Investigation of Thermophysical Properties of Zr-Based Metallic Glass-Polymer Composite
Abstract
:1. Introduction
2. Material and Methods
3. Results
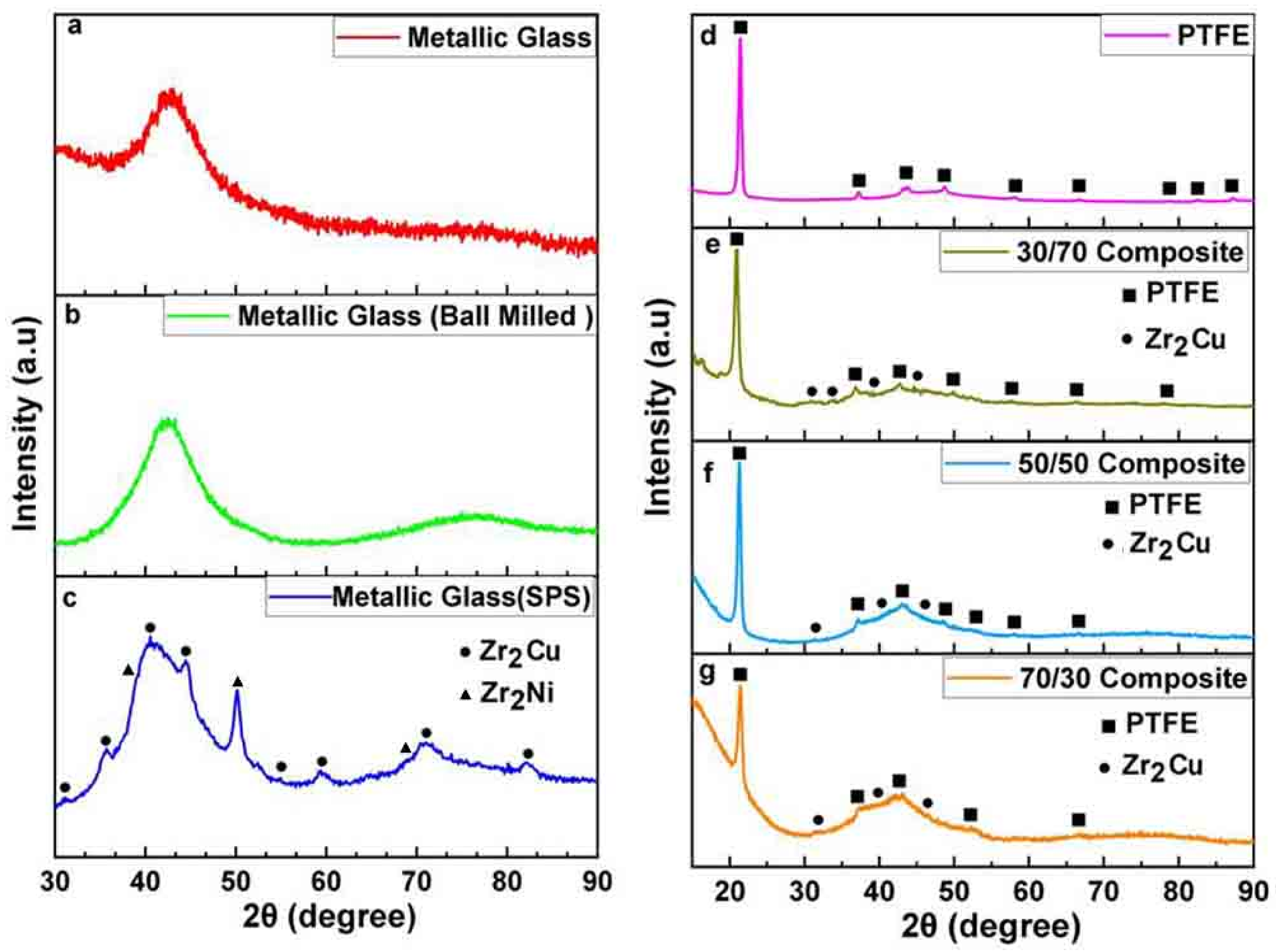
3.1. X-ray Diffraction Analysis (XRD)
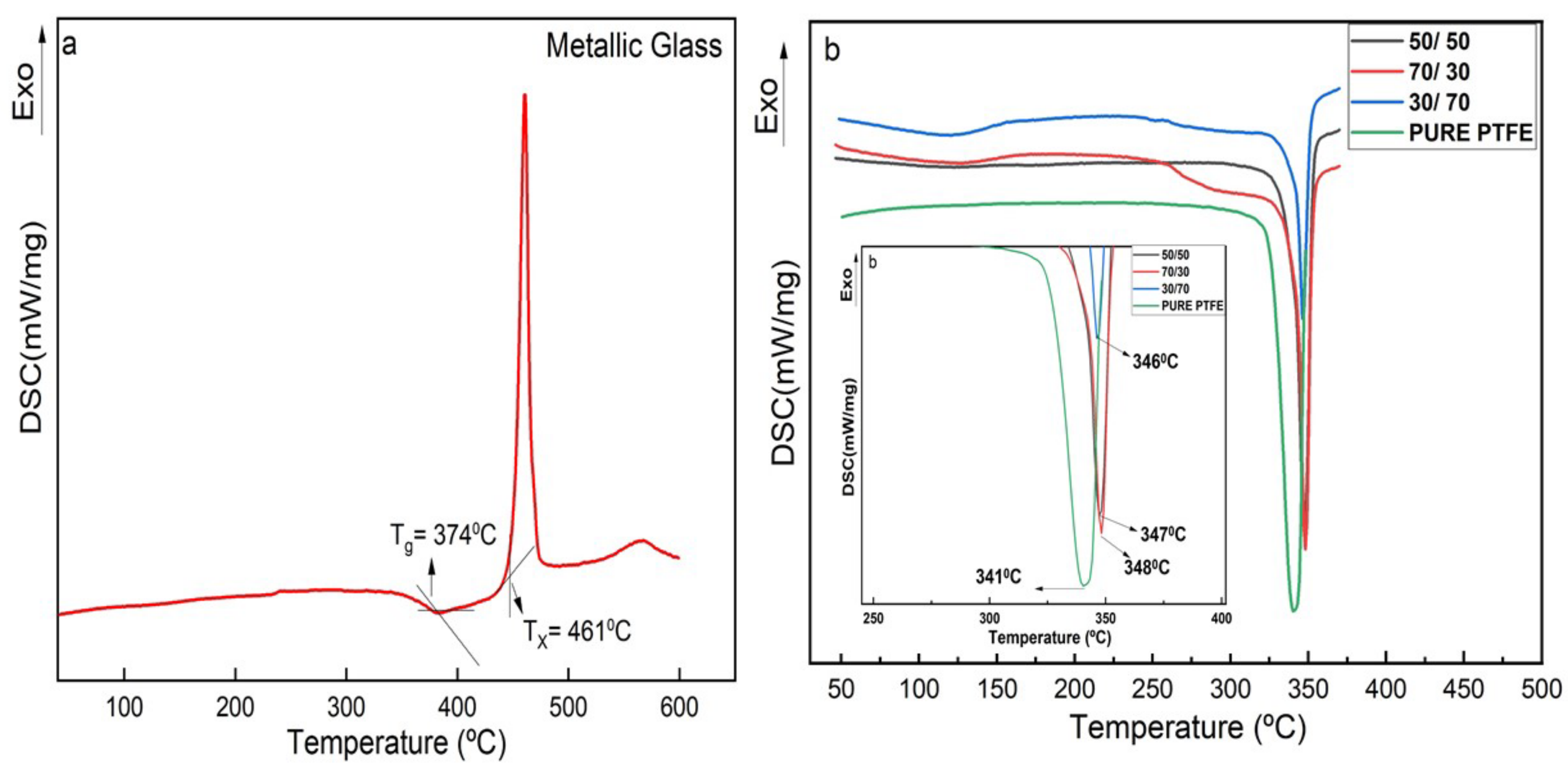
3.2. Differential Scanning Calorimetry (DSC)
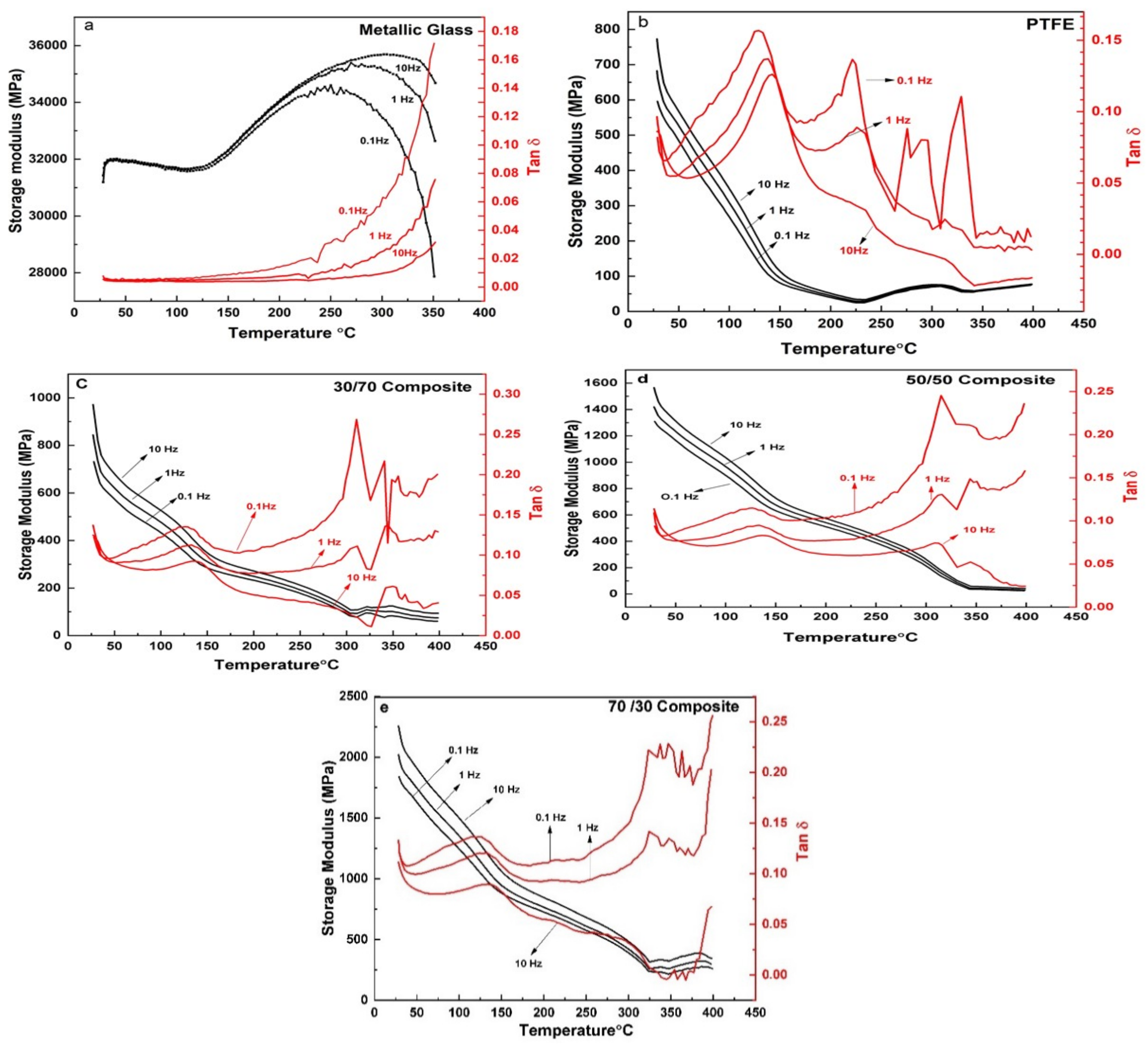
3.3. Dynamic Mechanical Analysis
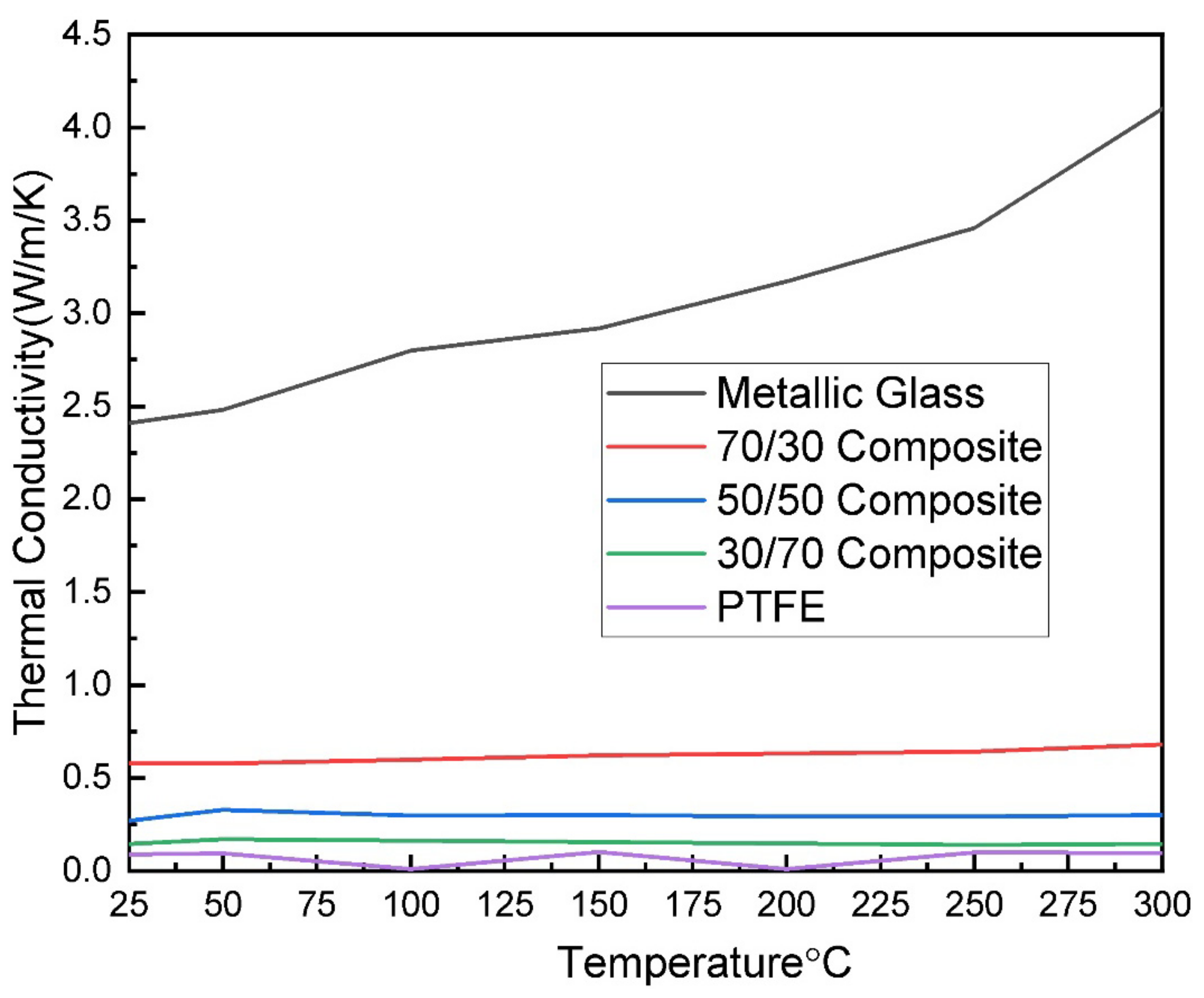
3.4. Laser Flash Analysis (LFA)
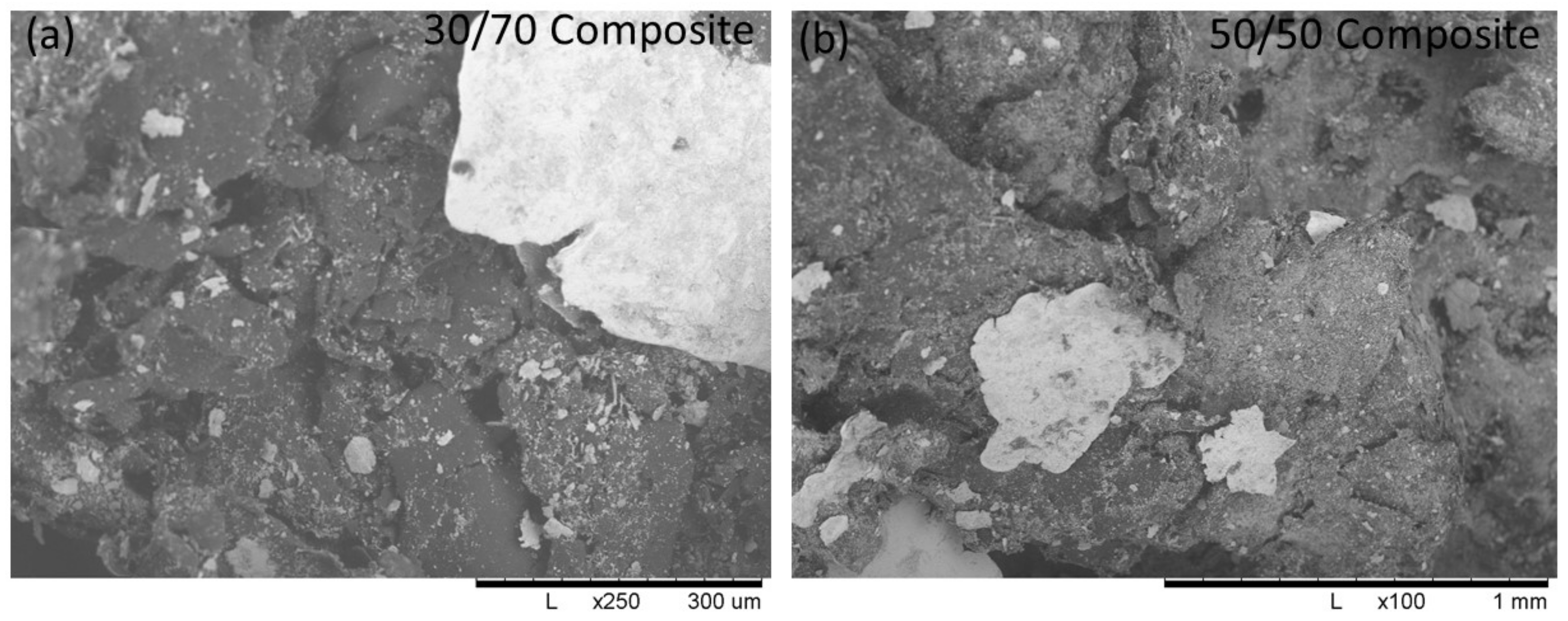
3.5. Scanning Electron Microscope
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Telford, M. The case for bulk metallic glass. Mater. Today 2004, 7, 36–43. [Google Scholar] [CrossRef]
- Sheng, H.W.; Luo, W.K.; Alamgir, F.M.; Bai, J.M.; Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. Nature 2006, 439, 419–425. [Google Scholar] [CrossRef]
- Ashby, M.F.; Greer, A.L. Metallic glasses as structural materials. Scr. Mater. 2006, 54, 321–326. [Google Scholar] [CrossRef]
- Schuh, C.A.; Hufnagel, T.C.; Ramamurty, U. Mechanical behavior of amorphous alloys. Acta Mater. 2007, 55, 4067–4109. [Google Scholar] [CrossRef]
- Greer, A.L.; Cheng, Y.Q.; Ma, E. Shear bands in metallic glasses. Mater. Sci. Eng. R Rep. 2013, 74, 71–132. [Google Scholar] [CrossRef]
- Jang, B.Z.; Uhlmann, D.R.; Sande, J.B.V. Ductile–brittle transition in polymers. J. Appl. Polym. Sci. 1984, 29, 3409–3420. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Lee, J.; Huang, K.H.; Hsu, K.C.; Tung, H.C.; Lee, J.W.; Duh, J.G. Applying composition control to improve the mechanical and thermal properties of Zr-Cu-Ni-Al thin film metallic glass by magnetron DC sputtering. Surf. Coat. Technol. 2015, 278, 132–137. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, A. Glass formation, production and superior properties of Zr-based thin film metallic glasses (TFMGs): A status review. J. Non. Cryst. Solids 2020, 527. [Google Scholar] [CrossRef]
- Tang, C.; Li, Y.; Zeng, K. Characterization of mechanical properties of a Zr-based metallic glass by indentation techniques. Mater. Sci. Eng. A 2004, 384, 215–223. [Google Scholar] [CrossRef]
- Khan, M.M.; Deen, K.M.; Haider, W. Combinatorial development and assessment of a Zr-based metallic glass for prospective biomedical applications. J. Non. Cryst. Solids 2019, 523, 119544. [Google Scholar] [CrossRef]
- Huang, L.; Pu, C.; Fisher, R.K.; Mountain, D.J.H.; Gao, Y.; Liaw, P.K.; Zhang, W.; He, W. A Zr-based bulk metallic glass for future stent applications: Materials properties, finite element modeling, and in vitro human vascular cell response. Acta Biomater. 2015, 25, 356–368. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.P.; Lee, C.M.; Huang, R.T.; Liaw, P.K. Zr-based glass-forming film for fatigue-property improvements of 316L stainless steel: Annealing effects. Surf. Coat. Technol. 2011, 205, 4030–4034. [Google Scholar] [CrossRef]
- Wang, Q.; Qiang, J.B.; Xia, J.H.; Wu, J.; Wang, Y.M.; Dong, C. Cu-Zr-Al (Ti) bulk metallic glasses: Cluster selection rules and glass formation. Intermetallics 2007, 15, 711–715. [Google Scholar] [CrossRef]
- Chu, C.W.; Jang, J.S.C.; Chen, G.J.; Chiu, S.M. Characteristic studies on the Zr-based metallic glass thin film fabricated by magnetron sputtering process. Surf. Coat. Technol. 2008, 202, 5564–5566. [Google Scholar] [CrossRef]
- Chu, J.P.; Jang, J.S.C.; Huang, J.C.; Chou, H.S.; Yang, Y.; Ye, J.C.; Wang, Y.C.; Lee, J.W.; Liu, F.X.; Liaw, P.K.; et al. Thin film metallic glasses: Unique properties and potential applications. Thin Solid Films 2012, 520, 5097–5122. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Kozachkov, H.; Khalifa, H.E.; Schramm, J.P.; Demetriou, M.D.; Vecchio, K.S.; Johnson, W.L. Semi-solid induction forging of metallic glass matrix composites. JOM 2009, 61, 11–17. [Google Scholar] [CrossRef]
- Chen, P.S.; Chen, H.W.; Duh, J.G.; Lee, J.W.; Jang, J.S.C. Characterization of mechanical properties and adhesion of Ta-Zr-Cu-Al-Ag thin film metallic glasses. Surf. Coat. Technol. 2013, 231, 332–336. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Liao, Y.C.; Lee, J.W.; Li, C.L.; Chu, J.P.; Duh, J.G. Electrochemical characterization of Zr-based thin film metallic glass in hydrochloric aqueous solution. Thin Solid Films 2013, 529, 338–341. [Google Scholar] [CrossRef]
- Tsai, P.H.; Lin, Y.Z.; Li, J.B.; Jian, S.R.; Jang, J.S.C.; Li, C.; Chu, J.P.; Huang, J.C. Sharpness improvement of surgical blade by means of ZrCuAlAgSi metallic glass and metallic glass thin film coating. Intermetallics 2012, 31, 127–131. [Google Scholar] [CrossRef]
- Chiang, P.T.; Chen, G.J.; Jian, S.R.; Shih, Y.H.; Jang, J.S.C.; Lai, C.H. Surface antimicrobial effects of Zr61Al7.5Ni10Cu17.5Si4 thin film metallic glasses on Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumannii and Candida albicans. Fooyin J. Health Sci. 2010, 2, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.P.; Liu, T.Y.; Li, C.L.; Wang, C.H.; Jang, J.S.C.; Chen, M.J.; Chang, S.H.; Huang, W.C. Fabrication and characterizations of thin film metallic glasses: Antibacterial property and durability study for medical application. Thin Solid Films 2014, 561, 102–107. [Google Scholar] [CrossRef]
- Shen, J.; Zou, J.; Ye, L.; Lu, Z.P.; Xing, D.W.; Yan, M.; Sun, J.F. Glass-forming ability and thermal stability of a new bulk metallic glass in the quaternary Zr-Cu-Ni-Al system. J. Non. Cryst. Solids 2005, 351, 2519–2523. [Google Scholar] [CrossRef]
- Chu, J.H.; Lee, J.; Chang, C.C.; Chan, Y.C.; Liou, M.L.; Lee, J.W.; Jang, J.S.C.; Duh, J.G. Antimicrobial characteristics in Cu-containing Zr-based thin film metallic glass. Surf. Coat. Technol. 2014, 259, 87–93. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Properties, Characteristics, and Applications of Expanded PTFE (ePTFE) Products. In Expanded PTFE Applications Handbook; William Andrew: Norwich, NY, USA, 2017; pp. 163–170. [Google Scholar] [CrossRef]
- Luo, W.; Liu, Q.; Li, Y.; Zhou, S.; Zou, H.; Liang, M. Enhanced mechanical and tribological properties in polyphenylene sulfide/polytetrafluoroethylene composites reinforced by short carbon fiber. Compos. Part B Eng. 2016, 91, 579–588. [Google Scholar] [CrossRef]
- Su, F.H.; Zhang, Z.Z.; Liu, W.M. Study on the friction and wear properties of glass fabric composites filled with nano- and micro-particles under different conditions. Mater. Sci. Eng. A 2005, 392, 359–365. [Google Scholar] [CrossRef]
- Burkarter, E.; Saul, C.K.; Thomazi, F.; Cruz, N.C.; Roman, L.S.; Schreiner, W.H. Superhydrophobic electrosprayed PTFE. Surf. Coat. Technol. 2007, 202, 194–198. [Google Scholar] [CrossRef]
- Li, J. Mechanical properties of a polyamide 6-RE in forced ptfe com posite. Mech. Compos. Mater. 2009, 45, 435–442. [Google Scholar] [CrossRef]
- Cai, J.; Nesterenko, V.F.; Vecchio, K.S.; Jiang, F.; Herbold, E.B.; Benson, D.J.; Addiss, J.W.; Walley, S.M.; Proud, W.G. The influence of metallic particle size on the mechanical properties of polytetraflouroethylene-Al-W powder composites. Appl. Phys. Lett. 2008, 92, 26–29. [Google Scholar] [CrossRef]
- Zaporojtchenko, V.; Podschun, R.; Schürmann, U.; Kulkarni, A.; Faupel, F. Physico-chemical and antimicrobial properties of co-sputtered Ag-Au/PTFE nanocomposite coatings. Nanotechnology 2006, 17, 4904–4908. [Google Scholar] [CrossRef]
- Kim, J.Y.; Gu, X.; Wraith, M.; Uhl, J.T.; Dahmen, K.A.; Greer, J.R. Suppression of catastrophic failure in metallic glass-polyisoprene nanolaminate containing nanopillars. Adv. Funct. Mater. 2012, 22, 1972–1980. [Google Scholar] [CrossRef]
- Li, S.; Louzguine-Luzgin, D.V.; Xie, G.; Sato, M.; Inoue, A. Development of novel metallic glass/polymer composite materials by microwave heating in a separated H-field. Mater. Lett. 2010, 64, 235–238. [Google Scholar] [CrossRef]
- Lasheras, A.; Gutiérrez, J.; Reis, S.; Sousa, D.; Silva, M.; Martins, P.; Lanceros-Mendez, S.; Barandiarán, J.M.; Shishkin, D.A.; Potapov, A.P. Energy harvesting device based on a metallic glass/PVDF magnetoelectric laminated composite. Smart Mater. Struct. 2015, 24, 065024. [Google Scholar] [CrossRef]
- Gizaw, E.T.; Yeh, H.H.; Chu, J.P.; Hu, C.C. Fabrication and characterization of nitrogen selective thin-film metallic glass/polyacrylonitrile composite membrane for gas separation. Sep. Purif. Technol. 2020, 237, 116340. [Google Scholar] [CrossRef]
- Venkataraman, S.; Rozhkova, E.; Eckert, J.; Schultz, L.; Sordelet, D.J. Thermal stability and crystallization kinetics of Cu-reinforced Cu 47Ti33Zr11Ni8Si1 metallic glass composite powders synthesized by ball milling: The effect of particulate reinforcement. Intermetallics 2005, 13, 833–840. [Google Scholar] [CrossRef]
- Lee, K.S.; Kang, S.H.; Lee, Y.S. Synthesis of Zr-based bulk metallic glass-crystalline aluminum alloy composite by co-extrusion. Mater. Lett. 2010, 64, 129–132. [Google Scholar] [CrossRef]
- Kündig, A.A.; Schweizer, T.; Schafler, E.; Löffler, J.F. Metallic glass/polymer composites by co-processing at similar viscosities. Scr. Mater. 2007, 56, 289–292. [Google Scholar] [CrossRef]
- Nieh, T.G.; Wadsworth, J.; Liu, C.T.; Ohkubo, T.; Hirotsu, Y. Plasticity and structural instability in a bulk metallic glass deformed in the supercooled liquid region. Acta Mater. 2001, 49, 2887–2896. [Google Scholar] [CrossRef]
- Zadorozhnyy, M.Y.; Chukov, D.I.; Churyukanova, M.N.; Gorshenkov, M.V.; Zadorozhnyy, V.Y.; Stepashkin, A.A.; Tsarkov, A.A.; Louzguine-Luzgin, D.V.; Kaloshkin, S.D. Investigation of contact surfaces between polymer matrix and metallic glasses in composite materials based on high-density polyethylene. Mater. Des. 2016, 92, 306–312. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.Y.; Gorshenkov, M.V.; Churyukanova, M.N.; Zadorozhnyy, M.Y.; Stepashkin, A.A.; Moskovskikh, D.O.; Ketov, S.V.; Zinnurova, L.K.; Sharma, A.; Louzguine-Luzgin, D.V.; et al. Investigation of structure and thermal properties in composite materials based on metallic glasses with small addition of polytetrafluoroethylene. J. Alloys Compd. 2017, 707, 264–268. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.; Churyukanova, M.; Stepashkin, A.; Zadorozhnyy, M.; Sharma, A.; Moskovskikh, D.; Wang, J.; Shabanova, E.; Ketov, S.; Louzguine-Luzgin, D.; et al. Structure and thermal properties of an Al-based metallic glass-polymer composite. Metals 2018, 8, 1037. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kopylov, A.; Zadorozhnyy, M.; Stepashkin, A.; Kudelkina, V.; Wang, J.Q.; Ketov, S.; Churyukanova, M.; Louzguine-Luzgin, D.; Sarac, B.; et al. New Mg-Based Metallic Glass-Polymer Composites: Investigation of Structure, Thermal Properties, and Biocompatibility. Metals 2020, 10, 867. [Google Scholar] [CrossRef]
- Orrù, R.; Licheri, R.; Locci, A.M.; Cincotti, A.; Cao, G. Consolidation/synthesis of materials by electric current activated/assisted sintering. Mater. Sci. Eng. R Rep. 2009, 63, 127–287. [Google Scholar] [CrossRef]
- Shelekhov, E.V.; Sviridova, T.A. Programs for x-ray analysis of polycrystals. Met. Sci. Heat Treat. 2000, 42, 309–313. [Google Scholar] [CrossRef]
- Wagner, M. Thermal Analysis in Practice; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2017; pp. 1–9. [Google Scholar] [CrossRef]
- He, L.; Wu, Z.G.; Jiang, F.; Sun, J. Enhanced thermal stability of Zr65Cu17.5Ni10Al7.5 metallic glass at temperature range near glass transition by oxygen impurity. J. Alloys Compd. 2008, 456, 181–186. [Google Scholar] [CrossRef]
- Abrosimova, G.E.; Aronin, A.S.; Kir’janov, Y.V.; Matveev, D.V.; Molokanov, V.V.; Zver’kova, I.I. Crystalline layer on the surface of Zr-based bulk metallic glasses. J. Non. Cryst. Solids. 2001, 288, 121–126. [Google Scholar] [CrossRef]
- Qiao, J.C.; Pelletier, J.M. Influence of thermal treatments and plastic deformation on the atomic mobility in Zr50.7Cu28Ni9Al12.3 bulk metallic glass. J. Alloys Compd. 2015, 615, S85–S89. [Google Scholar] [CrossRef]
- Mihaylov, L.; Lyubenova, L.; Gerdjikov, T.; Nihtianova, D.; Spassov, T. Selective dissolution of amorphous Zr-Cu-Ni-Al alloys. Corros. Sci. 2015, 94, 350–358. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.T.; Yang, Y.; Dong, Y.D.; Lu, J. Atomic-scale structural evolution and stability of supercooled liquid of a Zr-based bulk metallic glass. Phys. Rev. Lett. 2011, 106, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Senatov, F.; Niaza, K.; Zadorozhnyy, V.Y.; Maksimkin, A.; Kaloshkin, S.; Estrin, Y. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef]
- Aji, D.P.; Mujalis, Y. Physical Aging of Zr65Cu17.5Ni10Al7.5 Glassy Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 012036. [Google Scholar] [CrossRef]
- Qiao, J.C.; Casalini, R.; Pelletier, J.M.; Yao, Y. Dynamics of the strong metallic glass Zn38Mg12Ca32Yb18. J. Non. Cryst. Solids 2016, 447, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Calleja, G.; Jourdan, A.; Ameduri, B.; Habas, J.P. Where is the glass transition temperature of poly(tetrafluoroethylene)? A new approach by dynamic rheometry and mechanical tests. Eur. Polym. J. 2013, 49, 2214–2222. [Google Scholar] [CrossRef] [Green Version]
- Aharoni, S.M. Molecular stiffness and thermal properties of polymers. J. Appl. Polym. Sci. 1976, 20, 2863–2869. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Feng, B.; Li, Y.; Wu, S.; Yin, Q.; Yu, Z.; Huang, J. The effect of temperature-induced phase transition of PTFE on the dynamic mechanical behavior and impact-induced initiation characteristics of Al/PTFE. Polym. Test. 2020, 91, 106835. [Google Scholar] [CrossRef]
- Henri, V.; Dantras, E.; Lacabanne, C.; Dieudonne, A.; Koliatene, F. Thermal ageing of PTFE in the melted state: Influence of interdiffusion on the physicochemical structure. Polym. Degrad. Stab. 2020, 171, 109053. [Google Scholar] [CrossRef]
- Price, D.M.; Jarratt, M. Thermal conductivity of PTFE and PTFE composites. Thermochim. Acta 2002, 392–393, 231–236. [Google Scholar] [CrossRef]
| Temperature Analysis, °C | 25 | 50 | 100 | 150 | 200 | 250 | 300 |
|---|---|---|---|---|---|---|---|
| PTFE | |||||||
| Thermal diffusivity, mm2/s | 0.138 ± 0.03 | 0.147 ± 0.007 | 0.141 ± 0.001 | 0.134 ± 0.001 | 0.125 ± 0.001 | 0.115 ± 0.003 | 0.104 ± 0.007 |
| Thermal conductivity, W·m−1·K−1 | 0.090 ± 0.01 | 0.099 ± 0.01 | 0.103 ± 0.02 | 0.103 ± 0.01 | 0.0101 ± 0.01 | 0.099 ± 0.02 | 0.098 ± 0.01 |
| Heat capacity, J/(g·K) | 0.411 ± 0.02 | 0.421 ± 0.02 | 0.459 ± 0.02 | 0.483 ± 0.02 | 0.509 ± 0.02 | 0.540 ± 0.02 | 0.589 ± 0.02 |
| Sample density, g/cm3 | 1.6 ± 0.01 | ||||||
| Composite (70/30) | |||||||
| Thermal diffusivity, mm2/s | 0.254 ± 0.003 | 0.26 ± 0.002 | 0.256 ± 0.001 | 0.247 ± 0.002 | 0.235 ± 0.001 | 0.219 ± 0.001 | 0.197 ± 0.002 |
| Thermal conductivity, W·m−1·K−1 | 0.539 ± 0.01 | 0.580 ± 0.01 | 0.609 ± 0.02 | 0.622 ± 0.02 | 0.631 ± 0.03 | 0.6402 ± 0.01 | 0.680 ± 0.02 |
| Heat capacity, J/(g·K) | 0.59 ± 0.02 | 0.61 ± 0.02 | 0.661 ± 0.02 | 0.70 ± 0.02 | 0.752 ± 0.02 | 0.815 ± 0.02 | 0.959 ± 0.02 |
| Sample density, g/cm3 | 3.6 ± 0.01 | ||||||
| Composite (50/50) | |||||||
| Thermal diffusivity, mm2/s | 0.151 ± 0.005 | 0.178 ± 0.005 | 0.171 ± 0.004 | 0.164 ± 0.002 | 0.152 ± 0.002 | 0.144 ± 0.005 | 0.141 ± 0.007 |
| Thermal conductivity, W·m−1·K- | 0.271 ± 0.01 | 0.330 ± 0.02 | 0.298 ± 0.01 | 0.300 ± 0.03 | 0.293 ± 0.01 | 0.294 ± 0.01 | 0.3 ± 0.01 |
| Heat capacity, J/(g·K) | 0.621 ± 0.02 | 0.641 ± 0.02 | 0.602 ± 0.02 | 0.631 ± 0.02 | 0.666 ± 0.02 | 6.706 ± 0.02 | 0.73 ± 0.02 |
| Sample density, g/cm3 | 2.9 ± 0.01 | ||||||
| Composite (30/70) | |||||||
| Thermal diffusivity, mm2/s | 0.094 ± 0.008 | 0.109 ± 0.002 | 0.104 ± 0.004 | 0.098 ± 0.007 | 0.09 ± 0.003 | 0.082 ± 0.002 | 0.072 ± 0.005 |
| Thermal conductivity, W·m−1·K−1 | 0.147 ± 0.02 | 0.171 ± 0.03 | 0.163 ± 0.01 | 0.157 ± 0.01 | 0.148 ± 0.02 | 0.141 ± 0.04 | 0.145 ± 0.01 |
| Heat capacity, J/(g·K) | 0.602 ± 0.02 | 0.604 ± 0.02 | 0.605 ± 0.02 | 0.619 ± 0.02 | 0.634 ± 0.02 | 0.666 ± 0.02 | 0.775 ± 0.02 |
| Sample density, g/cm3 | 2.6 ± 0.01 | ||||||
| Metallic Glass | |||||||
| Thermal diffusivity, mm2/s | 0.462 ± 0.013 | 0.466 ± 0.004 | 0.479 ± 0.003 | 0.487 ± 0.003 | 0.499 ± 0.002 | 0.505 ± 0.007 | 0.503 ± 0.005 |
| Thermal conductivity, W·m−1·K−1 | 2.41 ± 0.1 | 2.48 ± 0.1 | 2.80 ± 0.1 | 2.92 ± 0.1 | 3.17 ± 0.2 | 3.46 ± 0.2 | 4.10 ± 0.3 |
| Heat capacity, J/(g·K) | 1.024 ± 0.02 | 1.044 ± 0.02 | 1.149 ± 0.02 | 1.177 ± 0.02 | 1.249 ± 0.02 | 1.347 ± 0.02 | 1.599 ± 0.02 |
| Sample density, g/cm3 | 5.1 ± 0.01 | ||||||
| Phase Composition | Zr65Cu17.5Ni10Al7.5 | Zr65Cu17.5Ni10Al7.5 after BM | Zr65Cu17.5Ni10Al7.5 after SPS |
|---|---|---|---|
| Amorphous | 100% | 100% | 70 ± 15% |
| Zr2Ni + Zr2Cu | - | - | ≈30 ± 5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Zadorozhnyy, M.; Stepashkin, A.; Kvaratskheliya, A.; Korol, A.; Moskovskikh, D.; Kaloshkin, S.; Zadorozhnyy, V. Investigation of Thermophysical Properties of Zr-Based Metallic Glass-Polymer Composite. Metals 2021, 11, 1412. https://doi.org/10.3390/met11091412
Sharma A, Zadorozhnyy M, Stepashkin A, Kvaratskheliya A, Korol A, Moskovskikh D, Kaloshkin S, Zadorozhnyy V. Investigation of Thermophysical Properties of Zr-Based Metallic Glass-Polymer Composite. Metals. 2021; 11(9):1412. https://doi.org/10.3390/met11091412
Chicago/Turabian StyleSharma, Adit, Mikhail Zadorozhnyy, Andrey Stepashkin, Aksar Kvaratskheliya, Artem Korol, Dmitri Moskovskikh, Sergey Kaloshkin, and Vladislav Zadorozhnyy. 2021. "Investigation of Thermophysical Properties of Zr-Based Metallic Glass-Polymer Composite" Metals 11, no. 9: 1412. https://doi.org/10.3390/met11091412
APA StyleSharma, A., Zadorozhnyy, M., Stepashkin, A., Kvaratskheliya, A., Korol, A., Moskovskikh, D., Kaloshkin, S., & Zadorozhnyy, V. (2021). Investigation of Thermophysical Properties of Zr-Based Metallic Glass-Polymer Composite. Metals, 11(9), 1412. https://doi.org/10.3390/met11091412









