of the International Conference on Industrial Engineering and Operations Management 2019, Toronto, ON, Canada, 23–25 October 2019; IEOM Society: Toronto, ON, Canada, 2019; pp. 737–744. [Google Scholar]
- Ambrosini, A.; Lambert, T.N.; Bencomo, M.; Hall, A.; Van Every, K.; Siegel, N.P.; Ho, C.K. Improved High Temperature Solar Absorbers for Use in Concentrating Solar Power Central Receiver Applications. In Proceedings of the ASME 2011 5th International Conference on Energy Sustainability, Washington, DC, USA, 7–10 August 2011; pp. 587–594. [Google Scholar] [CrossRef]
- Hall, A.; Ambrosini, A.; Ho, C. Solar Selective Coatings for Concentrating. Adv. Mater. Process. 2012, 170, 28–32. [Google Scholar]
- Wang, X.; Ouyang, T.; Duan, X.; Ke, C.; Zhang, X.; Min, J.; Li, A.; Guo, W.; Cheng, X. Improved Solar Absorptance of WC/Co Solar Selective Absorbing Coating with Multimodal WC Particles. Metals 2017, 7, 137. [Google Scholar] [CrossRef]
- Toru, D.; Quet, A.; De Sousa Meneses, D.; Del Campo, L.; Echegut, P. Influence of Microstructure and Composition on Optical Properties of Plasma Sprayed Al/Al2O3 Cermets. J. Phys. Chem. C 2015, 119, 5426–5433. [Google Scholar] [CrossRef]
- Del Campo, L.; De Sousa Meneses, D.; Wittmann-Ténèze, K.; Bacciochini, A.; Denoirjean, A.; Echegut, P. Effect of Porosity on the Infrared Radiative Properties of Plasma-Sprayed Yttria-Stabilized Zirconia Ceramic Thermal Barrier Coatings. J. Phys. Chem. C 2014, 118, 13590–13597. [Google Scholar] [CrossRef]
- Tului, M.; Arezzo, F.; Pawlowski, L. Optical Properties of Plasma Sprayed ZnO+Al2O3 Coatings. Surf. Coat. Technol. 2004, 179, 47–55. [Google Scholar] [CrossRef]
- Singh, C.C.; Panda, E. Zinc Interstitial Threshold in Al-Doped ZnO Film: Effect on Microstructure and Optoelectronic Properties. J. Appl. Phys. 2018, 123, 165106. [Google Scholar] [CrossRef]
- Tominaga, K.; Umezu, N.; Mori, I.; Ushiro, T.; Moriga, T.; Nakabayashi, I. Properties of ZnO: In Film Prepared by Sputtering of Facing ZnO:In and Zn Targets. J. Vac. Sci. Technol. A Vac. Surf. Film. 1998, 16, 1213–1217. [Google Scholar] [CrossRef]
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings; John Wiley & Sons: Chichester, UK, 2008; ISBN 9780470754085. [Google Scholar]
- Hall, A.; Ambrosini, A.; Ho, C.; McCloskey, J.; Van Every, K.; Urrea, D.; Lambert, T.; Bencomo, M.; Mahoney, A.; Siegel, N. Solar Selective Coatings for Concentrating Solar Power Central Receivers. Adv. Mater. Process. 2011, 28, 28–32. [Google Scholar]
- Ambrosini, A.; Lambert, T.N.; Boubault, A.; Hunt, A.; Davis, D.J.; Adams, D.; Hall, A.C. Thermal Stability of Oxide-Based Solar Selective Coatings for CSP Central Receivers. In Proceedings of the ASME 2015 9th International Conference on Energy Sustainability Collocated with the ASME 2015 Power Conference, the ASME 2015 13th International Conference on Fuel Cell Science, Engineering and Technology, and the ASME 2015 Nuclear Forum, San Diego, CA, USA, 28 June–2 July 2015; Volume 1, pp. 1–10. [Google Scholar] [CrossRef]
- Ke, C.; Zhang, X.; Guo, W.; Li, Y.; Gong, D.Q.; Cheng, X. Solar Selective Coatings with Multilayered Structure Based on Thermal Spraying WC-Co Solar Absorption Layer. Vacuum 2018, 152, 114–122. [Google Scholar] [CrossRef]
- Brousse-Pereira, E.; Wittmann-Teneze, K.; Bianchi, V.; Longuet, J.L.; Del Campo, L. Optical and Electrical Properties of Heterogeneous Coatings Produced by Aluminum Powder and Boehmite Suspension Plasma Spraying. J. Therm. Spray Technol. 2012, 21, 1110–1119. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, L.; Ma, Z.; Liu, Y.; Wang, F. Optical Property of La1−xSrxTiO3+δ Coatings Deposited by Plasma Spraying Technique. Appl. Surf. Sci. 2015, 356, 935–940. [Google Scholar] [CrossRef]
- Deng, X.-Q.; Xue, M.-M.; Lv, Y.-L.; Li, R.-H.; Tong, J.-M.; Shi, G.-H.; Yang, Y.; Dong, Y.-C. Study on Spectral Selective Absorbing Coatings with Spinel Structures Fabricated via Plasma Spraying. Vacuum 2020, 174, 109214. [Google Scholar] [CrossRef]
- Bunmephiphit, C.; Suriwong, T.; Jiajitsawat, S.; Dejang, N. Characterization of Ni-Al Solar Absorber Prepared by Flame Spray Technique. Key Eng. Mater. 2016, 675–676, 477–481. [Google Scholar] [CrossRef]
- Wu, S.; Cheng, C.H.; Hsiao, Y.J.; Juang, R.C.; Wen, W.F. Fe2O3 Films on Stainless Steel for Solar Absorbers. Renew. Sustain. Energy Rev. 2016, 58, 574–580. [Google Scholar] [CrossRef]
- Tulchinsky, D.; Uvarov, V.; Popov, I.; Mandler, D.; Magdassi, S. A Novel Non-Selective Coating Material for Solar Thermal Potential Application Formed by Reaction between Sol–Gel Titania and Copper Manganese Spinel. Sol. Energy Mater. Sol. Cells 2014, 120, 23–29. [Google Scholar] [CrossRef]
- Carlsson, B.; Möller, K.; Köhl, M.; Frei, U.; Brunold, S. Qualification Test Procedure for Solar Absorber Surface Durability. Sol. Energy Mater. Sol. Cells 2000, 61, 255–275. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, X.; Ke, C.; Jiang, S.; Wang, X.; Li, S.; Guo, W.; Cheng, X. Microstructure and Optical Properties of Co-WC-Al2O3 Duplex Ceramic Metal-Dielectric Solar Selective Absorbing Coating Prepared by High Velocity Oxy-Fuel Spraying and Sol-Gel Method. Vacuum 2017, 145, 209–216. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Kumar, P.; Rajam, K.S.; Biswas, A. Structure and Optical Properties of Ag–Al2O3 Nanocermet Solar Selective Coatings Prepared Using Unbalanced Magnetron Sputtering. Sol. Energy Mater. Sol. Cells 2011, 95, 1707–1715. [Google Scholar] [CrossRef]
- Gao, X.-H.; Guo, Z.-M.; Geng, Q.-F.; Ma, P.-J.; Wang, A.-Q.; Liu, G. Structure, Optical Properties and Thermal Stability of SS/TiC–ZrC/Al2O3 Spectrally Selective Solar Absorber. RSC Adv. 2016, 6, 63867–63873. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, J.; Gong, D.; Li, J.; Ding, M. Improvement of Solar Absorbing Property of Ni-Mo Based Thermal Spray Coatings by Laser Surface Treatment. Vacuum 2015, 121, 64–69. [Google Scholar] [CrossRef]
- Shah, A.A.; Gupta, M.C. Spectral Selective Surfaces for Concentrated Solar Power Receivers by Laser Sintering of Tungsten Micro and Nano Particles. Sol. Energy Mater. Sol. Cells 2013, 117, 489–493. [Google Scholar] [CrossRef]
- Sevillano, F.; Poza, P.; Múnez, C.J.; Vezzù, S.; Rech, S.; Trentin, A. Cold-Sprayed Ni-Al2O3 Coatings for Applications in Power Generation Industry. J. Therm. Spray Technol. 2013, 22, 772–782. [Google Scholar] [CrossRef]
- Shah, A.A.; Ungaro, C.; Gupta, M.C. High Temperature Spectral Selective Coatings for Solar Thermal Systems by Laser Sintering. Sol. Energy Mater. Sol. Cells 2015, 134, 209–214. [Google Scholar] [CrossRef]
- Pang, X.; Wei, Q.; Zhou, J.; Ma, H. High-Temperature Tolerance in Multi-Scale Cermet Solar-Selective Absorbing Coatings Prepared by Laser Cladding. Materials 2018, 11, 1037. [Google Scholar] [CrossRef]
- Ungaro, C.; Gray, S.K.; Gupta, M.C. Black Tungsten for Solar Power Generation. Appl. Phys. Lett. 2013, 103, 071105. [Google Scholar] [CrossRef]
- Ghmari, F.; Ghbara, T.; Laroche, M.; Carminati, R.; Greffet, J.-J. Influence of Microroughness on Emissivity. J. Appl. Phys. 2004, 96, 2656–2664. [Google Scholar] [CrossRef]
- Sai, H.; Yugami, H.; Kanamori, Y.; Hane, K. Solar Selective Absorbers Based on Two-Dimensional W Surface Gratings with Submicron Periods for High-Temperature Photothermal Conversion. Sol. Energy Mater. Sol. Cells 2003, 79, 35–49. [Google Scholar] [CrossRef]
- Rico, A.; Salazar, A.; Escobar, M.E.; Rodriguez, J.; Poza, P. Optimization of Atmospheric Low-Power Plasma Spraying Process Parameters of Al2O3-50wt%Cr2O3 Coatings. Surf. Coat. Technol. 2018, 354, 281–296. [Google Scholar] [CrossRef]
- Astarita, A.; Genna, S.; Leone, C.; Minutolo, F.M.C.; Rubino, F.; Squillace, A. Study of the Laser Remelting of a Cold Sprayed Titanium Layer. Procedia CIRP 2015, 33, 452–457. [Google Scholar] [CrossRef]
- Rubino, F.; Paradiso, V.; Astarita, A.; Carlone, P.; Squillace, A. Advances in Titanium on Aluminium Alloys Cold Spray Coatings. In Cold-Spray Coatings; Springer International Publishing: Cham, Switzerland, 2018; pp. 225–249. [Google Scholar]
- Rubino, F.; Astarita, A.; Carlone, P. Thermo-Mechanical Finite Element Modeling of the Laser Treatment of Titanium Cold-Sprayed Coatings. Coatings 2018, 8, 219. [Google Scholar] [CrossRef]
- Viscusi, A.; Astarita, A.; Gatta, R.D.; Rubino, F. A Perspective Review on the Bonding Mechanisms in Cold Gas Dynamic Spray. Surf. Eng. 2019, 35, 743–771. [Google Scholar] [CrossRef]
- Paradiso, V.; Rubino, F.; Tucci, F.; Astarita, A.; Carlone, P. Thermo-Mechanical Modeling of Laser Treatment on Titanium Cold-Spray Coatings. AIP Conf. Proc. 2018, 1960, 100011. [Google Scholar] [CrossRef]
- Rubino, F.; Astarita, A.; Carlone, P.; Genna, S.; Leone, C.; Memola Capece Minutolo, F.; Squillace, A. Selective Laser Post-Treatment on Titanium Cold Spray Coatings. Mater. Manuf. Process. 2016, 31, 1500–1506. [Google Scholar] [CrossRef]
- Carlone, P.; Astarita, A.; Rubino, F.; Pasquino, N.; Aprea, P. Selective Laser Treatment on Cold-Sprayed Titanium Coatings: Numerical Modeling and Experimental Analysis. Metall. Mater. Trans. B 2016, 47, 3310–3317. [Google Scholar] [CrossRef]
- Rubino, F.; Ammendola, P.; Astarita, A.; Raganati, F.; Squillace, A.; Viscusi, A.; Chirone, R.; Carrino, L. An Innovative Method to Produce Metal Foam Using Cold Gas Dynamic Spray Process Assisted by Fluidized Bed Mixing of Precursors. Key Eng. Mater. 2015, 651–653, 913–918. [Google Scholar] [CrossRef]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal Energy Storage Technologies and Systems for Concentrating Solar Power Plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- González-Roubaud, E.; Pérez-Osorio, D.; Prieto, C. Review of Commercial Thermal Energy Storage in Concentrated Solar Power Plants: Steam vs. Molten Salts. Renew. Sustain. Energy Rev. 2017, 80, 133–148. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the Art on High Temperature Thermal Energy Storage for Power Generation. Part 1—Concepts, Materials and Modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Walczak, M.; Pineda, F.; Fernández, Á.G.; Mata-Torres, C.; Escobar, R.A. Materials Corrosion for Thermal Energy Storage Systems in Concentrated Solar Power Plants. Renew. Sustain. Energy Rev. 2018, 86, 22–44. [Google Scholar] [CrossRef]
- Olivares, R.I. The Thermal Stability of Molten Nitrite/Nitrates Salt for Solar Thermal Energy Storage in Different Atmospheres. Sol. Energy 2012, 86, 2576–2583. [Google Scholar] [CrossRef]
- Sarvghad, M.; Delkasar Maher, S.; Collard, D.; Tassan, M.; Will, G.; Steinberg, T.A. Materials Compatibility for the next Generation of Concentrated Solar Power Plants. Energy Storage Mater. 2018, 14, 179–198. [Google Scholar] [CrossRef]
- Bell, S.; Steinberg, T.; Will, G. Corrosion Mechanisms in Molten Salt Thermal Energy Storage for Concentrating Solar Power. Renew. Sustain. Energy Rev. 2019, 114, 109328. [Google Scholar] [CrossRef]
- Medrano, M.; Gil, A.; Martorell, I.; Potau, X.; Cabeza, L.F. State of the Art on High-Temperature Thermal Energy Storage for Power Generation. Part 2—Case Studies. Renew. Sustain. Energy Rev. 2010, 14, 56–72. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat Transfer Fluids for Concentrating Solar Power Systems—A Review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Ibrahim, A.; Peng, H.; Riaz, A.; Abdul Basit, M.; Rashid, U.; Basit, A. Molten Salts in the Light of Corrosion Mitigation Strategies and Embedded with Nanoparticles to Enhance the Thermophysical Properties for CSP Plants. Sol. Energy Mater. Sol. Cells 2021, 219, 110768. [Google Scholar] [CrossRef]
- Groll, M.; Brost, O.; Heine, D. Corrosion of Steels in Contact with Salt Eutectics as Latent Heat Storage Materials: Influence of Water and Other Impurities. Heat Recover. Syst. CHP 1990, 10, 567–572. [Google Scholar] [CrossRef]
- Cuevas-Arteaga, C.; Uruchurtu-Chavarín, J.; Porcayo-Calderon, J.; Izquierdo-Montalvo, G.; Gonzalez, J. Study of Molten Salt Corrosion of HK-40 m Alloy Applying Linear Polarization Resistance and Conventional Weight Loss Techniques. Corros. Sci. 2004, 46, 2663–2679. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, C.Y. A Review of Solar Collectors and Thermal Energy Storage in Solar Thermal Applications. Appl. Energy 2013, 104, 538–553. [Google Scholar] [CrossRef]
- Vignarooban, K.; Pugazhendhi, P.; Tucker, C.; Gervasio, D.; Kannan, A.M. Corrosion Resistance of Hastelloys in Molten Metal-Chloride Heat-Transfer Fluids for Concentrating Solar Power Applications. Sol. Energy 2014, 103, 62–69. [Google Scholar] [CrossRef]
- Steinmann, W.-D. Thermal energy storage systems for concentrating solar power (CSP) plants. In Concentrating Solar Power Technology; Lovegrove, K., Stein, W., Eds.; Woodhead Publishing, Elsevier: Amsterdam, The Netherlands, 2012; pp. 362–394. [Google Scholar]
- Tortorelli, P.F.; Bishop, P.S.; DiStefano, J.R. Selection of Corrosion-Resistant Materials for Use in Molten Nitrate Salts; Oak Ridge National Lab.: Oak Ridge, TN, USA, 1989. [Google Scholar] [CrossRef]
- Tortorelli, P.F.; Bishop, P.S. Influence of Compositional Modifications on the Corrosion of Iron Aluminides of Molten Nitrate Salts; Oak Ridge National Lab.: Oak Ridge, TN, USA, 1991. [Google Scholar] [CrossRef]
- Gonzalez, M.; Nithiyanantham, U.; Carbó-Argibay, E.; Bondarchuk, O.; Grosu, Y.; Faik, A. Graphitization as Efficient Inhibitor of the Carbon Steel Corrosion by Molten Binary Nitrate Salt for Thermal Energy Storage at Concentrated Solar Power. Sol. Energy Mater. Sol. Cells 2019, 203, 110172. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Bauer, T. Molten Chloride Salts for next Generation CSP Plants: Selection of Promising Chloride Salts & Study on Corrosion of Alloys in Molten Chloride Salts. AIP Conf. Proc. 2019, 2126, 200014. [Google Scholar] [CrossRef]
- Dariva, C.G.; Galio, A.F. Corrosion Inhibitors—Principles, Mechanisms and Applications. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; InTech: London, UK, 2014. [Google Scholar]
- Ding, W.; Bonk, A.; Gussone, J.; Bauer, T. Electrochemical Measurement of Corrosive Impurities in Molten Chlorides for Thermal Energy Storage. J. Energy Storage 2018, 15, 408–414. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Gussone, J.; Bauer, T. Cyclic Voltammetry for Monitoring Corrosive Impurities in Molten Chlorides for Thermal Energy Storage. Energy Procedia 2017, 135, 82–91. [Google Scholar] [CrossRef]
- Singh, H.; Puri, D.; Prakash, S.; Ghosh, T.K. Hot Corrosion of a Plasma Sprayed Ni3Al Coating on a Ni-Base Superalloy. Mater. Corros. 2007, 58, 857–866. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Morton, E. Castable Cements to Prevent Corrosion of Metals in Molten Salts. Sol. Energy Mater. Sol. Cells 2016, 153, 44–51. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Fernandez, A.G.; Tirawat, R.; Turchi, C.; Huddleston, W. Corrosion Resistance of Alumina-Forming Alloys against Molten Chlorides for Energy Production. I: Pre-Oxidation Treatment and Isothermal Corrosion Tests. Sol. Energy Mater. Sol. Cells 2017, 166, 222–233. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Fernandez, A.G.; Tirawat, R.; Turchi, C.; Huddleston, W. Corrosion Resistance of Alumina Forming Alloys against Molten Chlorides for Energy Production. II: Electrochemical Impedance Spectroscopy under Thermal Cycling Conditions. Sol. Energy Mater. Sol. Cells 2017, 166, 234–245. [Google Scholar] [CrossRef]
- Agüero, A.; García de Blas, F.J.; García, M.C.; Muelas, R.; Román, A. Thermal Spray Coatings for Molten Carbonate Fuel Cells Separator Plates. Surf. Coat. Technol. 2001, 146–147, 578–585. [Google Scholar] [CrossRef]
- Tristancho-Reyes, J.L.; Chacón-Nava, J.G.; Peña-Ballesteros, D.Y.; Gaona-Tiburcio, C.; Gonzalez-Rodriguez, J.G.; Martínez-Villafañe, A.; Almeraya-Calderón, F. Hot Corrosion Behaviour of NiCrFeNbMoTiAl Coating in Molten Salts at 700 °C by Electrochemical Techniques. Int. J. Electrochem. Sci. 2011, 6, 432–441. [Google Scholar]
- Agüero, A. Progress in the Development of Coatings for Protection of New Generation Steam Plant Components. Energy Mater. 2008, 3, 35–44. [Google Scholar] [CrossRef]
- Ou, X.; Sun, Z.; Sun, M.; Zou, D. Hot-Corrosion Mechanism of Ni-Cr Coatings at 650 °C under Different Simulated Corrosion Conditions. J. China Univ. Min. Technol. 2008, 18, 444–448. [Google Scholar] [CrossRef]
- Jiang, S.M.; Xu, C.Z.; Li, H.Q.; Ma, J.; Gong, J.; Sun, C. High Temperature Corrosion Behaviour of a Gradient NiCoCrAlYSi Coating I: Microstructure Evolution. Corros. Sci. 2010, 52, 1746–1752. [Google Scholar] [CrossRef]
- Li, T.; Zhou, Y.; Li, M.; Li, Z. High Temperature Corrosion Behavior of a Multilayer CrAlN Coating Prepared by Magnetron Sputtering Method on a K38G Alloy. Surf. Coat. Technol. 2008, 202, 1985–1993. [Google Scholar] [CrossRef]
- Ren, X.; Wang, F.; Wang, X. High-Temperature Oxidation and Hot Corrosion Behaviors of the NiCr–CrAl Coating on a Nickel-Based Superalloy. Surf. Coat. Technol. 2005, 198, 425–431. [Google Scholar] [CrossRef]
- Lauwerens, W.; De Boeck, A.; Thijs, M.; Claessens, S.; Van Stappen, M.; Steenackers, P. PVD Al–Ti and Al–Mn Coatings for High Temperature Corrosion Protection of Sheet Steel. Surf. Coat. Technol. 2001, 146–147, 27–32. [Google Scholar] [CrossRef]
- Senderowski, C.; Cinca, N.; Dosta, S.; Cano, I.G.; Guilemany, J.M. The Effect of Hot Treatment on Composition and Microstructure of HVOF Iron Aluminide Coatings in Na2SO4 Molten Salts. J. Therm. Spray Technol. 2019, 28, 1492–1510. [Google Scholar] [CrossRef]
- Audigié, P.; Encinas-Sánchez, V.; Juez-Lorenzo, M.; Rodríguez, S.; Gutiérrez, M.; Pérez, F.J.; Agüero, A. High Temperature Molten Salt Corrosion Behavior of Aluminide and Nickel-Aluminide Coatings for Heat Storage in Concentrated Solar Power Plants. Surf. Coat. Technol. 2018, 349, 1148–1157. [Google Scholar] [CrossRef]
- Audigié, P.; Encinas-Sánchez, V.; Rodríguez, S.; Pérez, F.J.; Agüero, A. High Temperature Corrosion beneath Carbonate Melts of Aluminide Coatings for CSP Application. Sol. Energy Mater. Sol. Cells 2020, 210, 110514. [Google Scholar] [CrossRef]
- Grégoire, B.; Oskay, C.; Meißner, T.M.; Galetz, M.C. Corrosion Performance of Slurry Aluminide Coatings in Molten NaCl–KCl. Sol. Energy Mater. Sol. Cells 2021, 223. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Wang, K.; Molina, E.E.; Li, P.; Gervasio, D.; Kannan, A.M. Vapor Pressure and Corrosivity of Ternary Metal-Chloride Molten-Salt Based Heat Transfer Fluids for Use in Concentrating Solar Power Systems. Appl. Energy 2015, 159, 206–213. [Google Scholar] [CrossRef]
- McConohy, G.; Kruizenga, A. Molten Nitrate Salts at 600 and 680 °C: Thermophysical Property Changes and Corrosion of High-Temperature Nickel Alloys. Sol. Energy 2014, 103, 242–252. [Google Scholar] [CrossRef]
- Liu, B.; Wei, X.; Wang, W.; Lu, J.; Ding, J. Corrosion Behavior of Ni-Based Alloys in Molten NaCl-CaCl2-MgCl2 Eutectic Salt for Concentrating Solar Power. Sol. Energy Mater. Sol. Cells 2017, 170, 77–86. [Google Scholar] [CrossRef]
- Shankar, A.R.; Kanagasundar, A.; Mudali, U.K. Corrosion of Nickel-Containing Alloys in Molten LiCl-KCl Medium. Corrosion 2013, 69, 48–57. [Google Scholar] [CrossRef]
- Tristancho-Reyes, J.L.; Almeraya-Calderón, F.; Chacón-Nava, J.G. Hot Corrosion on NiCrFeNbMoTiAl Coating, Plasma Spray Deposited. Sci. Tech. Año XVI 2010, 1, 379–383. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Sotelo-Mazon, O.; Salinas-Bravo, V.M.; Arrieta-Gonzalez, C.D.; Ramos-Hernandez, J.J.; Cuevas-Arteaga, C. Electrochemical Performance of Ni20Cr Coatings Applied by Combustion Powder Spray in ZNCL 2-KCL Molten Salts. Int. J. Electrochem. Sci. 2012, 7, 1134–1148. [Google Scholar]
- Porcayo-Calderón, J.; Sotelo-Mazón, O.; Casales-Diaz, M.; Ascencio-Gutierrez, J.A.; Salinas-Bravo, V.M.; Martinez-Gomez, L. Electrochemical Study of Ni20Cr Coatings Applied by HVOF Process in ZnCl2-KCl at High Temperatures. J. Anal. Methods Chem. 2014, 2014, 503618. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Shores, D.A. Role of Chlorides in Hot Corrosion of a Cast Fe–Cr–Ni Alloy. Part I: Experimental Studies. Corros. Sci. 2004, 46, 2893–2907. [Google Scholar] [CrossRef]
- Shores, D.A.; Mohanty, B.P. Role of Chlorides in Hot Corrosion of a Cast Fe–Cr–Ni Alloy. Part II: Thermochemical Model Studies. Corros. Sci. 2004, 46, 2909–2924. [Google Scholar] [CrossRef]
- Ishitsuka, T.; Nose, K. Stability of Protective Oxide Films in Waste Incineration Environment—Solubility Measurement of Oxides in Molten Chlorides. Corros. Sci. 2002, 44, 247–263. [Google Scholar] [CrossRef]
- Marulanda A., J. L. Corrosión En Sales Fundidas de Un Acero Recubierto Mediante Rociado Térmico Por Llama. Prospectiva 2014, 12, 1. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C. Corrosion Resistance of MCrAlX Coatings in a Molten Chloride for Thermal Storage in Concentrating Solar Power Applications. Npj Mater. Degrad. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Noel, J.; Weber, J. Corrosion Evaluation of Alloys and MCrAlX Coatings in Molten Carbonates for Thermal Solar Applications. Sol. Energy Mater. Sol. Cells 2016, 157, 517–525. [Google Scholar] [CrossRef]
- Meier, G.H.; Pettit, F.S. High-Temperature Corrosion of Alumina-Forming Coatings for Superalloys. Surf. Coat. Technol. 1989, 39–40, 1–17. [Google Scholar] [CrossRef]
- Jiang, S.M.; Peng, X.; Bao, Z.B.; Liu, S.C.; Wang, Q.M.; Gong, J.; Sun, C. Preparation and Hot Corrosion Behaviour of a MCrAlY + AlSiY Composite Coating. Corros. Sci. 2008, 50, 3213–3220. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, C.; Lan, H.; Du, L.; Zhang, W. Oxidation and Hot Corrosion Behavior of Plasma-Sprayed MCrAlY–Cr2O3 Coatings. J. Therm. Spray Technol. 2016, 25, 1208–1216. [Google Scholar] [CrossRef]
- Raiman, S.S.; Mayes, R.T.; Kurley, J.M.; Parrish, R.; Vogli, E. Amorphous and Partially-Amorphous Metal Coatings for Corrosion Resistance in Molten Chloride Salt. Sol. Energy Mater. Sol. Cells 2019, 201, 110028. [Google Scholar] [CrossRef]
- Ma, H.R.; Li, J.W.; Chang, C.T.; Wang, X.M.; Li, R.W. Passivation Behavior of Fe-Based Amorphous Coatings Prepared by High-Velocity Air/Oxygen Fuel Processes. J. Therm. Spray Technol. 2017, 26, 2040–2047. [Google Scholar] [CrossRef]
- US Department of Energy’s Solar Energy Technologies Office the SunShot Initiative. Available online: https://www.energy.gov/eere/solar/sunshot-initiative (accessed on 1 May 2021).
- European Commission The Strategic Energy Technology (SET) Plan. Available online: https://op.europa.eu/en/publication-detail/-/publication/064a025d-0703-11e8-b8f5-01aa75ed71a1 (accessed on 1 May 2021).





| Coating | α | ε80 | FOM (W/cm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Material | As Sprayed | Smooth Surface (Ra = 1 µm) | Heat Treat. | As Sprayed | Smooth Surface (Ra = 1 µm) | Heat Treat. | As Sprayed | Smooth Surface (Ra = 1 µm) | Heat Treat. |
| Ni-25 Graphite | 0.81 | 0.52 | 0.93 | 0.62 | 0.33 | 0.78 | 45 | 26 | 52 |
| Ni-5Al | 0.63 | 0.26 | 0.89 | 0.39 | 0.11 | 0.57 | 35 | 12 | 50 |
| Tungsten | 0.69 | 0.46 | 0.74 | 0.29 | 0.13 | 0.82 | 39 | 28 | 40 |
| WC-20Co | 0.82 | 0.60 | 0.55 | 0.32 | 46 | 37 | |||
| WC-9Co | 0.73 | 0.45 | 0.53 | 0.24 | 40 | 28 | |||
| WC-25Ni | 0.75 | 0.60 | 0.51 | 0.24 | 39 | 35 | |||
| CeO2 | 0.85 | 0.16 | 0.89 | 0.41 | 0.11 | 0.49 | 48 | 7 | 50 |
| Co-28Mo-17.5Cr-3.5Si | 0.79 | 0.86 | 0.48 | 0.61 | 44 | 48 | |||
| Co-28Mo-17.5Cr-3.5Si | 0.79 | 0.86 | 0.47 | 0.57 | 44 | 48 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubino, F.; Poza, P.; Pasquino, G.; Carlone, P. Thermal Spray Processes in Concentrating Solar Power Technology. Metals 2021, 11, 1377. https://doi.org/10.3390/met11091377
Rubino F, Poza P, Pasquino G, Carlone P. Thermal Spray Processes in Concentrating Solar Power Technology. Metals. 2021; 11(9):1377. https://doi.org/10.3390/met11091377
Chicago/Turabian StyleRubino, Felice, Pedro Poza, Germana Pasquino, and Pierpaolo Carlone. 2021. "Thermal Spray Processes in Concentrating Solar Power Technology" Metals 11, no. 9: 1377. https://doi.org/10.3390/met11091377
APA StyleRubino, F., Poza, P., Pasquino, G., & Carlone, P. (2021). Thermal Spray Processes in Concentrating Solar Power Technology. Metals, 11(9), 1377. https://doi.org/10.3390/met11091377









