Effect of Fe/SiO2 Ratio and Fe2O3 on the Viscosity and Slag Structure of Copper-Smelting Slags
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
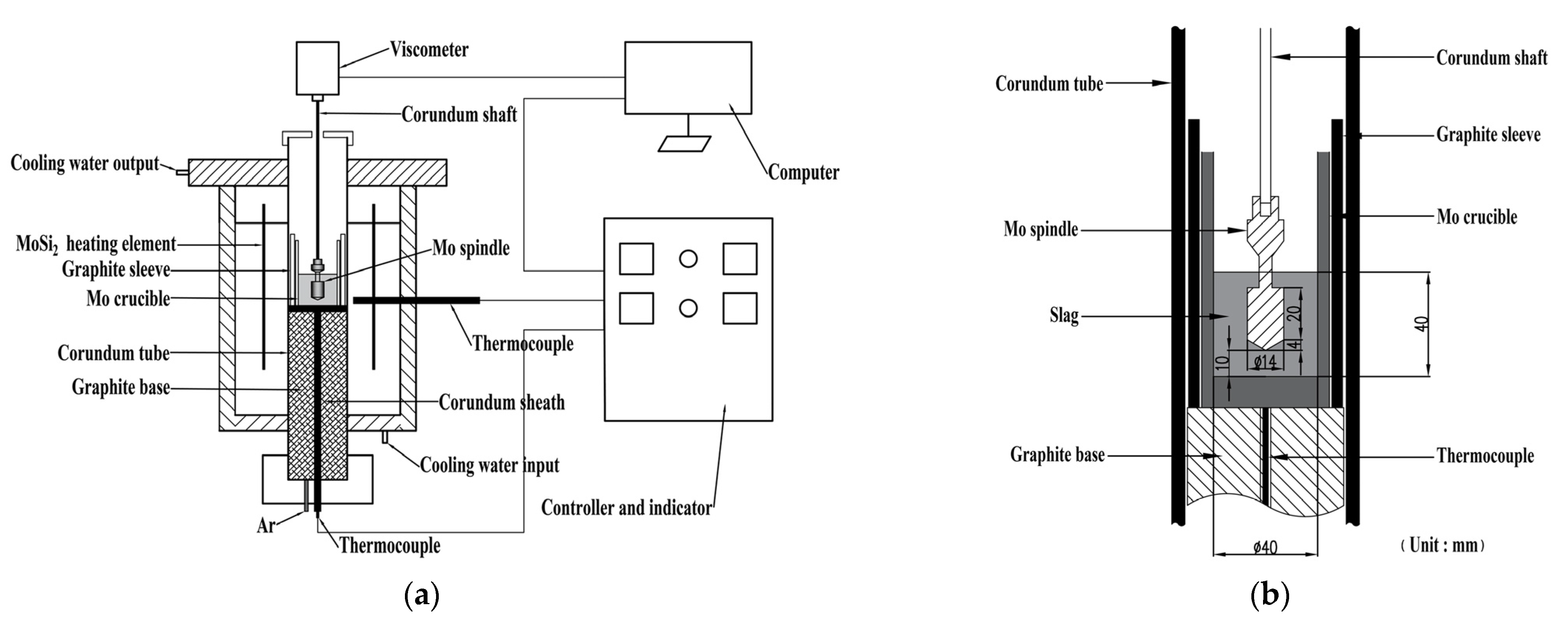
2.2. Experimental Apparatus
2.3. Process for Viscosity Determination
3. Results and Discussion
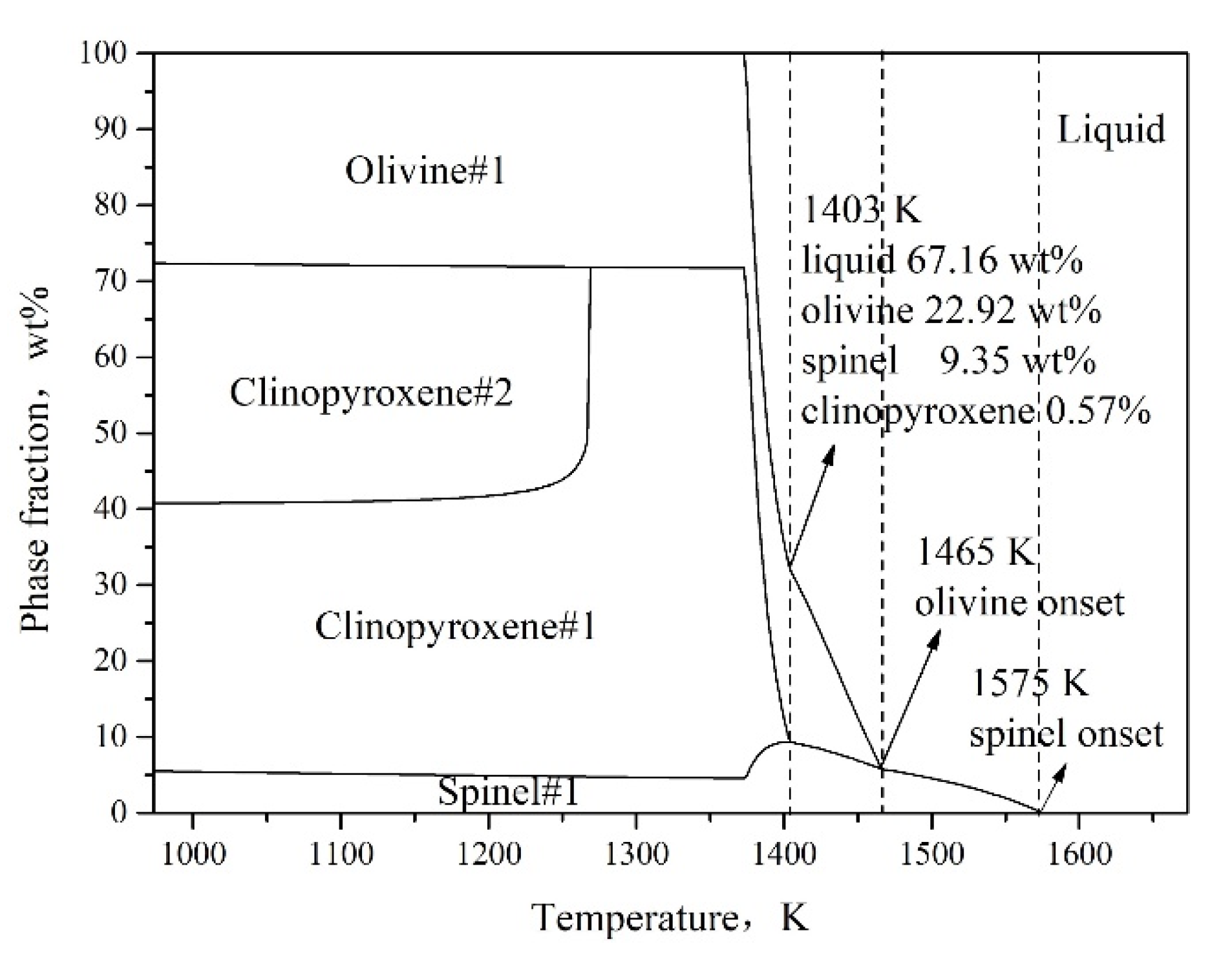
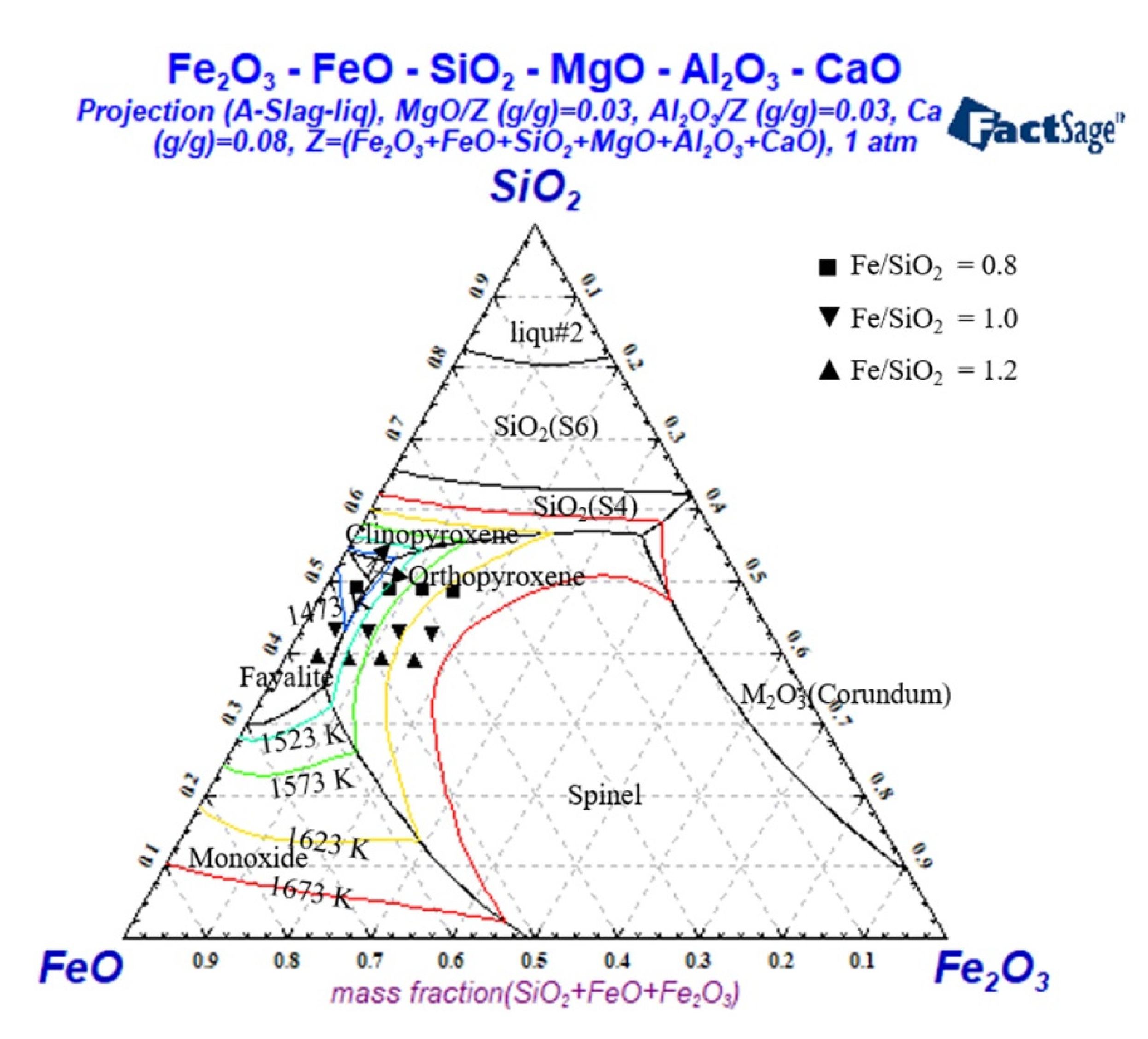
3.1. Effect of Fe/SiO2 on the Breaking Temperature
3.2. Effect of Fe/SiO2 on the Slag Viscosity
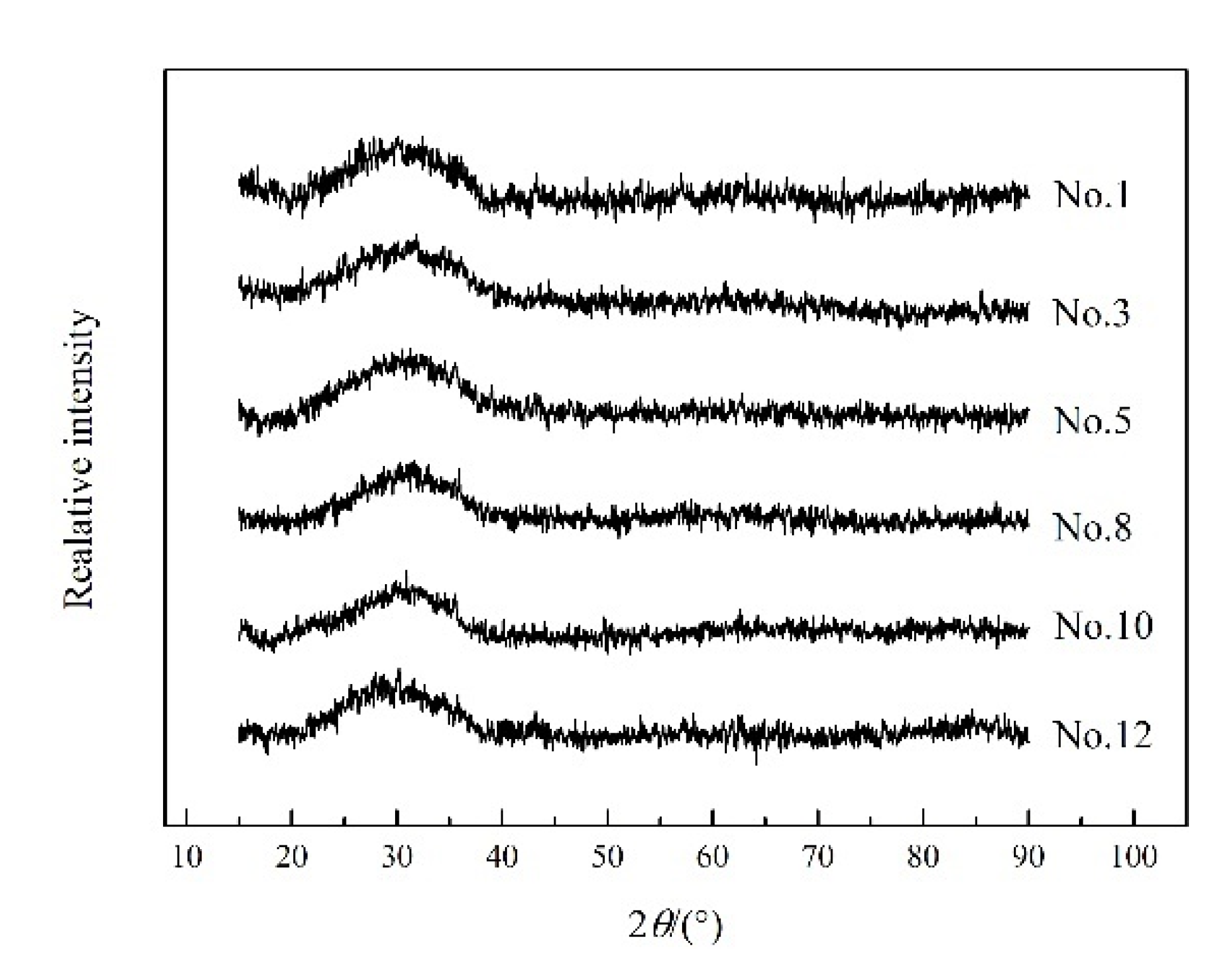
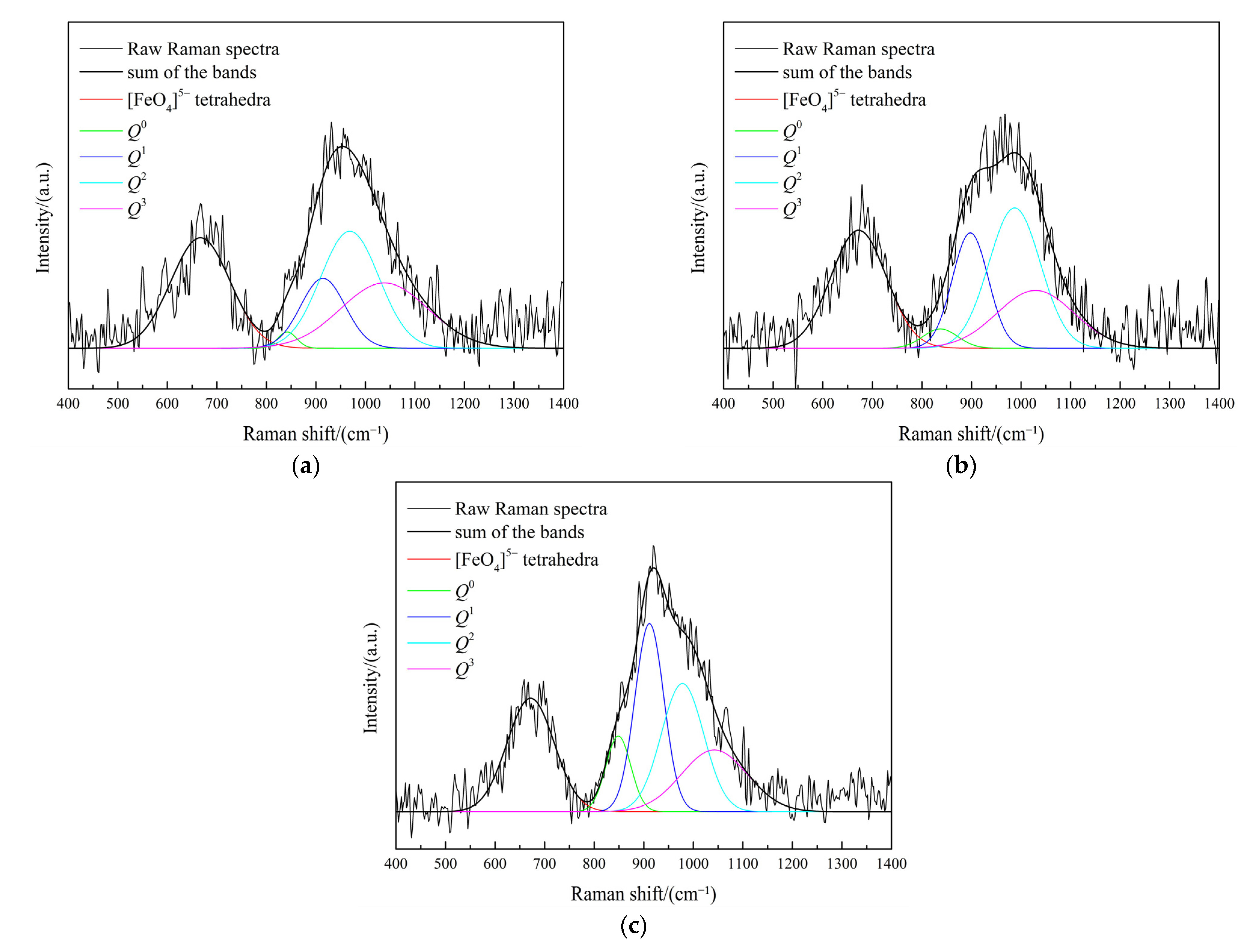
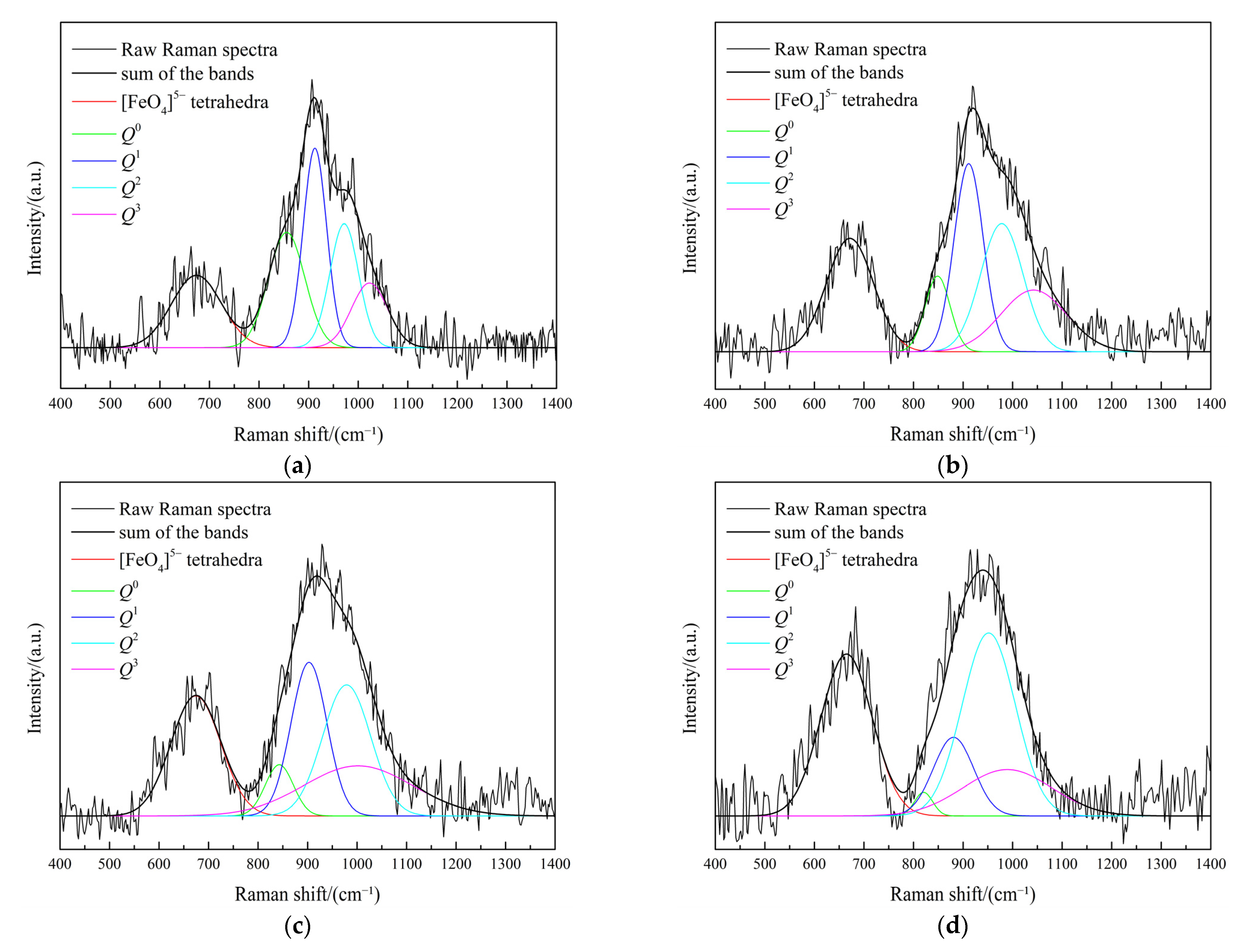
3.3. Effect of Fe/SiO2 on the Slag Structure
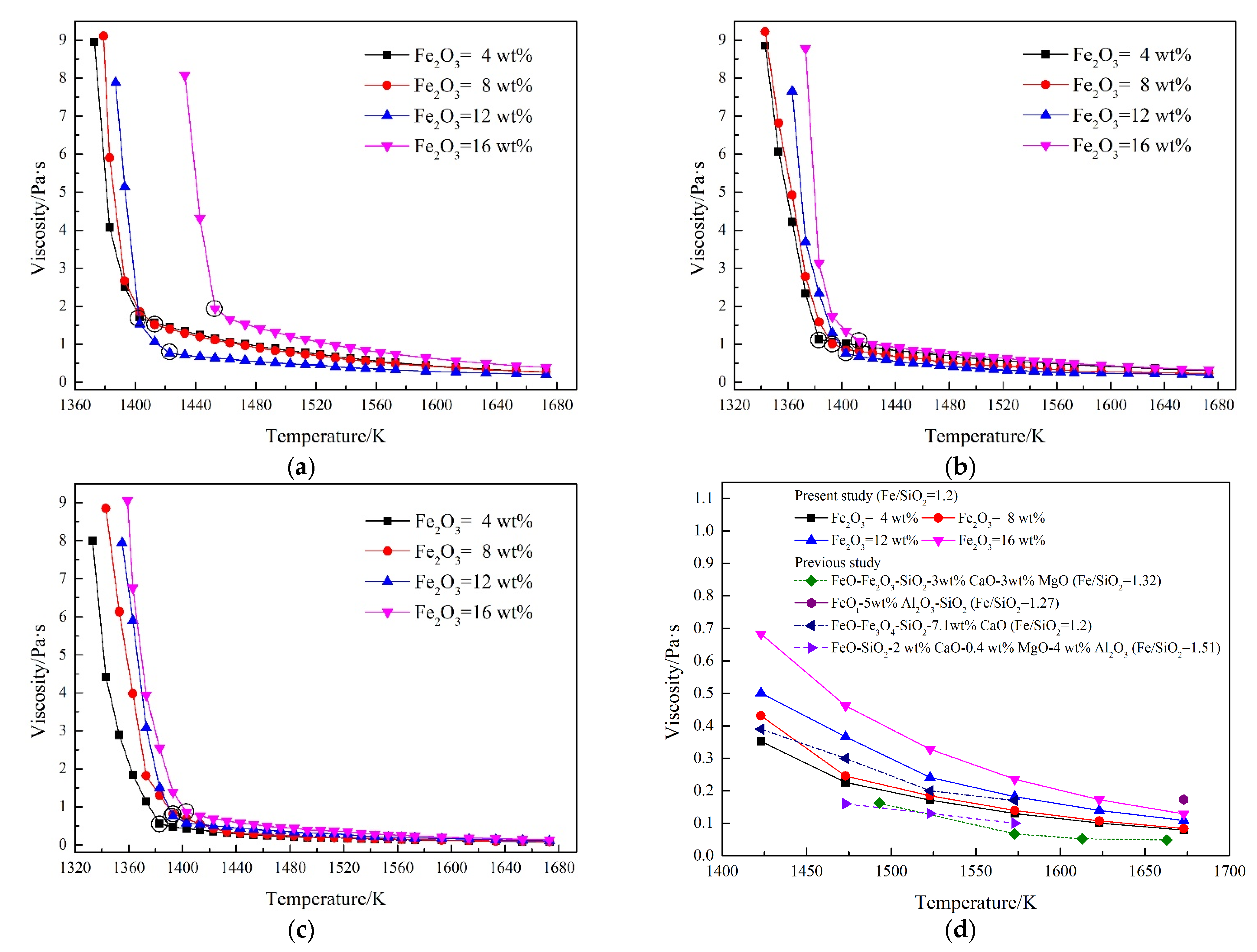
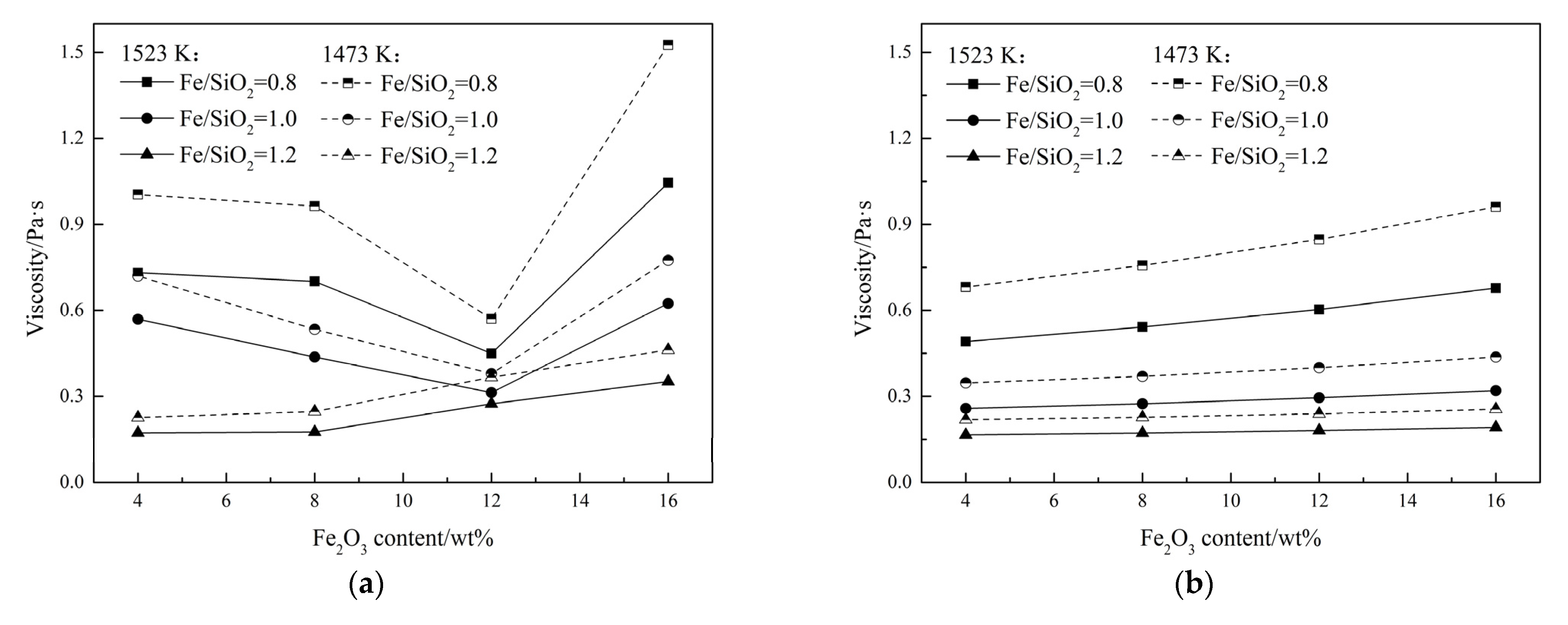
3.4. Effect of Fe2O3 on the Breaking Temperature
3.5. Effect of Fe2O3 on the Slag Viscosity
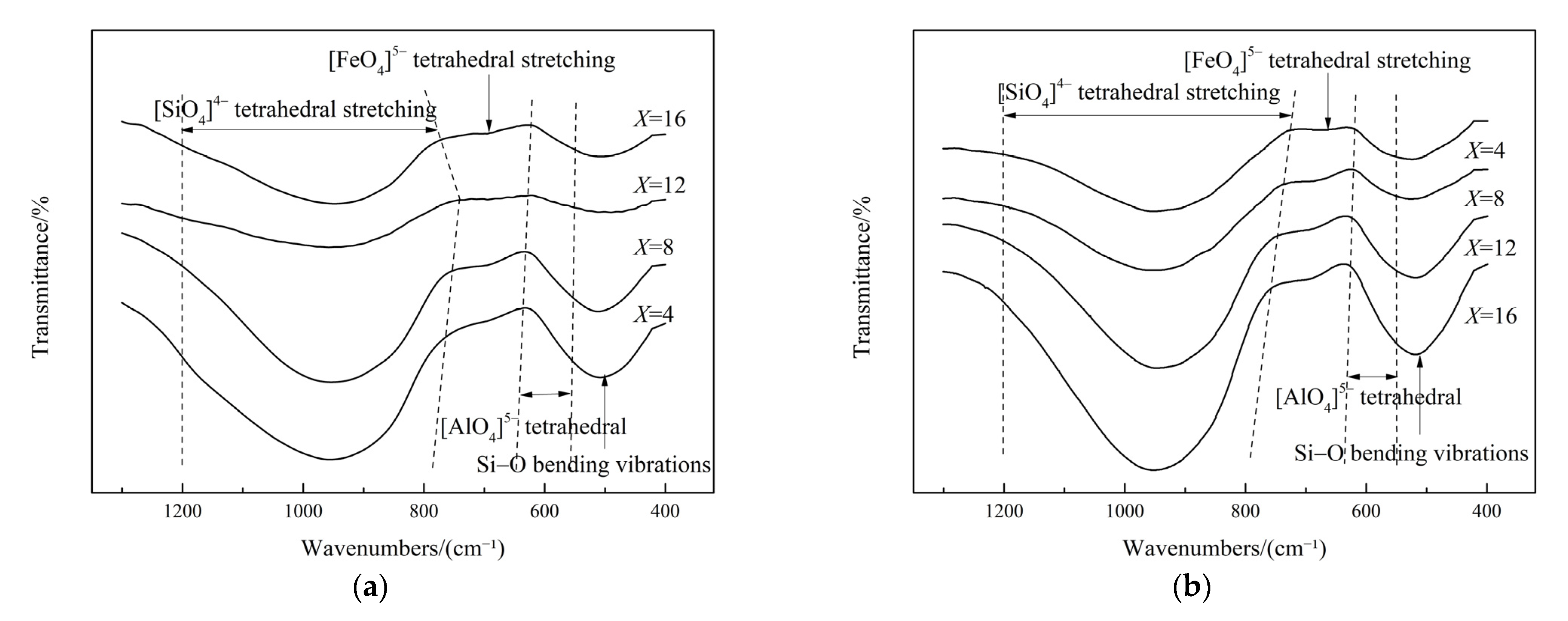
3.6. Effect of Fe2O3 on the Slag Structure
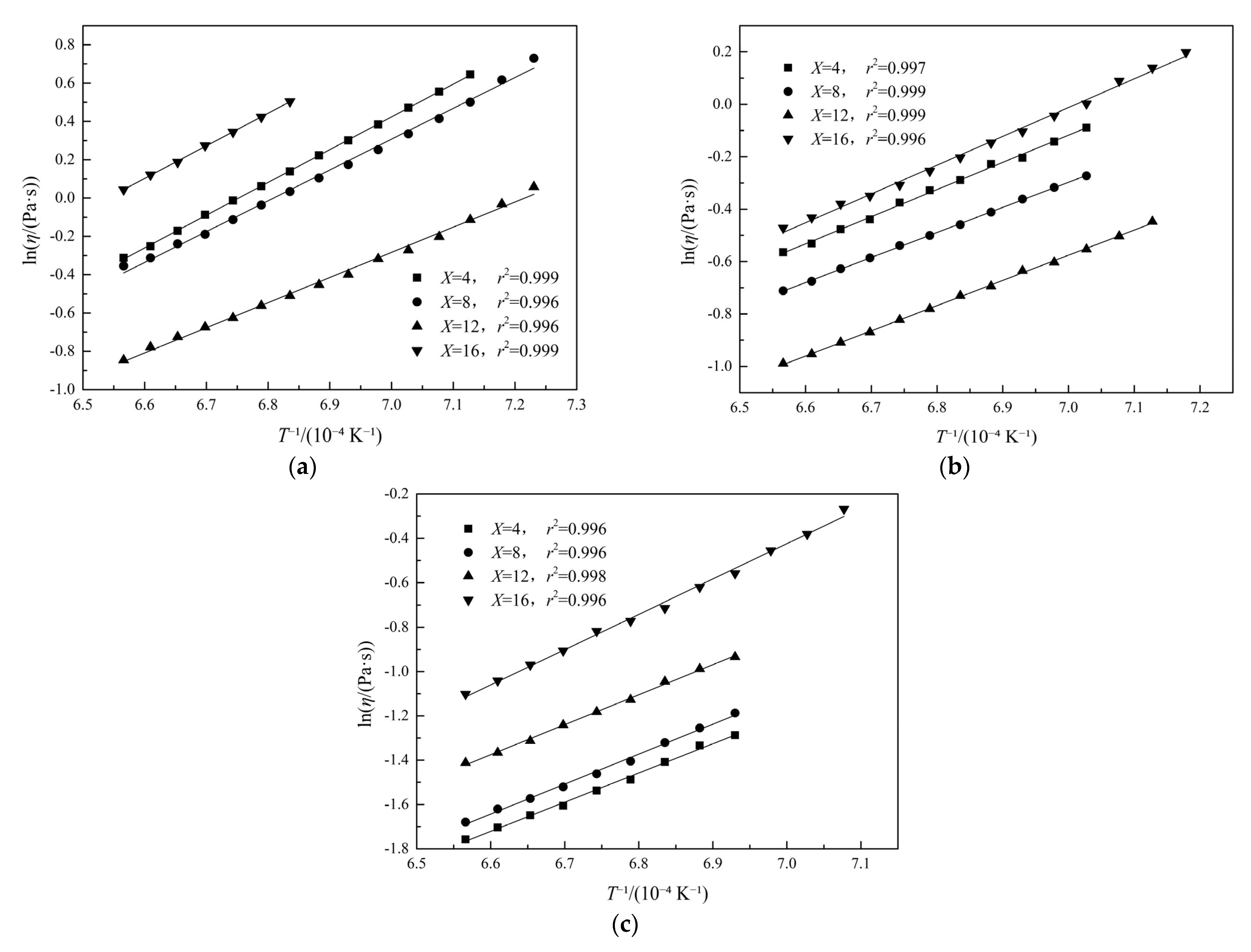
3.7. Activation Energy for Viscous Flow
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, J.G.; Dong, F.S.; Zhao, J.W.; Xue, S.B.; Jian, L.L. Treatment of complex gold concentrates by made matte. Gold 2013, 34, 60–62. (In Chinese) [Google Scholar] [CrossRef]
- Qu, S.L.; Dong, Z.Q.; Chen, T. Study on gold collection in matte with oxygen enriched bottom blowing smelting process. Nonferrous Met. (Extr. Metall.) 2013, 6, 40–42. (In Chinese) [Google Scholar] [CrossRef]
- Kucharski, M.; Stubina, N.M.; Toguri, J.M. Viscosity measurements of molten Fe–O–SiO2, Fe–O–CaO–SiO2, and Fe–O–MgO–SiO2 slags. Can. Metall. Q. 1989, 28, 7–11. [Google Scholar] [CrossRef]
- Goel, R.P.; Kellogg, H.H.; Rrain, J.L. Mathematical description of the thermodynamic properties of the systems Fe-O and Fe-O-SiO2. Metall. Mater. Trans. B 1980, 11, 107–117. [Google Scholar] [CrossRef]
- Nowok, J.W. Viscosity and Structural State of Iron in Coal Ash Slags under Gasification Conditions. Energy Fuels 1995, 9, 534–539. [Google Scholar] [CrossRef]
- Lee, S.; Min, D.J. Viscous Behavior of FeO-Bearing Slag Melts Considering Structure of Slag. Steel Res. Int. 2018, 89, 1800055. [Google Scholar] [CrossRef]
- Lee, Y.S.; Min, D.J.; Jung, S.M.; Yi, S.H. Influence of basicity and FeO content on viscosity of blast furnace type slags containing FeO. ISIJ Int. 2004, 44, 1283–1290. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.R.; Lee, Y.S.; Min, D.J.; Jung, S.M.; Yi, S.H. Influence of MgO and Al2O3 Contents on Viscosity of Blast Furnace Type Slags Containing FeO. ISIJ Int. 2004, 44, 1291–1297. [Google Scholar] [CrossRef]
- Wang, Z.J.; Shu, Q.F.; Sridhar, S.; Zhang, M.; Guo, M.; Zhang, Z.T. Effect of P2O5 and FetO on the Viscosity and Slag Structure in Steelmaking Slags. Met. Mater. Trans. A 2015, 46, 758–765. [Google Scholar] [CrossRef]
- Lv, J.F.; Jin, Z.N.; Yang, H.Y.; Tong, L.L.; Chen, G.B.; Xiao, F.X. Effect of the CaO/SiO2 mass ratio and FeO content on the viscosity of CaO−SiO2−“FeO”−12 wt%ZnO−3 wt%Al2O3 slags. Int. J. Miner. Metall. Mater. 2017, 24, 756–767. [Google Scholar] [CrossRef]
- Chen, M.; Raghunath, S.; Zhao, B.J. Viscosity measurements of “FeO”−SiO2 slag in equilibrium with metallic Fe. Metall. Mater. Trans. B 2013, 44, 506. [Google Scholar] [CrossRef]
- Ducret, A.C.; Rankin, W.J. Liquidus temperatures and viscosities of FeO−Fe2O3−SiO2−CaO−MgO slags at compositions rele-vant to nickel matte smelting. Scand. J. Metall. 2002, 31, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Ji, F.Z.; Sichen, D.; Seetharaman, S. Experimental studies of the viscosities in the CaO−FenO−SiO2 Slags. Metall. Mater. Trans. B 1997, 28, 827–834. [Google Scholar] [CrossRef]
- Chen, M.; Raghunath, S.; Zhao, B.J. Viscosity of SiO2−“FeO”−Al2O3 system in equilibrium with metallic Fe. Metall. Mater. Trans. B 2013, 44, 820–827. [Google Scholar] [CrossRef]
- Osugi, T.; Sukenaga, S.; Inatomi, Y.; Gonda, Y.; Saito, N.; Nakashima, K. Effect of Oxidation State of Iron Ions on the Viscosity of Alkali Silicate Melts. ISIJ Int. 2013, 53, 185–190. [Google Scholar] [CrossRef]
- Hayashi, M.; Horita, K.; Endo, R.; Watanabe, T.; Susa, M. Effect of coordination structure of iron ions on iron oxide activities in Na2O−SiO2−FeO−Fe2O3 melts. ISIJ Int. 2019, 59, 1744–1751. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Sridhar, S.; Zhang, M.; Guo, M.; Zhang, Z. Effect of Al2O3 on the Viscosity and Structure of CaO-SiO2-MgO-Al2O3-FetO Slags. Met. Mater. Trans. A 2015, 46, 537–541. [Google Scholar] [CrossRef]
- Dingwell, D.B.; Virgo, D. The effect of oxidation state on the viscosity of melts in the system Na2O-FeO-Fe2O3-SiO2. Geochim. Cosmochim. Acta 1987, 51, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Saito, N.; Hori, N.; Nakashima, K.; Mori, K. Viscosity of blast furnace type slags. Met. Mater. Trans. A 2003, 34, 509–516. [Google Scholar] [CrossRef]
- Wright, S.; Zhang, L. The Influence of Fe3+/Fe2+ ratio on the viscosity of iron silicate slags. In VII International Conference on Molten Slags Fluxes and Salts; South African Institute of Mining and Metallurgy: Cape Town, South Africa, 2004; pp. 231–236. [Google Scholar]
- Mysen, B.O.; Virgo, D.; Scarfe, C.M.; Cronin, D.J. Viscosity and structure of iron-bearing and aluminum-bearing calcium sili-cate melts at 1-atm. Am. Mineral. 1985, 70, 487–498. [Google Scholar]
- Sasaki, Y.; Mohri, M.; Suyama, K.; Ishii, K. The Effect of Fe3+ Ions on the Anionic Structure of Iron-bearing Sodium Silicate Melts. ISIJ Int. 2000, 40, 1181–1187. [Google Scholar] [CrossRef]
- Viswanathan, N.N.; Ji, F.-Z.; Sichen, D.; Seetharaman, S. Viscosity Measurements on Some Fayalite Slags. ISIJ Int. 2001, 41, 722–727. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-S.; Park, S.S.; Sohn, I. The Viscous Behavior of FeOt-Al2O3-SiO2 Copper Smelting Slags. Metall. Mater. Trans. A 2011, 42, 692–699. [Google Scholar] [CrossRef]
- Li, T.L.; Sun, C.Y.; Song, S.; Wang, Q. Influences of Al2O3 and TiO2 content on viscosity and structure of CaO−8%MgO−Al2O3−SiO2−TiO2−5%FeO blast furnace primary slag. Metals 2019, 9, 743. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Zhang, Z.; Liu, L.; Wang, X. Investigation of the Viscosity and Structural Properties of CaO-SiO2-TiO2 Slags. Met. Mater. Trans. A 2014, 45, 1389–1397. [Google Scholar] [CrossRef]
- Feng, C.; Chu, M.S.; Tang, J.; Tang, Y.T.; Liu, Z.G. Effect of CaO/SiO2 and Al2O3 on viscous behaviors of the Titani-um-bearing blast furnace slag. Steel Res. Int. 2016, 87, 1274–1283. [Google Scholar] [CrossRef]
- Wu, L.; Gran, J.; Sichen, D. The Effect of Calcium Fluoride on Slag Viscosity. Met. Mater. Trans. A 2011, 42, 928–931. [Google Scholar] [CrossRef]
- Kim, H.; Sohn, I. Effect of CaF2 and Li2O additives on the viscosity of CaO−SiO2−Na2O slags. ISIJ Int. 2011, 51, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, S.; Mills, K.C.; Afrange, O.D.C.; Lörz, H.P.; Carli, R. Break Temperatures of mould fluxes and their relevance to con-tinuous casting. Ironmak. Steelmak. 2000, 27, 238–242. [Google Scholar] [CrossRef]
- Kim, H.; Matsuura, H.; Tsukihashi, F.; Wang, W.L.; Min, D.J.; Sohn, I. Effect of Al2O3 and CaO/SiO2 on the viscosity of cal-cium-silicate-based slags containing 10 mass pct MgO. Metall. Mater. Trans. B 2013, 44, 5–12. [Google Scholar] [CrossRef]
- Yu, J.P.; Wang, L.J.; Wang, Y.X.; Liu, Y.Q.; Zhou, G.Z. Effect of Fe2+ and Fe3+ on the properties of melts containing FeOx. J. Iron Steel Res. 2014, 26, 1–8. [Google Scholar] [CrossRef]
- Park, H.; Park, J.-Y.; Kim, G.H.; Sohn, I. Effect of TiO2 on the Viscosity and Slag Structure in Blast Furnace Type Slags. Steel Res. Int. 2012, 83, 150–156. [Google Scholar] [CrossRef]
- Lucazeau, G.; Sergent, N.; Pagnier, T.; Shaula, A.; Kharton, V.; Marques, F.M.B. Raman spectra of apatites: La10x Si6y (Al, Fe)y O26 ± δ. J. Raman Spectrosc. 2007, 38, 21–33. [Google Scholar] [CrossRef]
- Mysen, B.O.; Finger, L.W.; Virgo, D.; Seifert, F.A. Curve-fitting of Raman spectra of silicate glasses. Am. Mineral. 1982, 67, 686–695. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D.; Scarfe, C.M. Relations between the anionic structure and viscosity of silicate melts—A Raman spectro-scopic study. Am. Mineral. 1980, 65, 690–710. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.N.; Yang, H.Y.; Lv, J.F.; Tong, L.L.; Chen, G.B.; Zhang, Q. Effect of ZnO on viscosity and structure of CaO−SiO2−ZnO−FeO−Al2O3 slags. JOM 2018, 70, 1430–1436. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Cai, D.; Zhang, J.; Sasaki, Y.; Ostrovski, O. Effects of CaO/SiO2 Ratio and Na2O Content on Melting Properties and Viscosity of SiO2-CaO-Al2O3-B2O3-Na2O Mold Fluxes. Met. Mater. Trans. A 2017, 48, 516–526. [Google Scholar] [CrossRef]
- Talapaneni, T.; Yedla, N.; Pal, S.; Sarkar, S. Experimental and Theoretical Studies on the Viscosity–Structure Correlation for High Alumina-Silicate Melts. Met. Mater. Trans. B 2017, 48, 1450–1462. [Google Scholar] [CrossRef]
- Zhang, H.W.; Sun, F.; Shi, X.Y.; Zhang, B.; Hong, X. The viscous and conductivity behavior of melts containing iron oxide in the FeOt−SiO2−CaO−Cu2O system for copper smelting slags. Metall. Mater. Trans. B 2012, 43, 1046–1053. [Google Scholar] [CrossRef]
- Zhao, B.J.; Font, J.; Moyano, A.; Jak, E.; Hayes, P.C. Viscosity and electric conductivity of copper slag at controlled oxygen potential. In Proceedings of the VIII International Conference on Molten Slags, Fluxes and Salts, Santiago, Chile, 18–21 January 2009; pp. 225–235. [Google Scholar]
- Urbain, G.; Bottinga, Y.; Richet, P. Viscosity of liquid silica, silicates and alumino-silicates. Geochim. Cosmochim. Acta 1982, 46, 1061–1072. [Google Scholar] [CrossRef]





| No. | Designed Composition (wt%) | Analyzed Composition (wt%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe/SiO2 | Fe2O3 | SiO2 | FeO | MgO | Al2O3 | CaO | Fe/SiO2 | Fe2O3 | SiO2 | FeO | MgO | Al2O3 | CaO | |
| 1 | 0.8 | 4 | 42.18 | 39.82 | 3 | 3 | 8 | 0.82 | 4.6 | 41.4 | 39.5 | 2.8 | 3.2 | 8.0 |
| 2 | 0.8 | 8 | 41.99 | 36.01 | 3 | 3 | 8 | 0.82 | 8.5 | 41.0 | 35.6 | 3.1 | 3.3 | 8.0 |
| 3 | 0.8 | 12 | 41.79 | 32.21 | 3 | 3 | 8 | 0.82 | 12.6 | 40.9 | 31.8 | 3.0 | 3.2 | 8.0 |
| 4 | 0.8 | 16 | 41.59 | 28.41 | 3 | 3 | 8 | 0.82 | 16.6 | 40.5 | 27.9 | 3.1 | 3.1 | 7.8 |
| 5 | 1.0 | 4 | 37.44 | 44.56 | 3 | 3 | 8 | 1.02 | 4.6 | 36.9 | 44.1 | 2.9 | 3.1 | 8.0 |
| 6 | 1.0 | 8 | 37.26 | 40.74 | 3 | 3 | 8 | 1.02 | 8.5 | 36.3 | 40.2 | 3.0 | 3.0 | 8.0 |
| 7 | 1.0 | 12 | 37.09 | 36.91 | 3 | 3 | 8 | 1.00 | 12.5 | 37.0 | 36.4 | 2.9 | 3.2 | 7.8 |
| 8 | 1.0 | 16 | 36.91 | 33.09 | 3 | 3 | 8 | 1.02 | 16.5 | 36.0 | 33.0 | 2.9 | 3.1 | 8.1 |
| 9 | 1.2 | 4 | 33.65 | 48.35 | 3 | 3 | 8 | 1.22 | 4.6 | 33.0 | 47.8 | 3.0 | 3.2 | 8.1 |
| 10 | 1.2 | 8 | 33.49 | 44.51 | 3 | 3 | 8 | 1.24 | 8.9 | 32.7 | 43.9 | 3.1 | 3.2 | 7.7 |
| 11 | 1.2 | 12 | 33.33 | 40.67 | 3 | 3 | 8 | 1.24 | 12.8 | 32.1 | 39.9 | 3.1 | 2.9 | 7.9 |
| 12 | 1.2 | 16 | 33.18 | 36.82 | 3 | 3 | 8 | 1.24 | 16.7 | 32.0 | 35.8 | 3.0 | 3.1 | 8.2 |
| No. | Fe/SiO2 | Q0 | Q1 | Q2 | Q3 | NBO/Si |
|---|---|---|---|---|---|---|
| 1 | 0.8 | 0.02 | 0.19 | 0.43 | 0.36 | 1.87 |
| 2 | 1.0 | 0.04 | 0.26 | 0.43 | 0.27 | 2.07 |
| 3 | 1.2 | 0.12 | 0.32 | 0.32 | 0.24 | 2.32 |
| Fe/SiO2 Ratio | Fe2O3 Content/wt% | Activation Energy/(kJ·mol−1) |
|---|---|---|
| 0.8 | 4 | 142.16 |
| 8 | 136.77 | |
| 12 | 131.79 | |
| 16 | 141.51 | |
| 1.0 | 4 | 138.14 |
| 8 | 129.56 | |
| 12 | 127.97 | |
| 16 | 136.25 | |
| 1.2 | 4 | 109.47 |
| 8 | 112.39 | |
| 12 | 112.63 | |
| 16 | 132.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yang, H.; Jin, Z.; Liu, Z.; Zou, M. Effect of Fe/SiO2 Ratio and Fe2O3 on the Viscosity and Slag Structure of Copper-Smelting Slags. Metals 2022, 12, 24. https://doi.org/10.3390/met12010024
Wang B, Yang H, Jin Z, Liu Z, Zou M. Effect of Fe/SiO2 Ratio and Fe2O3 on the Viscosity and Slag Structure of Copper-Smelting Slags. Metals. 2022; 12(1):24. https://doi.org/10.3390/met12010024
Chicago/Turabian StyleWang, Baoren, Hongying Yang, Zhenan Jin, Zhijian Liu, and Mingjun Zou. 2022. "Effect of Fe/SiO2 Ratio and Fe2O3 on the Viscosity and Slag Structure of Copper-Smelting Slags" Metals 12, no. 1: 24. https://doi.org/10.3390/met12010024





