Lead Recovery from a Lead Concentrate throughout Direct Smelting Reduction Process with Mixtures of Na2CO3 and SiC to 1000 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lead and Slag Characterization
2.2. Thermodynamic Modeling
3. Results and Discussion
3.1. Lead Concentrate Characterization
3.2. Direct Smelting Reduction with Na2CO3 and SiC
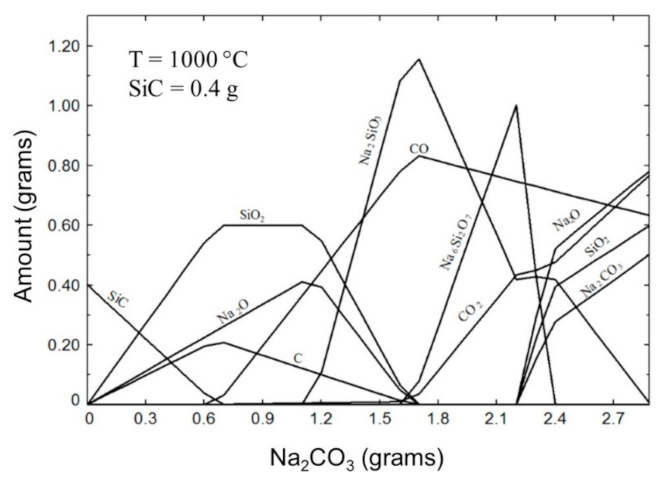
3.3. Thermodynamic Modeling
4. Conclusions
- A high recovery of crude lead (86 wt.%) with a purity up to 97% was obtained in a single-step process by using a mixture of 40 wt.% Na2CO3 and 0.4 g SiC to 1000 °C;
- The C consumption to produce 1g bullion lead was between 0.20 and 0.24 g (200–240 kg C/ton) which represents a lower C consumption than the traditional sinter-smelting route for primary lead production and hence a lesser amount of CO2 would be produced;
- The slag obtained was composed mainly of sodium silicates Na4SiO4 and Na2SiO3, sulfides as FeS2, and Na2S, and fewer amounts than 2 wt.% of ZnS, CuS, and PbS. The sodium silicates could be more stable to the slag weather degradation while the sulfides contribute to decreasing the SO2 emissions;
- The thermodynamic modeling showed that the higher lead recovery was obtained for an addition of 34 wt.% Na2CO3 which matches reasonably well with the experimental addition required;
- The thermal decomposition and oxidation of Na2CO3 and SiC promote the formation of Na2O, SiO2, CO, and CO2. The Na2O bonds partially with silica to form stabilized sodium silicates, the PbS is reduced with C and CO to produce metallic lead, while the S from PbS reacts with Na2O to produce Na2S, partially decreasing the formation of SO2;
- FactSage calculation was used as a tool for a first approximation to the estimation of products after PbS direct reduction with Na2CO3 and SiC to 1000 °C and good agreement was found in the compounds predictions with those obtained experimentally.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Li, A.; Finlow, D. The lead and lead-acid battery industries during 2002 and 2007 in China. J. Power Sources 2009, 191, 22–27. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Zhu, X.; Sun, X.; Yu, W.; Hu, Y.; Yuan, X.; Dong, J.; Hu, J.; Liang, S.; et al. Structural study of a lead (II) organic complex—A key precursor in a green recovery route for spent lead-acid battery paste. J. Chem. Technol. Biotechnol. 2014, 91, 672–679. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Wu, X.; Hu, Y.; Yu, W.; Wang, J.; Dong, J.; Li, M.; Liang, S.; Hu, J.; et al. A critical review on secondary lead recycling technology and its prospect. Renew. Sustain. Energy Rev. 2016, 61, 108–122. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Olivas-Martinez, M. Lead and Zinc Production, Treatise on Process Metallurgy—Chapter 2.3. In Treatise on Process Metallurgy, Volume 3: Industrial Processes; Elsevier: Amsterdam, The Netherlands, 2014; pp. 671–700. [Google Scholar]
- Li, W.; Zhan, J.; Fan, Y.; Wei, C.; Zhang, C.; Hwang, J.-Y. Research and Industrial Application of a Process for Direct Reduction of Molten High-Lead Smelting Slag. JOM 2017, 69, 784–789. [Google Scholar] [CrossRef]
- Sinclair, R.J. Extractive Metallurgy of Lead; The Australasian Institute of Mining and Metallurgy: Carlton, VIC, Australia, 2009; pp. 10–12, 28, 29, 37, 47, 48, 55, 77, 81, 99–127, 161, 162, 188–193. ISBN 978 1 921522 02 4. [Google Scholar]
- Starev, N.; Doganov, G. KCM—Innovator in the Pb Metal Production Through Ausmelt Technology and Variable SO2 Concentration Off-Gas Utilization. In PbZn 2020: 9th International Symposium on Lead and Zinc Processing; The Minerals, Metals & Materials Series; Siegmund, A., Alam, S., Grogan, J., Kerney, U., Shibata, E., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Norrgran, D.A. Process for the Sulfatization of Non-Ferrous Metal Sulfides. U.S. Patent US4541993A, 17 September 1985. [Google Scholar]
- Baimbetov, B.S.; Aitenov, K.D.; Bekisheva, A.A.; Abdikerim, B.E. Metallic sulphide and sodium carbonate interacting process. Met. J.-Metall. Min. Ind. 2015, 11, 26–34. [Google Scholar]
- Szczygiel, Z.; Lara, C.; Escobedo, S.; Mendoza, O. The direct reduction of sulfide minerals for the recovery of precious metals. JOM 1998, 50, 55–59. [Google Scholar] [CrossRef]
- Angelica Sánchez, M.; Victor Hugo Gutiérrez, P.; Alejandro Cruz, R.; Ricardo Gerardo Sánchez, A. Lead production from recycled paste of lead acid batteries with SiC-Na2CO3. Russ. J. Non-Ferr. Met. 2016, 57, 316–324. [Google Scholar] [CrossRef]
- Bale, C.W.; Pelton, A.D.; Thompson, W.T.; Facility for the Analysis of Chemical Thermodynamics (FactSage, v. 7.3) 2018, User’s Manual. Available online: www.factsage.com/facthelp/FS73new.htm (accessed on 17 December 2021).
- Gutiérrez, V.H.; Cruz, A.; Romero, A.; Rivera, E. Analysis of the sulfur decoppering from molten lead by powder injection. Russ. J. Non-Ferrous Met. 2015, 56, 251–260. [Google Scholar] [CrossRef]
- Yin, N.-H.; Sivry, Y.; Guyot, F.; Lens, P.N.L.; van Hullebusch, E.D. Evaluation on chemical stability of lead blast furnace (LBF) and imperial smelting furnace (ISF) slags. J. Environ. Manag. 2016, 180, 310–323. [Google Scholar] [CrossRef] [PubMed]


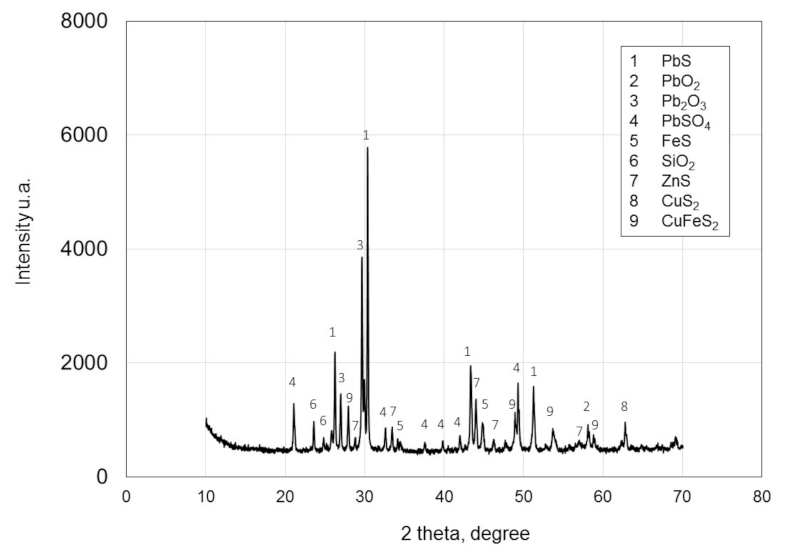
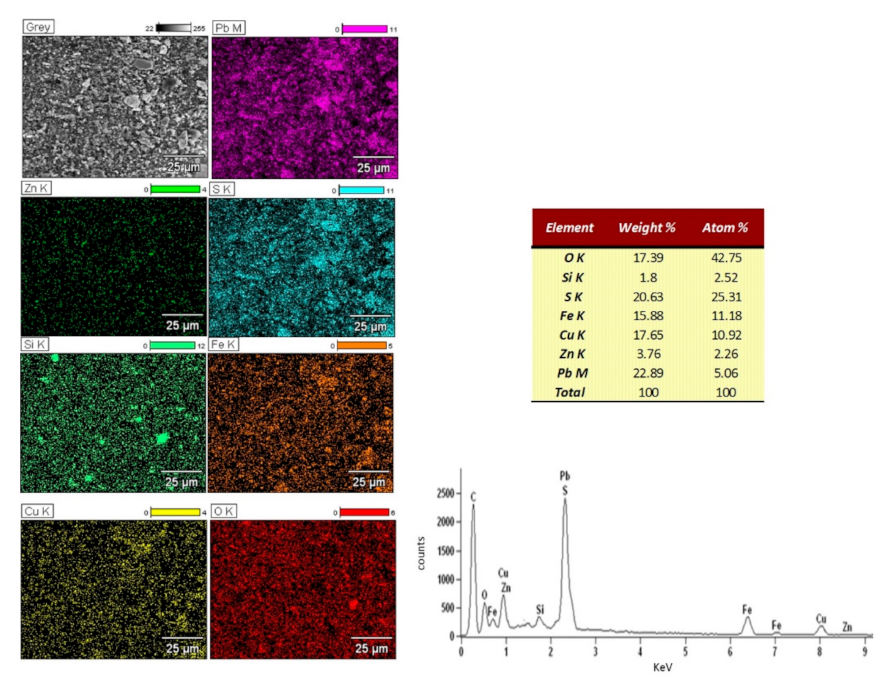

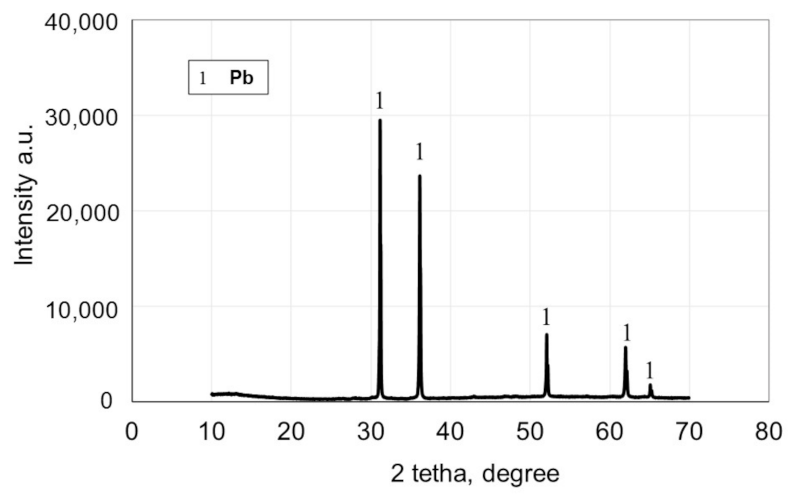


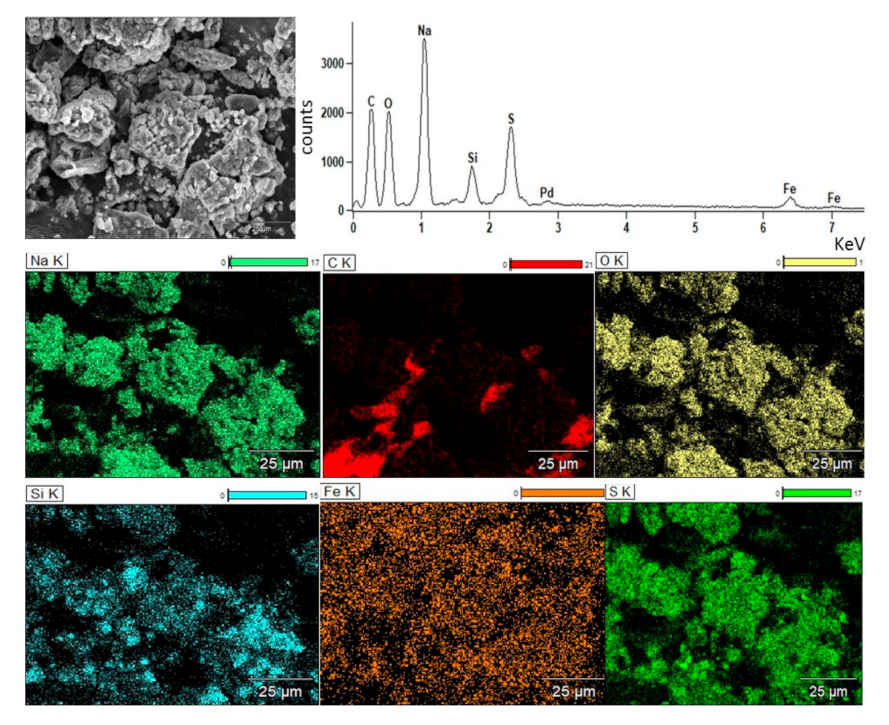

| Element | Pb | Cu | * Ag | Fe | Zn | Si | Stotal | Sb | As | Cd |
|---|---|---|---|---|---|---|---|---|---|---|
| Lead concentrate | 53.69 | 7.43 | 105 | 7.35 | 3.31 | 5.82 | 19.21 | 0.18 | 0.14 | 0.19 |
| Na2CO3 (wt.%) | Pb-Button (g) | Chemical Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pb balance | Cu | * Ag | Fe | As | Si | Zn | ||
| 25 | 1.46 | 96.30 | 1.70 | 20 | 0.50 | 1.38 | 0.01 | 0.11 |
| 30 | 1.58 | 96.68 | 1.28 | 31 | 1.98 | 0.01 | 0.02 | 0.03 |
| 35 | 1.64 | 95.88 | 1.08 | 33 | 2.14 | 0.86 | 0.01 | 0.03 |
| 40 | 1.91 | 97.38 | 1.84 | 46 | 0.72 | 0.04 | 0.01 | 0.01 |
| Na2CO3 (wt.%) | Slag (g) | Chemical Composition (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | Cu | * Ag | Fe | Zn | Si | As | S | Na | ||
| 25 | 3.02 | 13.11 | 8.16 | 82 | 7.86 | 0.85 | 9.73 | 0.26 | 20.07 | 20.05 |
| 30 | 2.80 | 9.34 | 8.51 | 78 | 8.81 | 0.72 | 9.89 | 0.39 | 21.05 | 27.12 |
| 35 | 3.14 | 7.36 | 6.24 | 71 | 7.24 | 1.02 | 9.64 | 0.22 | 21.08 | 30.17 |
| 40 | 3.88 | 2.88 | 4.12 | 54 | 7.71 | 0.54 | 9.69 | 0.07 | 24.04 | 31.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez Pérez, V.H.; Osorio Hernández, J.D.; Sánchez Alvarado, R.G.; Cruz Ramírez, A.; Olvera Vázquez, S.L.; Rivera Salinas, J.E. Lead Recovery from a Lead Concentrate throughout Direct Smelting Reduction Process with Mixtures of Na2CO3 and SiC to 1000 °C. Metals 2022, 12, 58. https://doi.org/10.3390/met12010058
Gutiérrez Pérez VH, Osorio Hernández JD, Sánchez Alvarado RG, Cruz Ramírez A, Olvera Vázquez SL, Rivera Salinas JE. Lead Recovery from a Lead Concentrate throughout Direct Smelting Reduction Process with Mixtures of Na2CO3 and SiC to 1000 °C. Metals. 2022; 12(1):58. https://doi.org/10.3390/met12010058
Chicago/Turabian StyleGutiérrez Pérez, Víctor Hugo, Juan Daniel Osorio Hernández, Ricardo Gerardo Sánchez Alvarado, Alejandro Cruz Ramírez, Seydy Lizbeth Olvera Vázquez, and Jorge Enrique Rivera Salinas. 2022. "Lead Recovery from a Lead Concentrate throughout Direct Smelting Reduction Process with Mixtures of Na2CO3 and SiC to 1000 °C" Metals 12, no. 1: 58. https://doi.org/10.3390/met12010058
APA StyleGutiérrez Pérez, V. H., Osorio Hernández, J. D., Sánchez Alvarado, R. G., Cruz Ramírez, A., Olvera Vázquez, S. L., & Rivera Salinas, J. E. (2022). Lead Recovery from a Lead Concentrate throughout Direct Smelting Reduction Process with Mixtures of Na2CO3 and SiC to 1000 °C. Metals, 12(1), 58. https://doi.org/10.3390/met12010058






