The Effect of High-Energy Ball Milling Conditions on Microstructure and Hydrogen Desorption Properties of Magnesium Hydride and Single-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
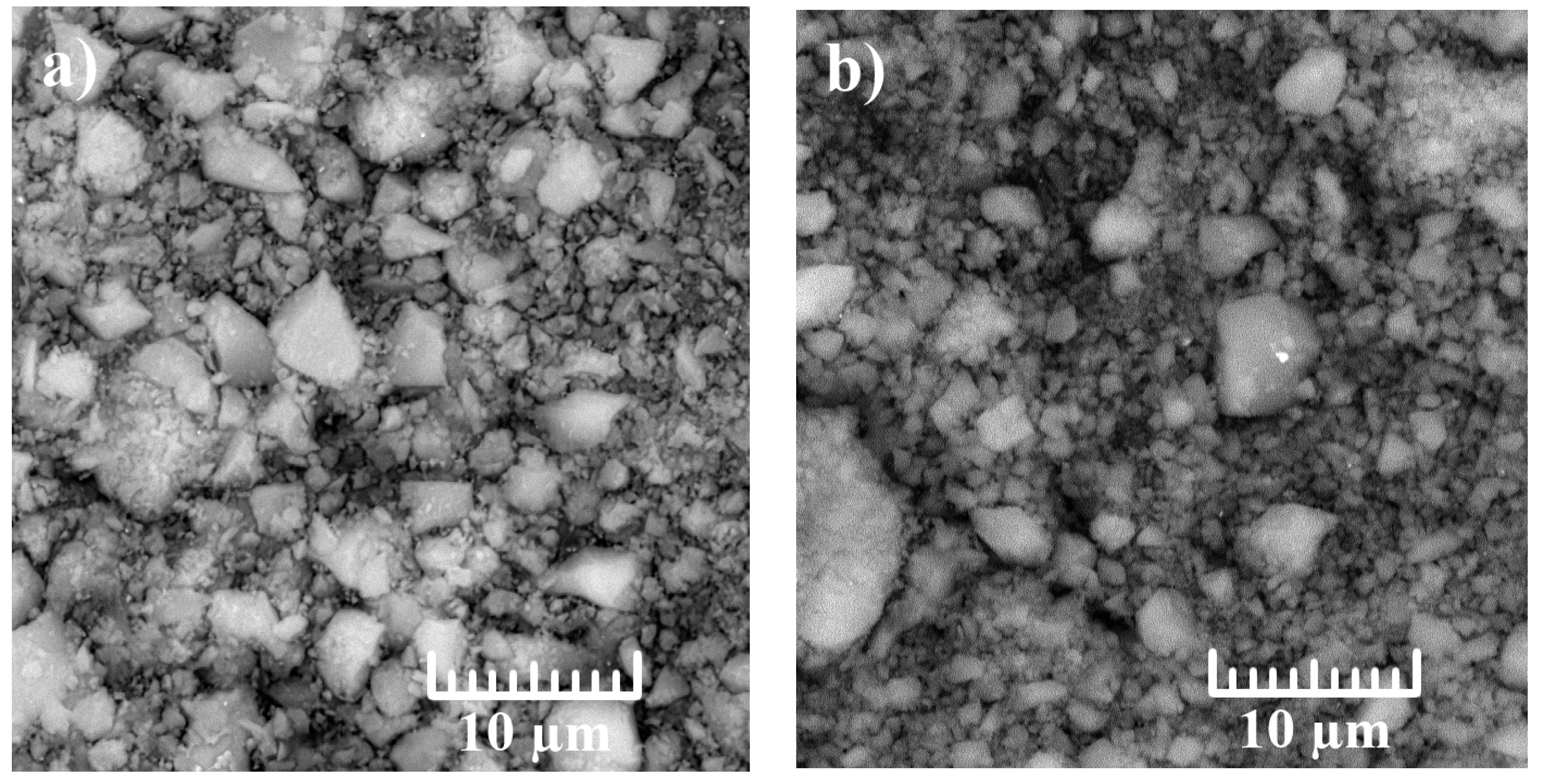
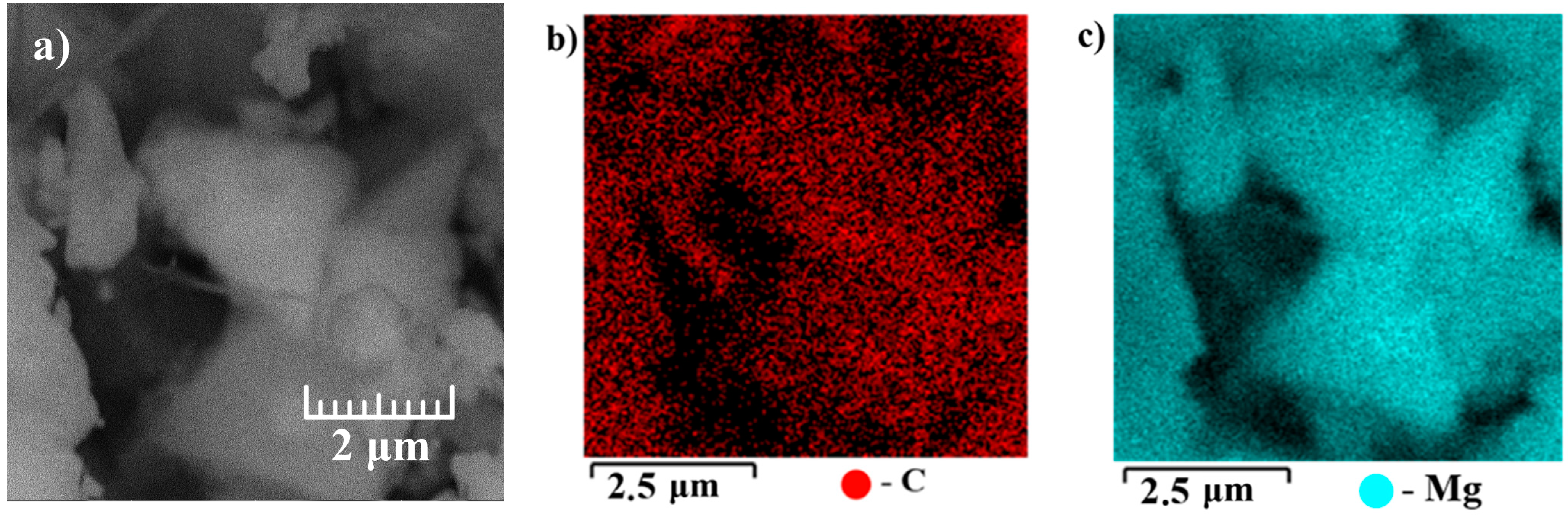
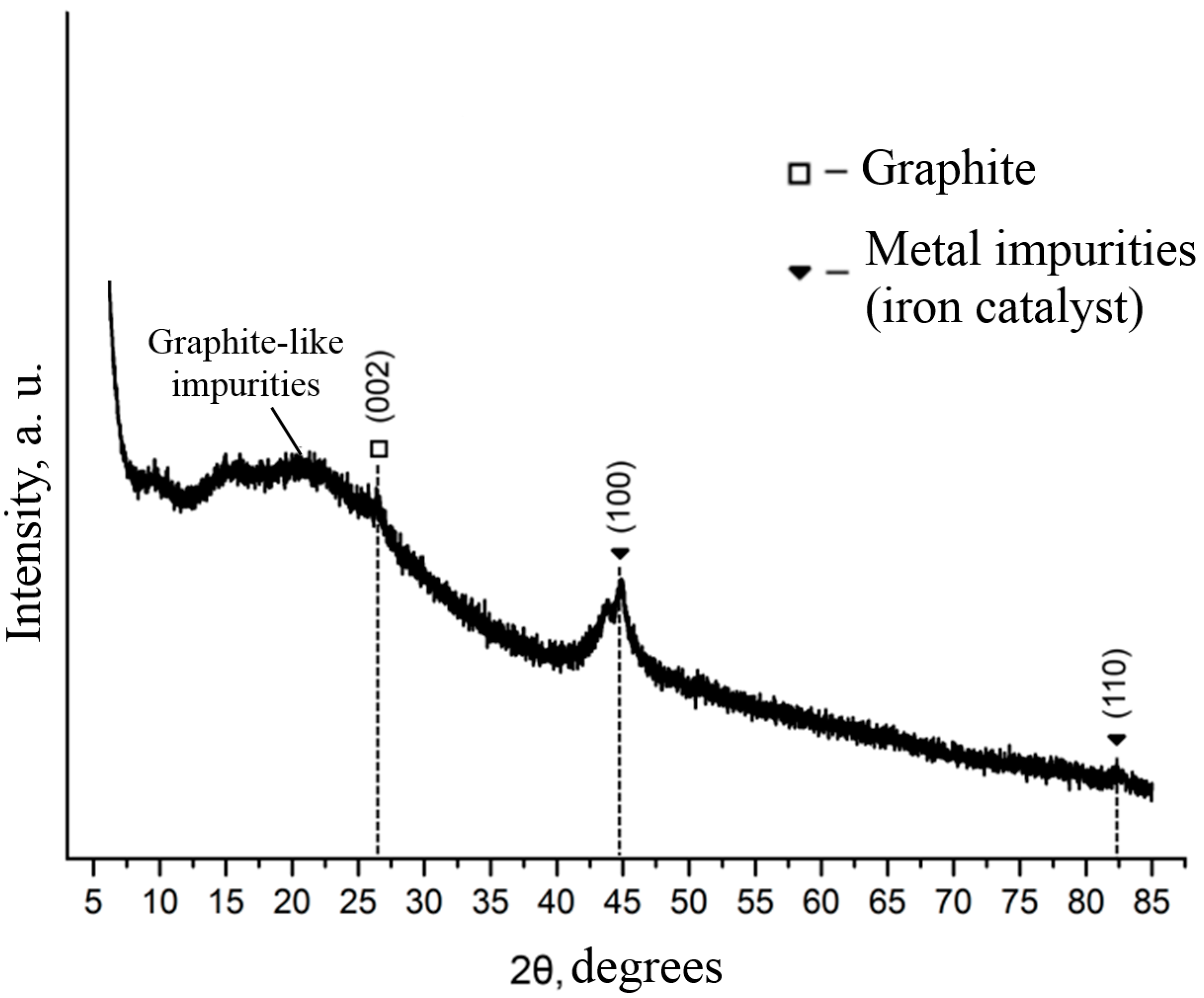
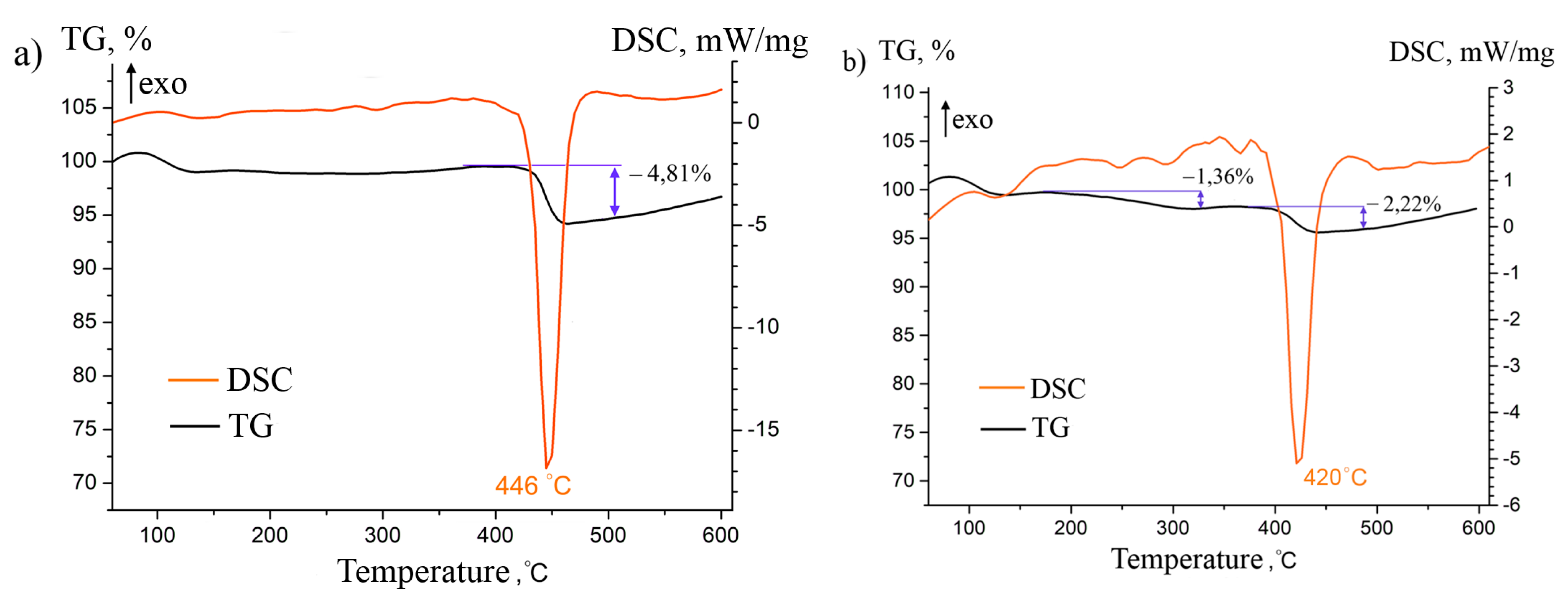
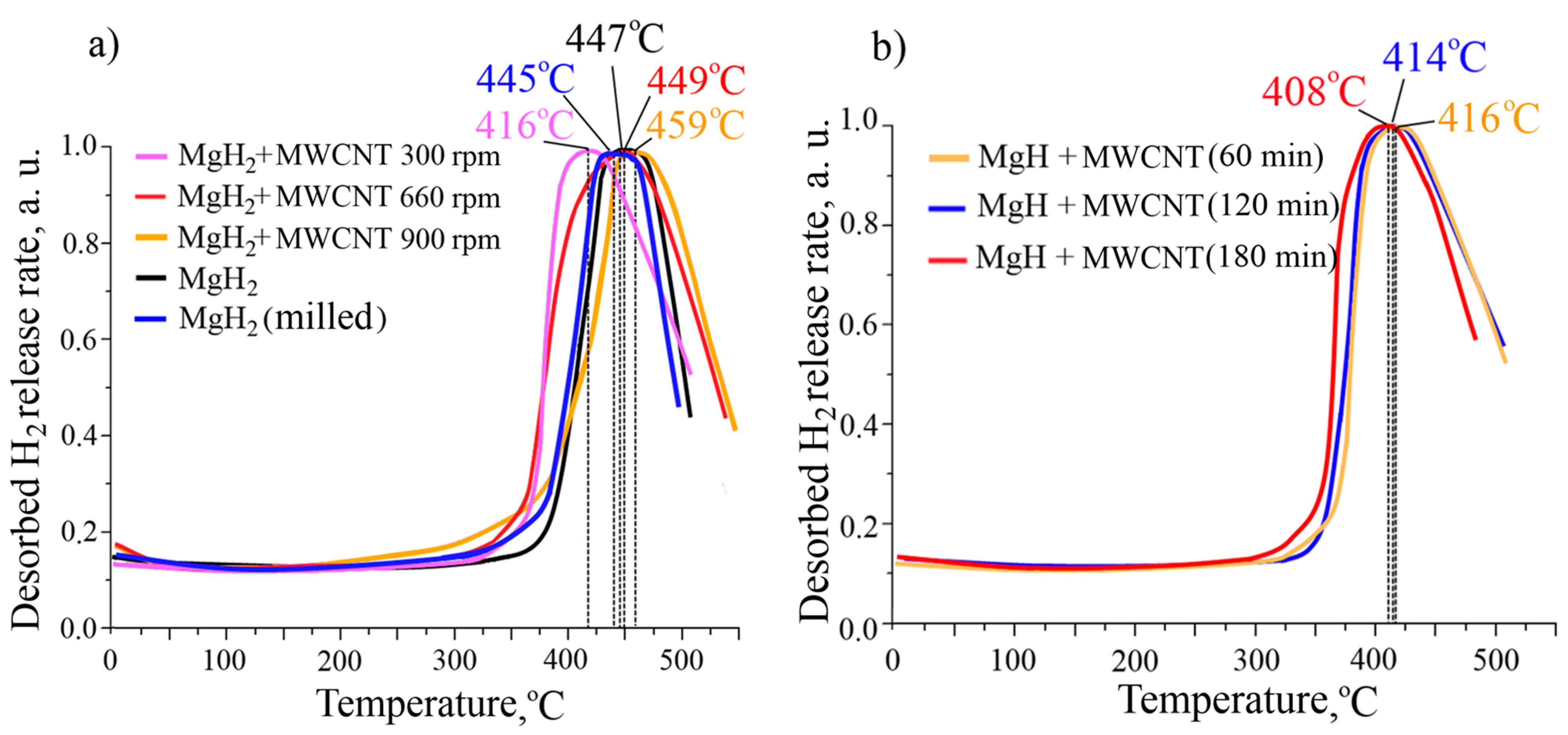
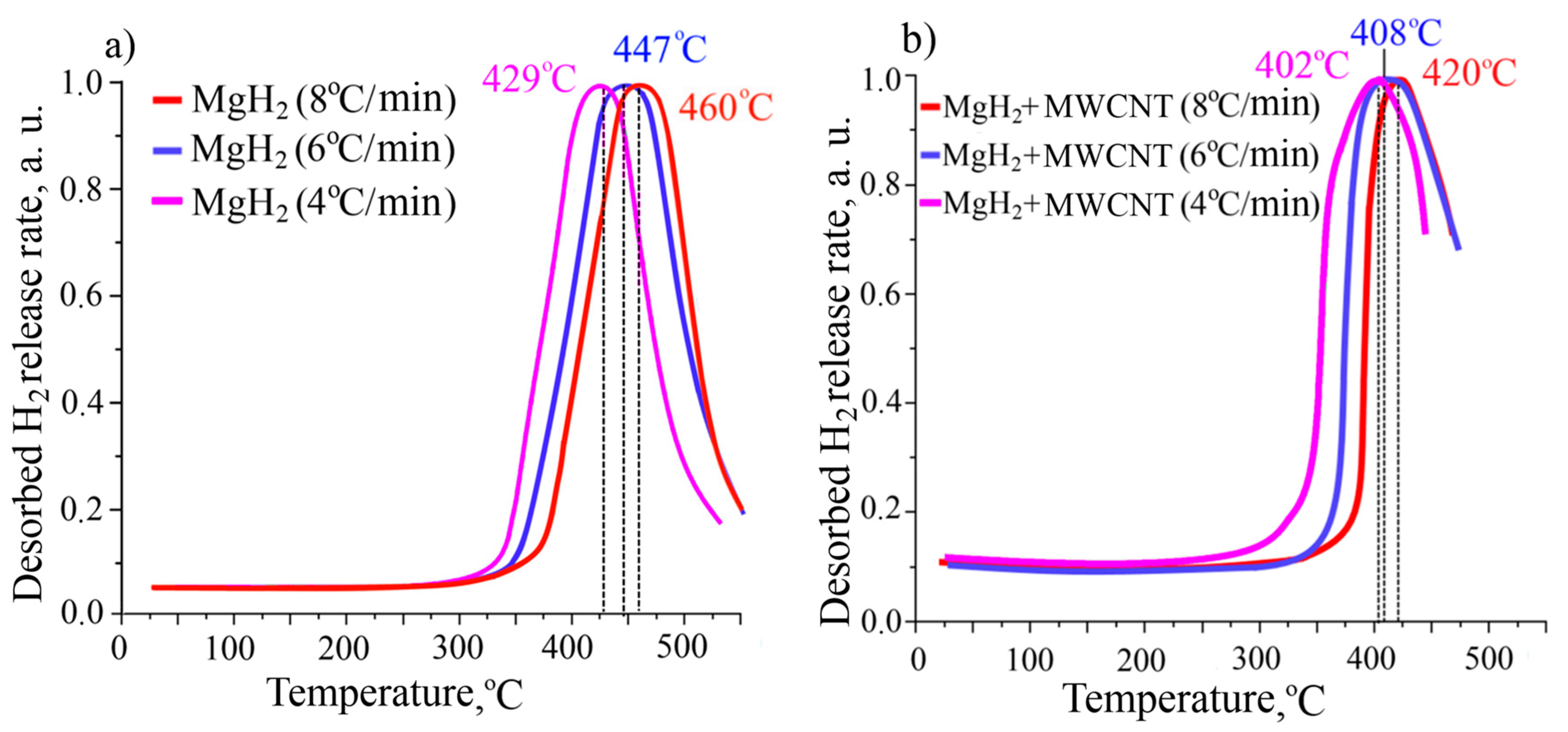
3. Results and Discussion
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortiz, A.L.; Zaragoza, M.J.M.; Collins-Martínez, V. Hydrogen production research in Mexico: A review. Int. J. Hydrogen Energy 2016, 41, 23363–23379. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The hydrogen issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Zhang, J.; Bowman, R.C., Jr.; Fang, Z.Z. Roles of Ti-Based Catalysts on Magnesium Hydride and Its Hydrogen Storage Properties. Inorganics 2021, 9, 36. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. Mater. Int. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- Sabitu, S.T.; Goudy, A.J. Dehydrogenation kinetics and modeling studies of MgH2 enhanced by transition metal oxide catalysts using constant pressure thermodynamic driving forces. Metals 2012, 2, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Lider, A.; Kudiiarov, V.; Kashkarov, E.; Syrtanov, M.; Murashkina, T.; Lomygin, A.; Sakvin, I.; Karpov, D.; Ivanov, A. Hydrogen Accumulation and Distribution in Titanium Coatings at Gas-Phase Hydrogenation. Metals 2020, 10, 880. [Google Scholar] [CrossRef]
- Crivello, J.C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 17. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Lu, Y.; Ouyang, L.; Wang, H. Thermodynamic Tuning of Mg-Based Hydrogen Storage Alloys: A Review. Materials 2013, 6, 4654–4674. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Dupim, I.; Ferreira Santos, S.; Huot, J. Effect of Cold Rolling on the Hydrogen Desorption Behavior of Binary Metal Hydride Powders under Microwave Irradiation. Metals 2015, 5, 2021–2033. [Google Scholar] [CrossRef]
- Huot, J.; Tousignant, M. Effect of cold rolling on metal hydrides. Mater. Trans. 2019, 60, 1571–1576. [Google Scholar] [CrossRef] [Green Version]
- Lyu, J.; Lider, A.; Kudiiarov, V. Using ball milling for modification of the hydrogenation/dehydrogenation process in magnesium-based hydrogen storage materials: An overview. Metals 2019, 9, 768. [Google Scholar] [CrossRef] [Green Version]
- Song, M.Y.; Choi, E.; Kwak, Y.J. Nickel, Graphene, and Yttria-Stabilized Zirconia (YSZ)-Added Mg by Grinding in Hydrogen Atmosphere for Hydrogen Storage. Metals 2019, 9, 1347. [Google Scholar] [CrossRef] [Green Version]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of nanoparticle 3d-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Fruchart, D. Role of transition elements in stability of magnesium hydride: A review of theoretical studies. Solid State Phenom. 2011, 170, 227–231. [Google Scholar] [CrossRef]
- Patah, A.; Takasaki, A.; Szmyd, J.S. Synergetic effect of oxides on hydrogen reaction kinetics of magnesium hydride. Mater. Sci. Forum 2007, 561, 1605–1608. [Google Scholar] [CrossRef]
- Jung, K.S.; Lee, E.Y.; Lee, K.S. Catalytic effects of metal oxide on hydrogen absorption of magnesium metal hydride. J. Alloy. Compd. 2006, 421, 179–184. [Google Scholar] [CrossRef]
- Pandyan, R.K.; Seenithurai, S.; Kumar, S.V.; Mahendran, M. Magnesium Hydride Doped on Single-Walled Carbon Nanotubes for Hydrogen Adsorption. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 175–180. [Google Scholar] [CrossRef]
- Wu, C.Z.; Wang, P.; Yao, X.; Liu, C.; Chen, D.M.; Lu, G.Q.; Cheng, H.M. Effect of carbon/noncarbon addition on hydrogen storage behaviors of magnesium hydride. J. Alloy. Compd. 2006, 414, 259–264. [Google Scholar] [CrossRef]
- Wu, C.; Wang, P.; Yao, X.; Liu, C.; Chen, D.; Lu, G.Q.; Cheng, H. Effects of SWNT and metallic catalyst on hydrogen absorption/desorption performance of MgH2. J. Phys. Chem. B 2005, 109, 22217–22221. [Google Scholar] [CrossRef]
- Lototskyy, M.; Sibanyoni, J.M.; Denys, R.V.; Williams, M.; Pollet, B.G.; Yartys, V.A. Magnesium–carbon hydrogen storage hybrid materials produced by reactive ball milling in hydrogen. Carbon 2013, 57, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Tucho, W.M.; Mauroy, H.; Walmsley, J.C.; Deledda, S.; Holmestad, R.; Hauback, B.C. The effects of ball milling intensity on morphology of multiwall carbon nanotubes. Scr. Mater. 2010, 63, 637–640. [Google Scholar] [CrossRef]
- Campos, R.B.V.; Camargo, S.A.D.S.; Brum, M.C.; Santos, D.S.D. Hydrogen uptake enhancement by the use of a magnesium hydride and carbon nanotubes mixture. Mater. Res. 2017, 20, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Adelhelm, P.; De Jongh, P.E. The impact of carbon materials on the hydrogen storage properties of light metal hydrides. J. Mater. Chem. 2011, 21, 2417–2427. [Google Scholar] [CrossRef] [Green Version]
- Ruse, E.; Pevzner, S.; Bar, I.P.; Nadiv, R.; Skripnyuk, V.M.; Rabkin, E.; Regev, O. Hydrogen storage and spillover kinetics in carbon nanotube-Mg composites. Int. J. Hydrogen Energy 2016, 41, 2814–2819. [Google Scholar] [CrossRef]
- Ruse, E.; Buzaglo, M.; Pevzner, S.; Pri-Bar, I.; Skripnyuk, V.M.; Rabkin, E.; Regev, O. Tuning Mg hydriding kinetics with nanocarbons. J. Alloy. Compd. 2017, 725, 616–622. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Guo, Z.X.; Aguey-Zinsou, K.F.; Cazorla-Amorós, D.; Linares-Solano, A. Effects of different carbon materials on MgH2 decomposition. Carbon 2008, 46, 126–137. [Google Scholar] [CrossRef]
- Huang, Z.G.; Guo, Z.P.; Calka, A.; Wexler, D.; Liu, H.K. Effects of carbon black, graphite and carbon nanotube additives on hydrogen storage properties of magnesium. J. Alloy. Compd. 2007, 427, 94–100. [Google Scholar] [CrossRef]
- Cai, W.; Zhou, X.; Xia, L.; Jiang, K.; Peng, S.; Long, X.; Liang, J. Positive and negative effects of carbon nanotubes on the hydrogen sorption kinetics of magnesium. J. Phys. Chem. C 2015, 119, 25282–25290. [Google Scholar] [CrossRef]
- Kudiiarov, V.N.; Gulidova, L.V.; Pushilina, N.S.; Lider, A.M. Application of automated complex Gas Reaction Controller for hydrogen storage materials investigation. Adv. Mater. Res. 2013, 740, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Syrtanov, M.; Garanin, G.; Kashkarov, E.; Pushilina, N.; Kudiiarov, V.; Murashkina, T. Laboratory X-ray diffraction complex for in situ investigations of structural phase evolution of materials under gaseous atmosphere. Metals 2020, 10, 447. [Google Scholar] [CrossRef] [Green Version]
- Popilevsky, L.; Skripnyuk, V.M.; Beregovsky, M.; Sezen, M.; Amouyal, Y.; Rabkin, E. Hydrogen storage and thermal transport properties of pelletized porous Mg-2 wt.% multiwall carbon nanotubes and Mg-2 wt.% graphite composites. Int. J. Hydrogen Energy 2016, 41, 14461–14474. [Google Scholar] [CrossRef]
- Amirkhiz, B.S.; Danaie, M.; Mitlin, D. The influence of SWCNT–metallic nanoparticle mixtures on the desorption properties of milled MgH2 powders. Nanotechnology 2009, 20, 13. [Google Scholar] [CrossRef]
- Albaaji, A.J.; Castle, E.G.; Reece, M.J.; Hall, J.P.; Evans, S.L. Effect of ball-milling time on mechanical and magnetic properties of carbon nanotube reinforced FeCo alloy composites. Mater. Des. 2017, 122, 296–306. [Google Scholar] [CrossRef]
- Peng, T.; Chang, I. Mechanical alloying of multi-walled carbon nanotubes reinforced aluminum composite powder. Powder Technol. 2014, 266, 7–15. [Google Scholar] [CrossRef]
- Stoyadinova, H.; Zlatanova, Z.; Spassova, M.; Spassov, T.; Baklanov, M. Influence of milling conditions on the hydriding properties of Mg-C nanocomposites. J. Nanomater. 2015, 2015, 6. [Google Scholar] [CrossRef]
- Pandey, S.K.; Singh, R.K.; Srivastava, O.N. Investigations on hydrogenation behaviour of CNT admixed Mg2Ni. Int. J. Hydrogen Energy 2009, 34, 9379–9384. [Google Scholar] [CrossRef]
- Salamatov, I.N.; Yatsenko, D.A.; Khasin, A.A. Determination of the Diameter Distribution Function of Single-Wall Carbon Nanotubes by the X-Ray Diffraction Data. J. Struct. Chem. 2019, 60, 2001–2008. [Google Scholar] [CrossRef]
- Mahmoud, A.E.; Wasly, H.S.; Doheim, M.A. Studies of crystallite size and lattice strain in Al-Al2O3 powders produced by high-energy mechanical milling. J. Eng. Sci. 2014, 42, 1430–1439. [Google Scholar] [CrossRef]
- Rahmaninasab, M.A.; Raygan, S.; Abdizadeh, H.; Pourabdoli, M.; Mirghaderi, S.H. Properties of activated MgH2 + mischmetal nanostructured composite produced by ball-milling. Mater. Renew. Sustain. Energy 2018, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Zlotea, C.; Oumellal, Y.; Hwang, S.J.; Ghimbeu, C.M.; de Jongh, P.E.; Latroche, M. Ultrasmall MgH2 nanoparticles embedded in an ordered microporous carbon exhibiting rapid hydrogen sorption kinetics. J. Phys. Chem. C 2015, 119, 18091–18098. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Liu, H.; He, T.; Wang, Y.; Li, S.; Yan, M. Effects of nano-composites (FeB, FeB/CNTs) on hydrogen storage properties of MgH2. J. Power Sources 2019, 438, 11. [Google Scholar] [CrossRef]
- Yahya, M.S.; Ismail, M. Improvement of hydrogen storage properties of MgH2 catalyzed by K2NbF7 and multiwall carbon nanotube. J. Phys. Chem. C 2018, 122, 11222–11233. [Google Scholar] [CrossRef]





| № | Sample Name | TP, K | Ed, kJ/mol | Ed (Average), kJ/mol | |
|---|---|---|---|---|---|
| 1 | MgH2 | 702 | −12.1 | 188.52 | 189 |
| 2 | 720 | −11.4 | 188.96 | ||
| 3 | 733 | −10.9 | 189.72 | ||
| 4 | MgH2–5 wt.% SWCNT (300 rpm, 180 min) | 675 | −11.6 | 162.00 | 162 |
| 5 | 681 | −11.3 | 161.74 | ||
| 6 | 693 | −11.0 | 162.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudiyarov, V.N.; Elman, R.R.; Kurdyumov, N.E. The Effect of High-Energy Ball Milling Conditions on Microstructure and Hydrogen Desorption Properties of Magnesium Hydride and Single-Walled Carbon Nanotubes. Metals 2021, 11, 1409. https://doi.org/10.3390/met11091409
Kudiyarov VN, Elman RR, Kurdyumov NE. The Effect of High-Energy Ball Milling Conditions on Microstructure and Hydrogen Desorption Properties of Magnesium Hydride and Single-Walled Carbon Nanotubes. Metals. 2021; 11(9):1409. https://doi.org/10.3390/met11091409
Chicago/Turabian StyleKudiyarov, Viktor N., Roman R. Elman, and Nikita E. Kurdyumov. 2021. "The Effect of High-Energy Ball Milling Conditions on Microstructure and Hydrogen Desorption Properties of Magnesium Hydride and Single-Walled Carbon Nanotubes" Metals 11, no. 9: 1409. https://doi.org/10.3390/met11091409





