Morphology and Crystallography Analyses of HSLA Steels with Hardenability Enhanced by Tailored C–Ni Collocation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. CCT and Vickers Hardness
3.2. Morphological and Crystallographic Features
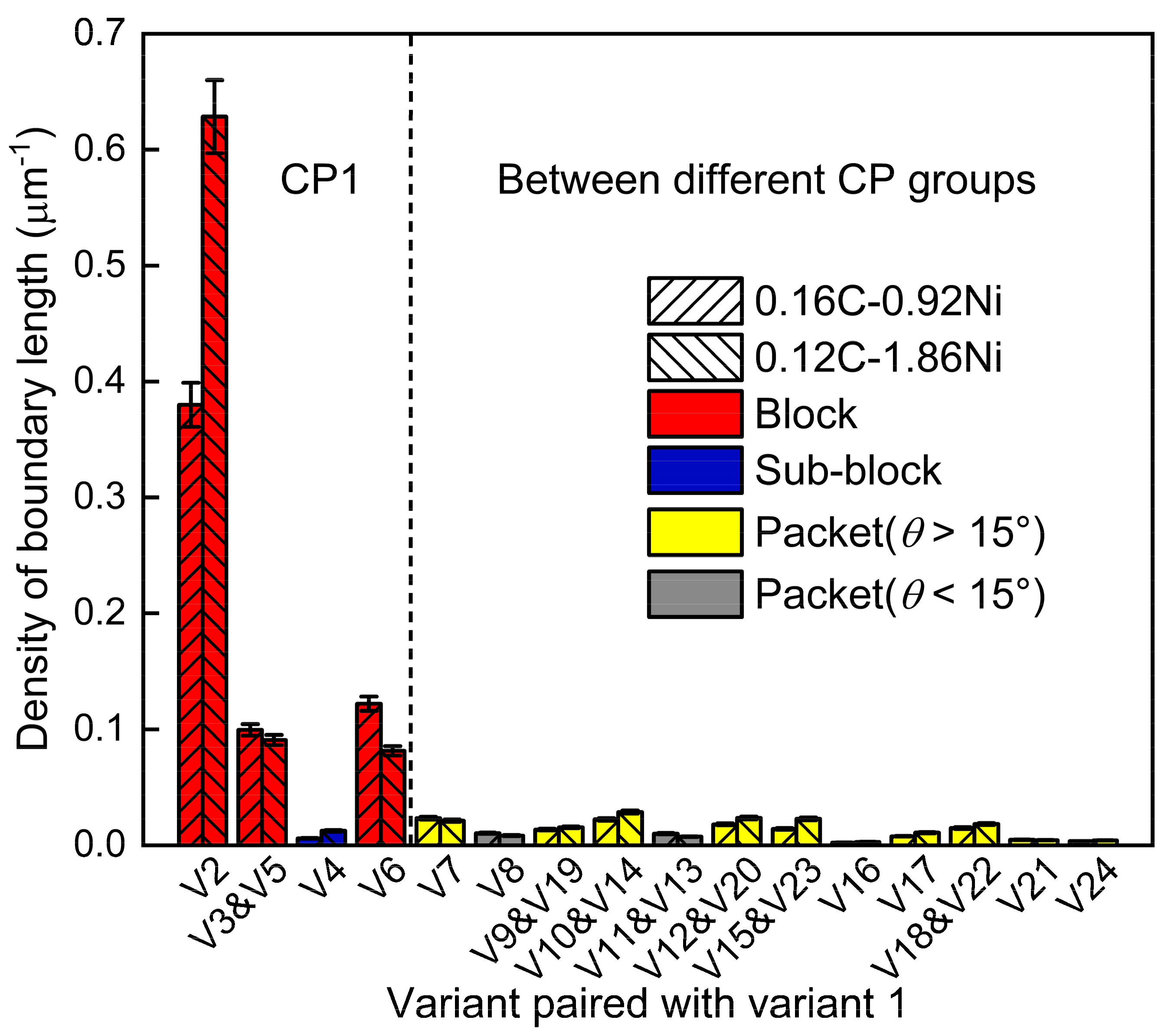
3.3. Variant Analysis: Digitization and Visualization
3.4. Correlation of Crystallographic Structure and Hardness
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Z.J.; Fang, Y.P.; Han, G.; Guo, H.; Misra, R.D.K.; Shang, C.J. Structure–property relationship in a 960 MPa grade ultrahigh strength low carbon niobium–vanadium microalloyed steel: The significance of high frequency induction tempering. Mater. Sci. Eng. A 2014, 618, 112–117. [Google Scholar] [CrossRef]
- Yu, Y.S.; Hu, B.; Gao, M.L.; Xie, Z.J.; Rong, X.Q.; Han, G.; Guo, H.; Shang, C.J. Determining role of heterogeneous microstructure in lowering yield ratio and enhancing impact toughness in high-strength low-alloy steel. Int. J. Miner. Metall. Mater. 2021, 28, 816–825. [Google Scholar] [CrossRef]
- Capdevila, C.; García-Mateo, C.; Chao, J.; Caballero, F.G. Advanced vanadium alloyed steel for heavy product applications. Mater. Sci. Technol. 2009, 25, 1383–1386. [Google Scholar] [CrossRef] [Green Version]
- An, F.C.; Zhao, S.X.; Xue, X.K.; Wang, J.J.; Yuan, G.; Liu, C.M. Incompleteness of bainite transformation in quenched and tempered steel under continuous cooling conditions. J. Mater. Res. Technol. 2020, 9, 8985–8996. [Google Scholar] [CrossRef]
- Pan, T.; Wang, X.; Su, H.; Yang, C. Effect of alloying element Al on hardenability and mechanical properties of micro-B treated ultra-heavy plate steels. Acta Metall. Sin. 2014, 50, 431–438. [Google Scholar] [CrossRef]
- Zhou, T.; Yu, H.; Wang, S.Y. Microstructural characterization and mechanical properties across thickness of ultra-heavy steel plate. Steel Res. Int. 2017, 88, 1700132. [Google Scholar] [CrossRef]
- Al Hajeri, K.F.; Garcia, C.I.; Hua, M.; Deardo, A.J. Particle-stimulated nucleation of ferrite in heavy steel sections. ISIJ Int. 2006, 46, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Cao, R.; Zhu, W.; Guo, X.; Jiang, Y.; Chen, J. Microstructure evolution and impact toughness variation for high strength steel multi-pass weld metals with various cooling rates. J. Manuf. Processes 2021, 65, 245–257. [Google Scholar] [CrossRef]
- Kong, X.; Lan, L. Optimization of mechanical properties of low carbon bainitic steel using TMCP and accelerated cooling. Procedia Eng. 2014, 81, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.L.; Wang, Z.Q.; Dong, L.L.; Shang, C.J.; Ma, X.P.; Subramanian, S.V. New insights into the mechanism of cooling rate on the impact toughness of coarse grained heat affected zone from the aspect of variant selection. Mater. Sci. Eng. A 2017, 704, 448–458. [Google Scholar] [CrossRef]
- Wang, X.L.; Ma, X.P.; Wang, Z.Q.; Subramanian, S.V.; Xie, Z.J.; Shang, C.J.; Li, X.C. Carbon microalloying effect of base material on variant selection in coarse grained heat affected zone of X80 pipeline steel. Mater. Charact. 2019, 149, 26–33. [Google Scholar] [CrossRef]
- Asahi, H. Effects of Mo addition and austenitizing temperature on hardenability of low alloy B-added steels. ISIJ Int. 2002, 42, 1150–1155. [Google Scholar] [CrossRef]
- Hara, T.; Asahi, H.; Uemori, R.; Tamehiro, H. Role of combined addition of niobium and boron and of molybdenum and boron on hardnenability in low carbon steels. ISIJ Int. 2004, 44, 1431–1440. [Google Scholar] [CrossRef]
- Han, F.; Hwang, B.; Suh, D.W.; Wang, Z.; Lee, D.L.; Kim, S.J. Effect of molybdenum and chromium on hardenability of low-carbon boron-added steels. Met. Mater. Int. 2008, 14, 667–672. [Google Scholar] [CrossRef]
- Li, Y.J.; Ponge, D.; Choi, P.; Raabe, D. Atomic scale investigation of non-equilibrium segregation of boron in a quenched Mo-free martensitic steel. Ultramicroscopy 2015, 159, 240–247. [Google Scholar] [CrossRef]
- Takahashi, J.; Ishikawa, K.; Kawakami, K.; Fujioka, M.; Kubota, N. Atomic-scale study on segregation behavior at austenite grain boundaries in boron- and molybdenum-added steels. Acta Mater. 2017, 133, 41–54. [Google Scholar] [CrossRef]
- Huang, S.; Wu, B.B.; Wang, Z.Q.; Yu, Y.S.; Wang, C.S.; Yan, L.; Li, X.C.; Shang, C.J.; Misra, R.D.K. EBSD study on the significance of carbon content on hardenability. Mater. Lett. 2019, 254, 412–414. [Google Scholar] [CrossRef]
- Dossett, J.L.; Totten, G.E. ASM Handbook Volume 4A: Steel Heat Treating Fundamentals and Processes; ASM International: Materials Park, OI, USA, 2013. [Google Scholar]
- Chung, Y.D.; Fujii, H.; Ueji, R.; Tsuji, N. Friction stir welding of high carbon steel with excellent toughness and ductility. Scripta Mater. 2010, 63, 223–226. [Google Scholar] [CrossRef]
- Huang, S.; Yu, Y.S.; Wang, Z.Q.; Su, S.; Chen, K.; Yuan, S.F.; Xie, Z.J.; Shang, C.J. Crystallographic insights into the role of nickel on hardenability of wear-resistant steels. Mater. Lett. 2022, 306, 130961. [Google Scholar] [CrossRef]
- Huang, G.; Wan, X.; Wu, K.; Zhao, H.; Misra, R.D. Effects of small Ni addition on the microstructure and toughness of coarse-grained heat-affected zone of high-strength low-alloy steel. Metals 2018, 8, 718. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, G.; Hori, R.; Poorganji, B.; Furuhara, T. Crystallographic analysis of proeutectoid ferrite/austenite interface and interphase precipitation of vanadium carbide in medium-carbon steel. Metall. Mater. Trans. A 2013, 44, 3436–3443. [Google Scholar] [CrossRef] [Green Version]
- Kawata, H.; Sakamoto, K.; Moritani, T.; Morito, S.; Furuhara, T.; Maki, T. Crystallography of ausformed upper bainite structure in Fe-9Ni-C alloys. Mater. Sci. Eng. A 2006, 438–440, 140–144. [Google Scholar] [CrossRef]
- Takayama, N.; Miyamoto, G.; Furuhara, T. Effects of transformation temperature on variant pairing of bainitic ferrite in low carbon steel. Acta Mater. 2012, 60, 2387–2396. [Google Scholar] [CrossRef]
- Yu, Y.-S.; Wang, Z.-Q.; Wu, B.-B.; Zhao, J.-X.; Wang, X.-L.; Guo, H.; Shang, C.-J. Tailoring variant pairing to enhance impact toughness in high-strength low-alloy steels via trace carbon addition. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 755–764. [Google Scholar] [CrossRef]
- Yu, Y.S.; Wang, Z.Q.; Wu, B.B.; Rong, X.Q.; Wei, L.J.; Yuan, S.F.; Guo, H.; Shang, C.J. New insight into the hardenability of high strength low alloy steel from the perspective of crystallography. Mater. Lett. 2021, 292, 129624. [Google Scholar] [CrossRef]
- Furuhara, T.; Chiba, T.; Kaneshita, T.; Wu, H.; Miyamoto, G. Crystallography and interphase boundary of martensite and bainite in steels. Metall. Mater. Trans. A 2017, 48, 2739–2752. [Google Scholar] [CrossRef]
- Miyamoto, G.; Iwata, N.; Takayama, N.; Furuhara, T. Variant selection of lath martensite and bainite transformation in low carbon steel by ausforming. J. Alloys Compd. 2013, 577, S528–S532. [Google Scholar] [CrossRef]
- Chiba, T.; Miyamoto, G.; Furuhara, T. Comparison of variant selection between lenticular and lath martensite transformed from deformed austenite. ISIJ Int. 2013, 53, 915–919. [Google Scholar] [CrossRef] [Green Version]
- Morito, S.; Tanaka, H.; Konishi, R.; Furuhara, T.; Maki, T. The morphology and crystallography of lath martensite in Fe-C alloys. Acta Mater. 2003, 51, 1789–1799. [Google Scholar] [CrossRef]
- Wu, B.B.; Wang, Z.Q.; Yu, Y.S.; Wang, X.L.; Shang, C.J.; Misra, R.D.K. Thermodynamic basis of twin-related variant pair in high strength low alloy steel. Scripta Mater. 2019, 170, 43–47. [Google Scholar] [CrossRef]
- Filippov, S.A.; Zolotorevsky, N.Y. Orientation relationship and variant pairing in bainite of low carbon steels depending on thermomechanical treatment. Mater. Lett. 2018, 214, 130–133. [Google Scholar] [CrossRef]
- Lambert-Perlade, A.; Gourgues, A.F.; Pineau, A. Austenite to bainite phase transformation in the heat-affected zone of a high strength low alloy steel. Acta Mater. 2004, 52, 2337–2348. [Google Scholar] [CrossRef]
- Morris, J.W.; Lee, C.S.; Guo, Z. The nature and consequences of coherent transformations in steel. ISIJ Int. 2003, 43, 410–419. [Google Scholar] [CrossRef]
- Guo, Z.; Lee, C.S.; Morris, J.W. On coherent transformations in steel. Acta Mater. 2004, 52, 5511–5518. [Google Scholar] [CrossRef]
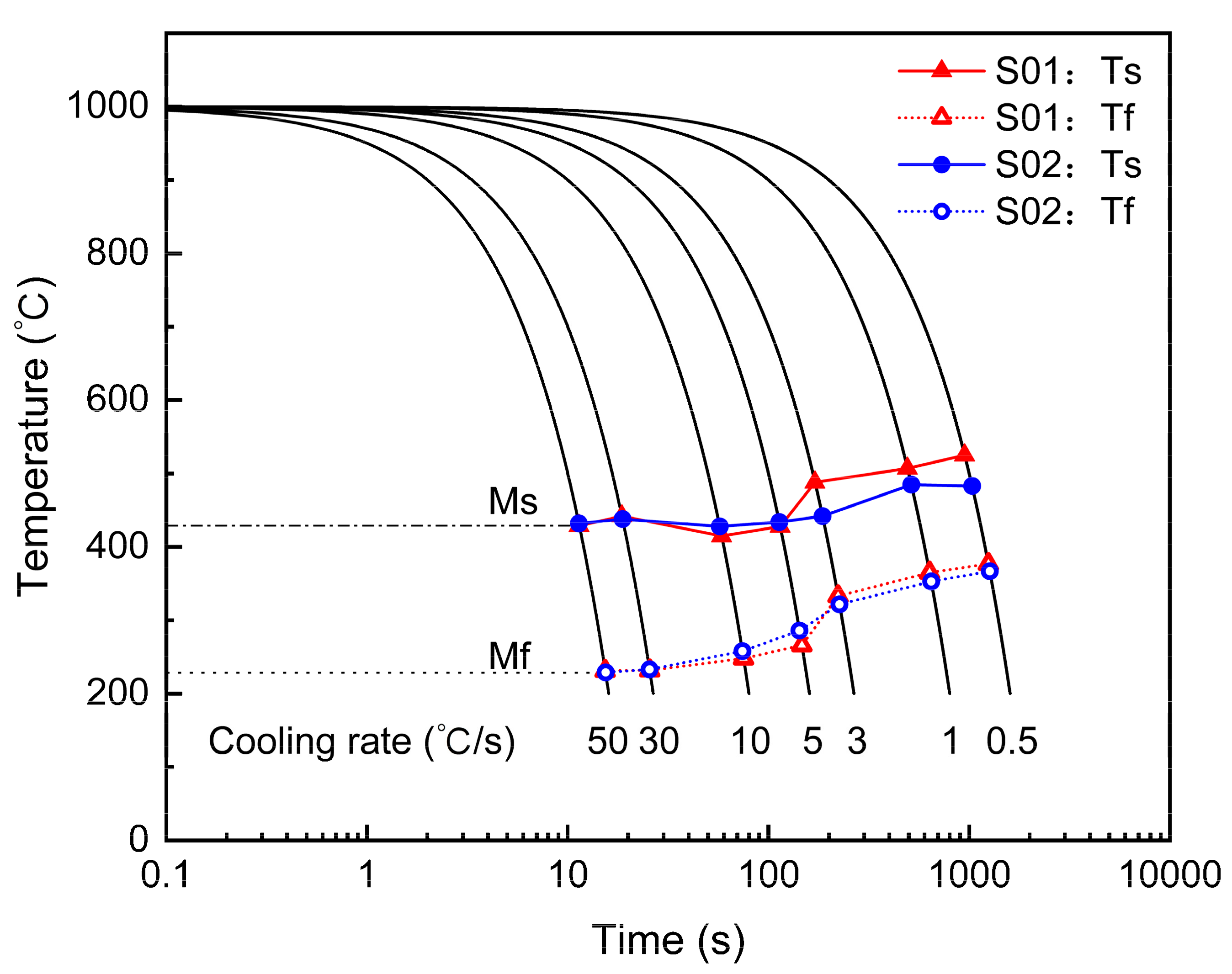
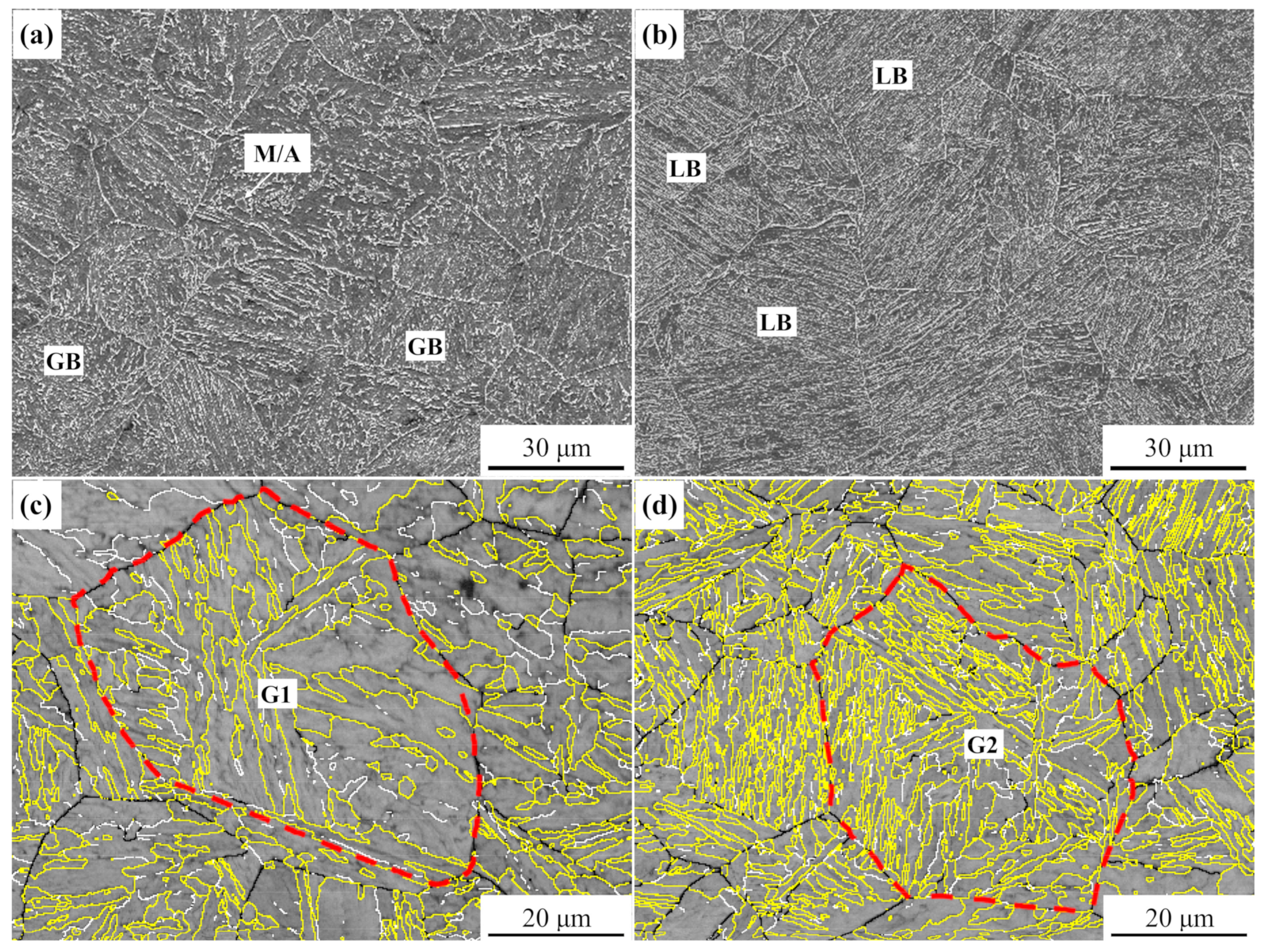
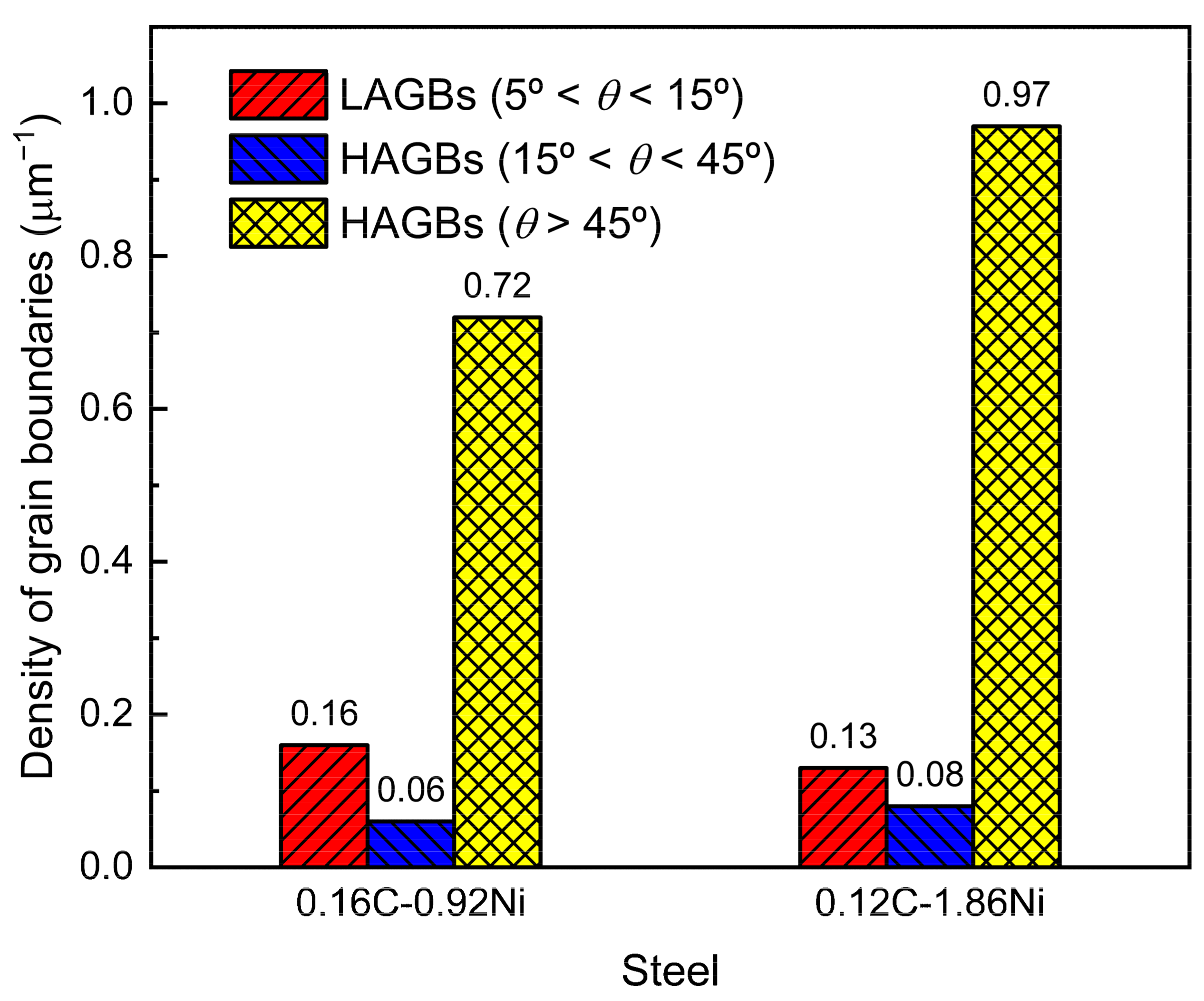
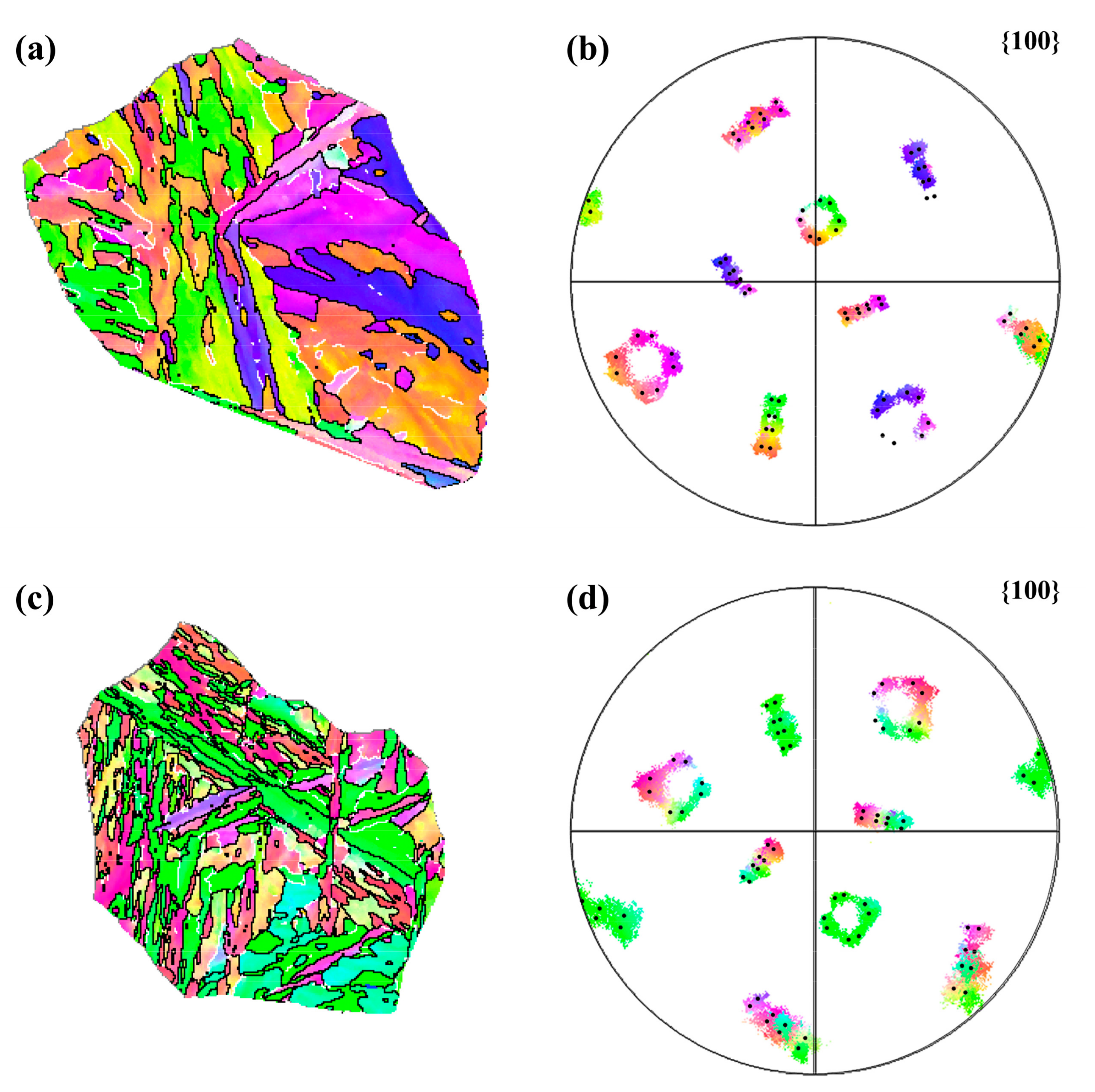


| Steel | C | Si | Mn | Ni | Cr + Mo | V + Ti | B | S | P |
|---|---|---|---|---|---|---|---|---|---|
| 0.16C-0.92Ni | 0.16 | 0.38 | 1.01 | 0.92 | 1.107 | 0.098 | 0.0015 | 0.0082 | 0.0171 |
| 0.12C-1.86Ni | 0.12 | 0.40 | 1.03 | 1.86 | 1.109 | 0.096 | 0.0014 | 0.0081 | 0.0172 |
| Steel | Cooling Rate (°C/s) | ||||||
|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 3 | 5 | 10 | 30 | 50 | |
| 0.16C-0.92Ni | 333.6 | 359.4 | 385.8 | 419.6 | 421.2 | 425.6 | 439.4 |
| 0.12C-1.86Ni | 365.0 | 365.6 | 380.8 | 383.0 | 395.0 | 399.8 | 399.4 |
| OR | Steel | Euler Angle | ||
|---|---|---|---|---|
| φ1 (°) | Φ (°) | φ2 (°) | ||
| Exact K–S OR | 114.2 | 10.5 | 204.2 | |
| Actual OR | 0.16C-0.92Ni | 120.6 | 8.6 | 195.6 |
| 0.12C-1.86Ni | 122.3 | 9.3 | 194.2 | |
| Variant | Plane Parallel | Direction Parallel | Rotation Angle/Axis from V1 | CP Group | Bain Group | Boundary Type | ||
|---|---|---|---|---|---|---|---|---|
| Exact K-S OR | 0.16C-0.92Ni | 0.12C-1.86Ni | ||||||
| V1 | (111)γ//(011)α | [−101]γ//[−1−11]α | – | – | – | CP1 | B1 | – |
| V2 | [−101]γ//[−11−1]α | 60.0°/[1,1,−1] | 60.3° | 60.2° | B2 | Block | ||
| V3 | [01−1]γ//[−1−11]α | 60.0°/[0,1,1] | 60.0° | 60.0° | B3 | Block | ||
| V4 | [01−1]γ//[−11−1]α | 10.5°/[0,−1,−1] | 5.0° | 5.3° | B1 | Sub-block | ||
| V5 | [1−10]γ//[−1−11]α | 60.0°/[0,−1,−1] | 60.0° | 60.0° | B2 | Block | ||
| V6 | [1−10]γ//[−11−1]α | 49.5°/[0,1,1] | 55.2° | 54.7° | B3 | Block | ||
| V7 | (1−11)γ//(011)α | [10−1]γ//[−1−11]α | 49.5°/[−1,−1,1] | 52.2° | 50.8° | CP2 | B2 | Packet |
| V8 | [10−1]γ//[−11−1]α | 10.5°/[1,1,−1] | 8.9° | 10.2° | B1 | Packet | ||
| V9 | [−1−10]γ//[−1−11]α | 50.5°/[−10,3,−13] | 53.1° | 52.5° | B3 | Packet | ||
| V10 | [−1−10]γ//[−11−1]α | 50.5°/[−7,−5,5] | 51.9° | 51.0° | B2 | Packet | ||
| V11 | [011]γ//[−1−11]α | 14.9°/[13,5,1] | 12.1° | 13.1° | B1 | Packet | ||
| V12 | [011]γ//[−11−1]α | 57.2°/[−3,5,6] | 57.9° | 57.7° | B3 | Packet | ||
| V13 | (−111)γ//(011)α | [0−11]γ//[−1−11]α | 14.9°/[5,−13,−1] | 12.1° | 13.1° | CP3 | B1 | Packet |
| V14 | [0−11]γ//[−11−1]α | 50.5°/[−5,5,−7] | 51.9° | 51.0° | B3 | Packet | ||
| V15 | [−10−1]γ//[−1−11]α | 57.2°/[−6,−2,5] | 57.0° | 56.2° | B2 | Packet | ||
| V16 | [−10−1]γ//[−11−1]α | 20.6°/[11,−11,−6] | 15.1° | 16.1° | B1 | Packet | ||
| V17 | [110]γ//[−1−11]α | 51.7°/[−11,6,−11] | 51.9° | 51.4° | B3 | Packet | ||
| V18 | [110]γ//[−11−1]α | 47.1°/[−24,−10,21] | 52.3° | 51.1° | B2 | Packet | ||
| V19 | (11−1)γ//(011)α | [−110]γ//[−1−11]α | 50.5°/[−3,13,10] | 53.1° | 52.5° | CP4 | B3 | Packet |
| V20 | [−110]γ//[−11−1]α | 57.2°/[3,6,−5] | 57.9° | 57.7° | B2 | Packet | ||
| V21 | [0−1−1]γ//[−1−11]α | 20.6°/[3,0,−1] | 16.7° | 18.3° | B1 | Packet | ||
| V22 | [0−1−1]γ//[−11−1]α | 47.1°/[−10,21,24] | 52.3° | 51.1° | B3 | Packet | ||
| V23 | [101]γ//[−1−11]α | 57.2°/[−2,−5,−6] | 57.0° | 56.2° | B2 | Packet | ||
| V24 | [101]γ//[−11−1]α | 21.1°/[9,−4,0] | 17.2° | 18.6° | B1 | Packet | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yu, Y.; Yang, J.; Wang, Z.; Guo, H.; Shang, C. Morphology and Crystallography Analyses of HSLA Steels with Hardenability Enhanced by Tailored C–Ni Collocation. Metals 2022, 12, 32. https://doi.org/10.3390/met12010032
Liu Z, Yu Y, Yang J, Wang Z, Guo H, Shang C. Morphology and Crystallography Analyses of HSLA Steels with Hardenability Enhanced by Tailored C–Ni Collocation. Metals. 2022; 12(1):32. https://doi.org/10.3390/met12010032
Chicago/Turabian StyleLiu, Zhipeng, Yishuang Yu, Jie Yang, Zhiquan Wang, Hui Guo, and Chengjia Shang. 2022. "Morphology and Crystallography Analyses of HSLA Steels with Hardenability Enhanced by Tailored C–Ni Collocation" Metals 12, no. 1: 32. https://doi.org/10.3390/met12010032
APA StyleLiu, Z., Yu, Y., Yang, J., Wang, Z., Guo, H., & Shang, C. (2022). Morphology and Crystallography Analyses of HSLA Steels with Hardenability Enhanced by Tailored C–Ni Collocation. Metals, 12(1), 32. https://doi.org/10.3390/met12010032








