The Effect of Alcohol on Palladium Nanoparticles in i-Pd(OAc)2(TPPTS)2 for Aerobic Oxidation of Benzyl Alcohol
Abstract
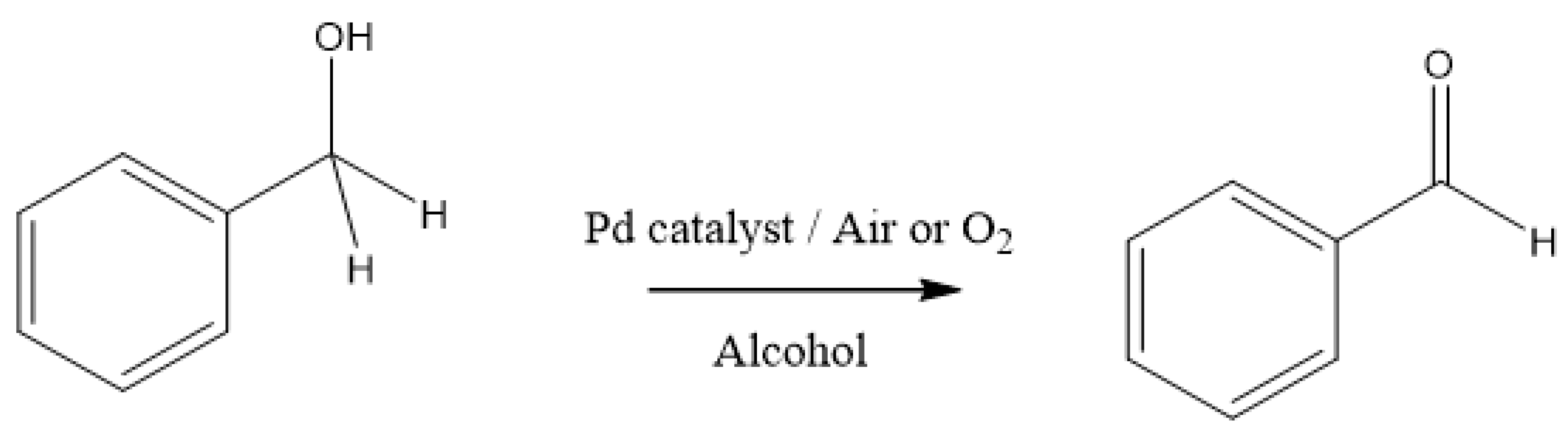
:1. Introduction
2. Materials and Methods
- The i-Pd(OAc)2(TPPTS)2 (without or after impregnation with the selected solvent) was added to a vial that contained 5 mL of ethanol with 0.925 mmol of benzyl alcohol.
- The reaction mixture was preheated using an oil bath at 60 °C and magnetically stirred for 24 h.
- Following the 24 h reaction, the reaction mixture was cooled, and the solid was removed from the liquid. Next, the remaining liquid was further separated from the left-over solid particles by transferring it through a 0.45 μm Millex LH filter (Millipore, Bedford, MA, USA). The filtered liquid was then analyzed by an HP-5 column to determine the conversion by a GC (Phenomenex, Torrance, CA, USA). First, a sample was injected to the GC without any dilution. Next, the conversion rate was calculated based on a calibration carve, which was built in advance and verified every day. In addition, the identity of the GC peaks observed was verified by a comparison of the retention time of the peaks with the retention time of the known standards.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bäckvall, J.E. Modern Oxidation Methods; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Kopylovich, M.N.; Ribeiro, A.P.; Alegria, E.C.; Martins, N.M.; Martins, L.M.; Pombeiro, A.J. Catalytic oxidation of alcohols: Recent advances. In Advances in Organometallic Chemistry; Perez, P., Ed.; Academic Press: Cambridge, UK, 2015; pp. 91–174. [Google Scholar]
- Iwabuchi, Y. Green Oxidative Synthesis of Aldehydes and Ketones. In Green Oxidation in Organic Synthesis; Jiao, N., Stahl, S.S., Eds.; John Wiley & Sons: New York, NY, USA, 2019; pp. 35–78. [Google Scholar]
- Tojo, G.; Fernandez, M.I. Oxidation of Alcohols to Aldehydes and Aetones: A Guide to Current Common Practice; Springer Science & Business Media: New York, NY, USA, 2006. [Google Scholar]
- Ueno, M.; Ohmura, S.D.; Wada, M.; Miyoshi, N. Aerobic oxidation of alcohols using bismuth bromide as a catalyst. Tetrahedron Lett. 2019, 60, 570–573. [Google Scholar] [CrossRef]
- Landaeta, V.R.; Rodríguez-Lugo, R.E. Aerobic Oxidation Reactions in the Fine Chemicals and Pharmaceutical Industries. In Catalytic. Aerobic. Oxidations; Mejía, E., Ed.; Royal Society of Chemistry: Cambridge, UK, 2020; pp. 252–290. [Google Scholar]
- Vinod, C.P.; Wilson, K.; Lee, A.F. Recent advances in the heterogeneously catalysed aerobic selective oxidation of alcohols. J. Chem. Technol. Biotechnol. 2011, 86, 161–171. [Google Scholar] [CrossRef]
- Sheldon, R.A. Recent advances in green catalytic oxidations of alcohols in aqueous media. Catal. Today 2015, 247, 4–13. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Cardona, F. Transition metal based catalysts in the aerobic oxidation of alcohols. Green Chem. 2012, 14, 547–564. [Google Scholar] [CrossRef]
- Cardona, F.; Parmeggiani, C. Overview: Representative experimental procedure, comparative tables and conclusions. In Transition Metal Catalysis in Aerobic Alcohol Oxidation; Cardona, F., Parmeggiani, C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 256–283. [Google Scholar]
- Pierluigi, B.; Liguori, F. Heterogenized Homogeneous Catalysts for Fine Chemicals Production: Materials and Processes; Springer Science & Business Media: Heidelberg, Germany, 2010. [Google Scholar]
- Wolfson, A.; Geresh, S.; Gottlieb, M.; Herskowitz, M. Heterogenization of Rh-MeDuPHOS by occlusion in polyvinyl alcohol films. Tetrahedron. Asymmetry 2002, 13, 465–468. [Google Scholar] [CrossRef]
- Blaser, H.U.; Indolese, A.; Schnyder, A.; Steiner, H.; Studer, M. Supported palladium catalysts for fine chemicals synthesis. J. Mol. Catal. A Chem. 2001, 173, 3–18. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. Aqueous palladium catalysis. In Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi, E.I., Ed.; John Wiley & Sons: New York, NY, USA, 2002; pp. 2955–3006. [Google Scholar]
- Genet, J.P.; Savignac, M.J. Recent developments of palladium (0) catalyzed reactions in aqueous medium. Organomet. Chem. 1999, 576, 305–317. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Biton, S.; Shlomov, B.; Wolfson, A. Renewable polysaccharides as supports for palladium phosphine catalysts. Polymers 2018, 10, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy-Ontman, O.; Blum, D.; Golden, R.; Pierschel, E.; Leviev, S.; Wolfson, A. Palladium based-polysaccharide hydrogels as catalysts in the Suzuki cross-coupling reaction. J. Inorg. Organomet. Polym. Mater. 2020, 30, 622–636. [Google Scholar] [CrossRef]
- Wolfson, A.; Biton, S.; Levy-Ontman, O. Study of Pd-based catalysts within red algae-derived polysaccharide supports in a Suzuki cross-coupling reaction. RSC Adv. 2018, 8, 37939–37948. [Google Scholar] [CrossRef] [Green Version]
- Wolfson, A.; Levy-Ontman, O. Development and application of palladium nanoparticles on renewable polysaccharides as catalysts for the Suzuki cross-coupling of halobenzenes and phenylboronic acids. Mol. Catal. 2020, 493, 111048. [Google Scholar] [CrossRef]
- Wolfson, A.; Pierschel, E.; Orzehovsky, T.; Nirenberg, S.; Levy-Ontman, O. Heterogeneous iota carrageenan-based palladium catalysts for organic synthesis. Org. Comm. 2019, 12, 149–159. [Google Scholar] [CrossRef]
- Stamker, E.; Levy-Ontman, O.; Wolfson, A. Green procedure for aerobic oxidation of benzylic alcohols with palladium supported on iota-carrageenan in ethanol. Polymers 2021, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Bugaev, A.L.; Polyakov, V.A.; Tereshchenko, A.A.; Isaeva, A.N.; Skorynina, A.A.; Kamyshova, E.G.; Budnyk, A.P.; Lastovina, T.A.; Soldatov, A.V. Chemical synthesis and characterization of Pd/SiO2: The effect of chemical reagent. Metals 2018, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Gligorich, K.M.; Sigman, M.S. Recent advancements and challenges of palladium II-catalyzed oxidation reactions with molecular oxygen as the sole oxidant. Chem. Comm. 2009, 26, 3854–3867. [Google Scholar] [CrossRef] [PubMed]
- Skebo, J.E.; Grabinski, C.M.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Assessment of metal nanoparticle agglomeration, uptake, and interaction using high-illuminating system. Int. J. Toxicol. 2007, 26, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, E.; Jansat, S.; Philippot, K.; Lecante, P.; Gomez, M.; Masdeu-Bultó, A.M.; Chaudret, B. Influence of organic ligands on the stabilization of palladium nanoparticles. J. Organomet. Chem. 2004, 689, 4601–4610. [Google Scholar] [CrossRef]
- Provencher, S.W. 1982 CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 2004, 27, 229–242. [Google Scholar] [CrossRef]






| Impregnation Time (h) | Zeta Potential (mV) |
|---|---|
| 0 | –13.37 ± 1.25 |
| 4 | –10.88 ± 0.62 |
| 24 | –5.47 ± 0.19 |
| 48 | –1.80 ± 0.08 |
| 72 | –0.90 ± 0.02 |
| Entry | Solvent | pKa | Conversion (%) |
|---|---|---|---|
| 1 | Ethanol | 15.5 | 20.0 |
| 2 | 1-Propanol | 16.1 | 20.3 |
| 3 | 2-Propanol | 17.2 | 10.5 |
| 4 | 1-Butanol | 16.1 | 22.7 |
| 5 | 1-Hexanol | 16.8 | 19.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy-Ontman, O.; Stamker, E.; Wolfson, A. The Effect of Alcohol on Palladium Nanoparticles in i-Pd(OAc)2(TPPTS)2 for Aerobic Oxidation of Benzyl Alcohol. Metals 2021, 11, 1443. https://doi.org/10.3390/met11091443
Levy-Ontman O, Stamker E, Wolfson A. The Effect of Alcohol on Palladium Nanoparticles in i-Pd(OAc)2(TPPTS)2 for Aerobic Oxidation of Benzyl Alcohol. Metals. 2021; 11(9):1443. https://doi.org/10.3390/met11091443
Chicago/Turabian StyleLevy-Ontman, Oshrat, Eliraz Stamker, and Adi Wolfson. 2021. "The Effect of Alcohol on Palladium Nanoparticles in i-Pd(OAc)2(TPPTS)2 for Aerobic Oxidation of Benzyl Alcohol" Metals 11, no. 9: 1443. https://doi.org/10.3390/met11091443
APA StyleLevy-Ontman, O., Stamker, E., & Wolfson, A. (2021). The Effect of Alcohol on Palladium Nanoparticles in i-Pd(OAc)2(TPPTS)2 for Aerobic Oxidation of Benzyl Alcohol. Metals, 11(9), 1443. https://doi.org/10.3390/met11091443








