Effects of Process Parameters on Microstructure and Mechanical Properties of Semi-Solid Al-7Si-0.5Mg Aluminum Alloy by Gas Induced Semi-Solid Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. DSC Test
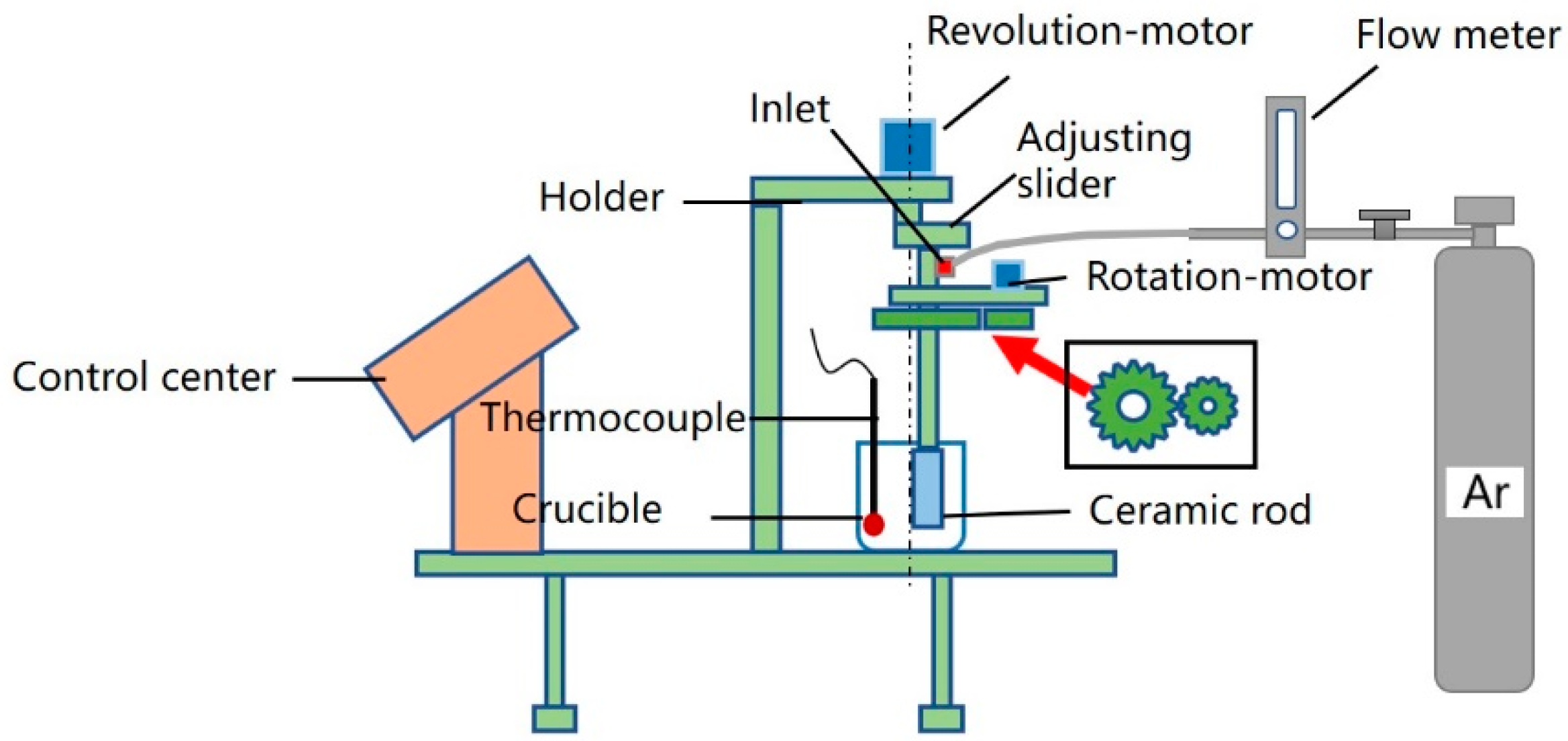
2.3. Slurry Preparation and Die-Casting
2.4. Metallographic Observations and Tensile Tests
3. Results
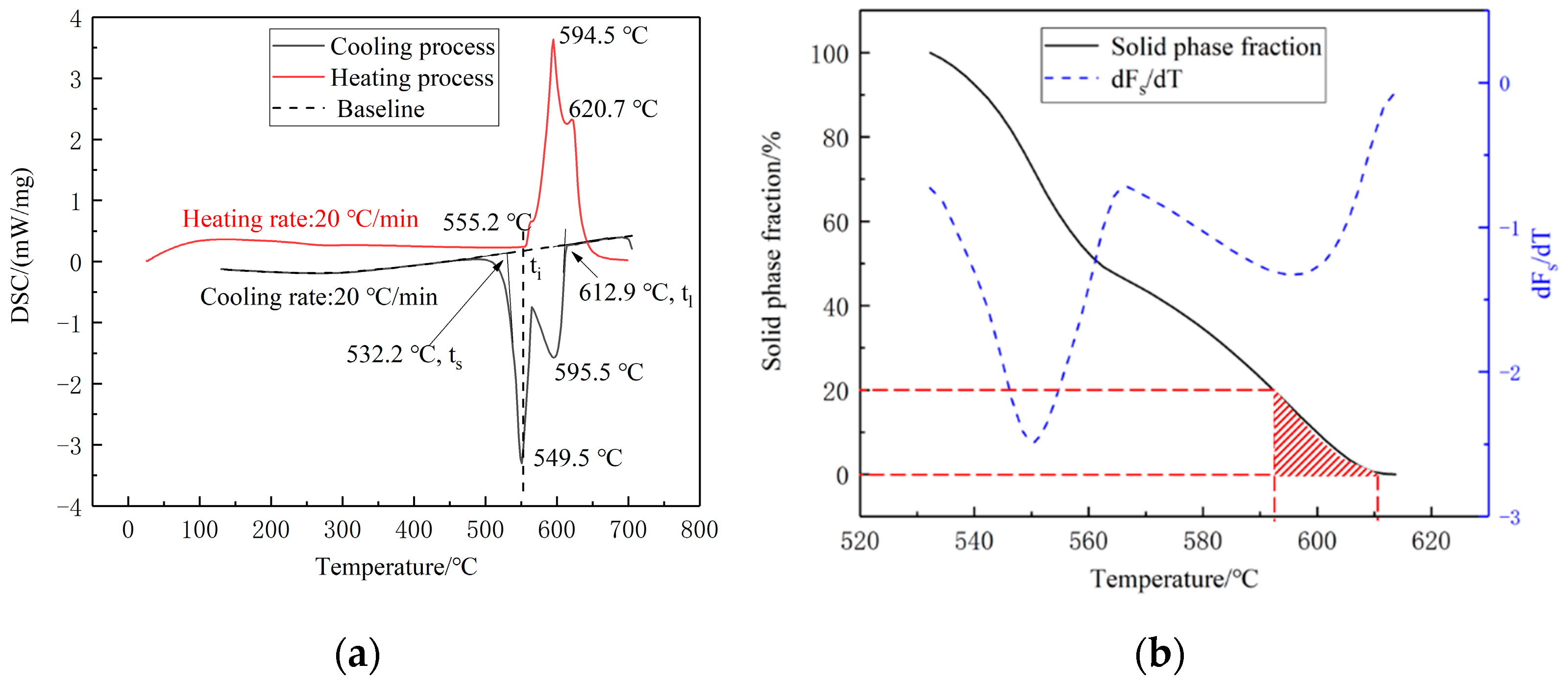
3.1. DSC Tests
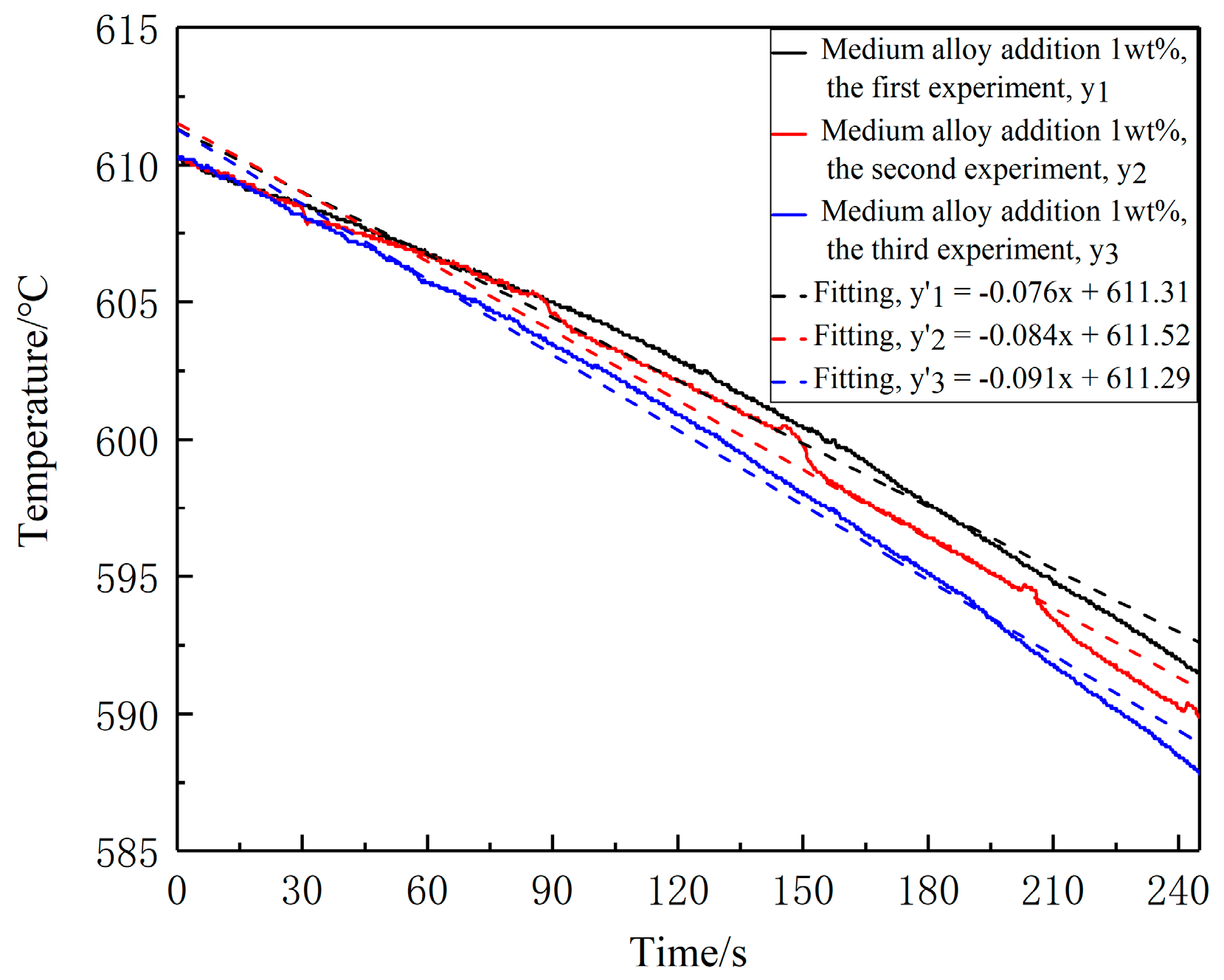
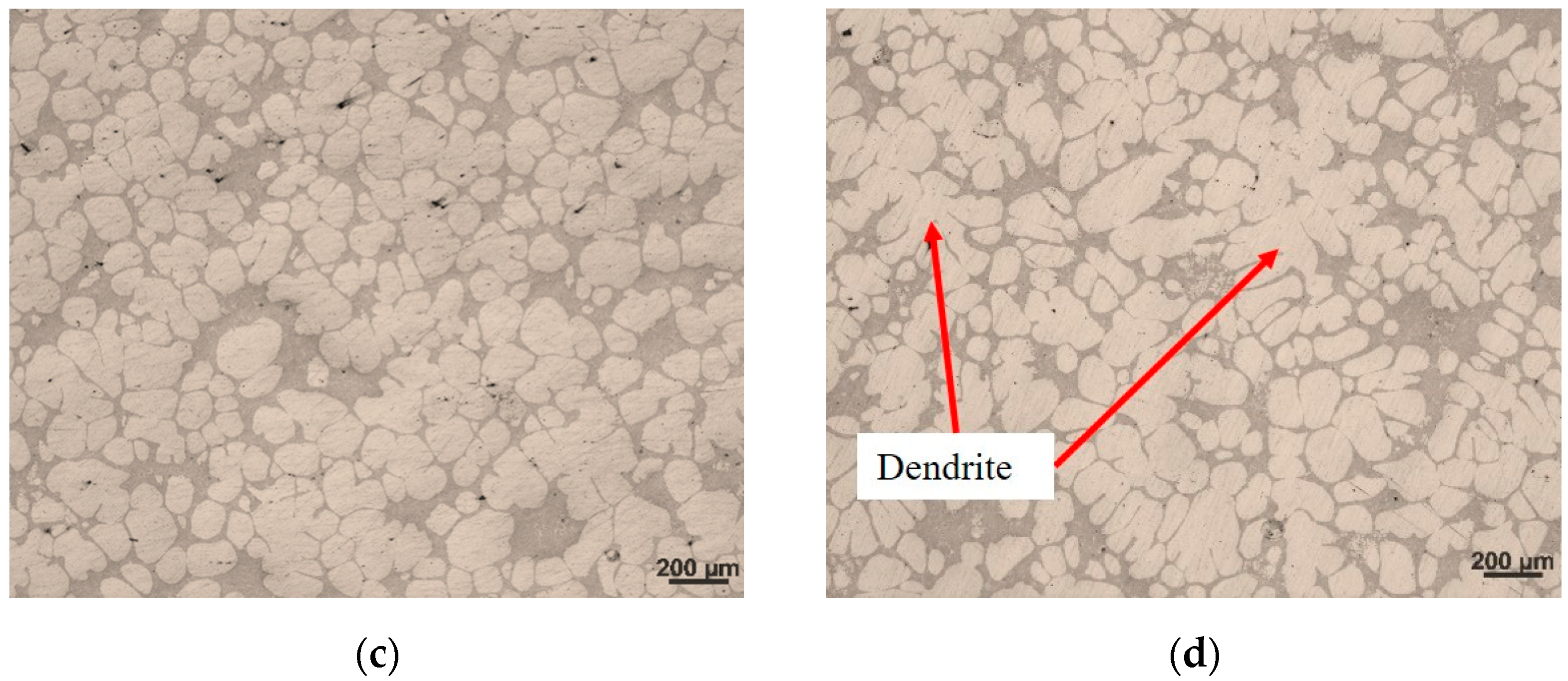
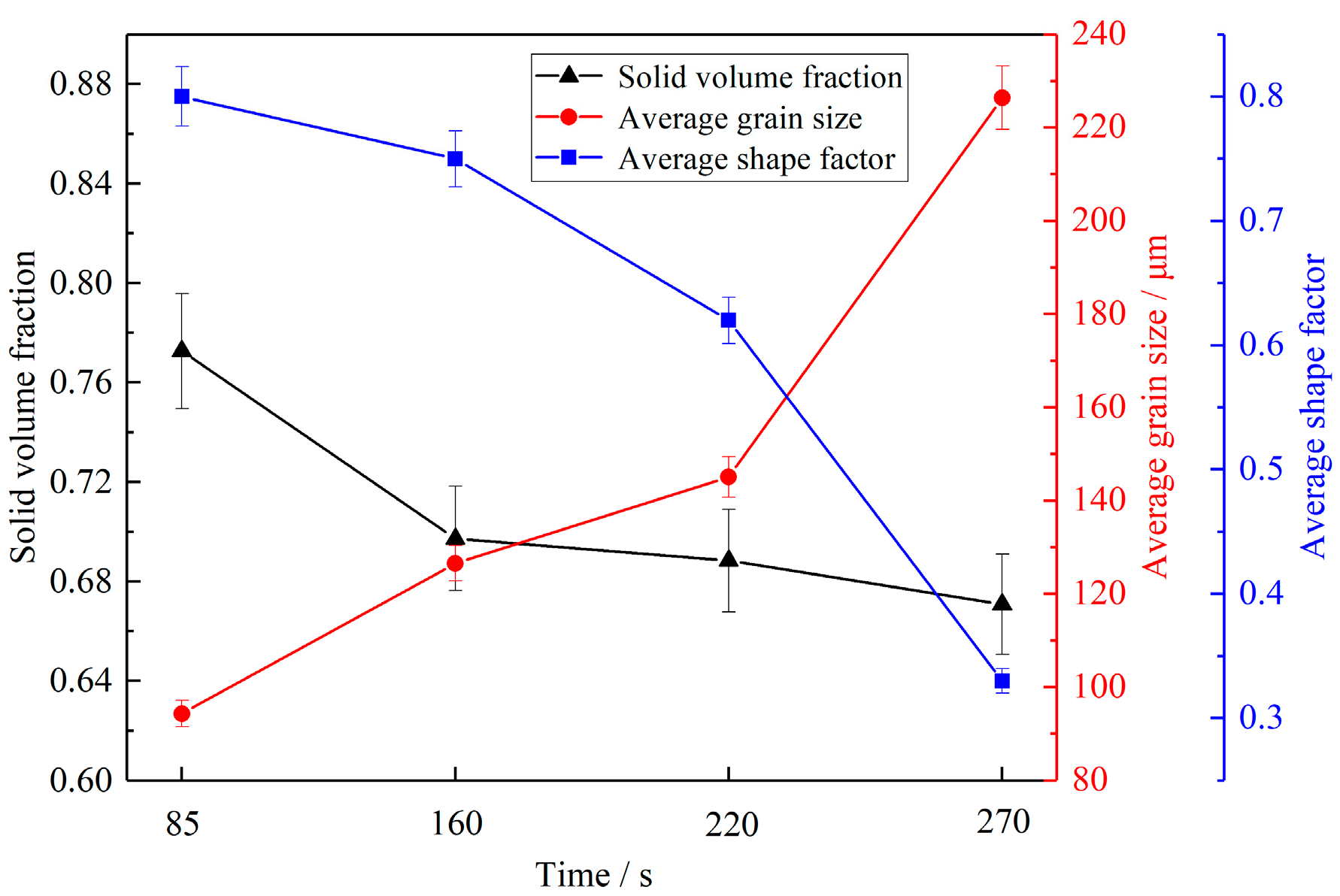
3.2. Influence of Different Holding Time on Slurry Structure
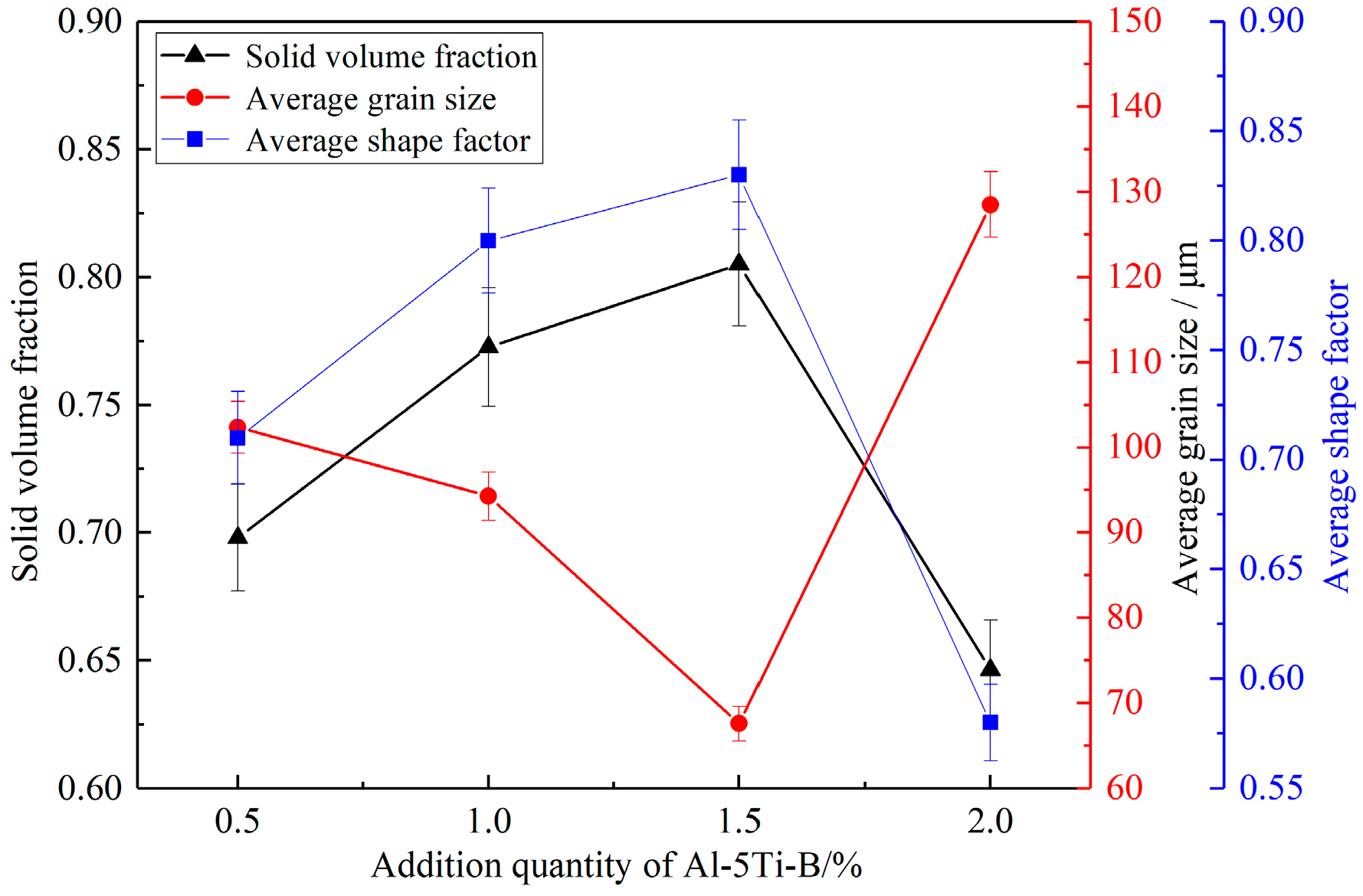
3.3. Effect of Medium Alloy Addition on Slurry Microstructure
3.4. Test Bar Analysis
3.4.1. Mechanical Properties
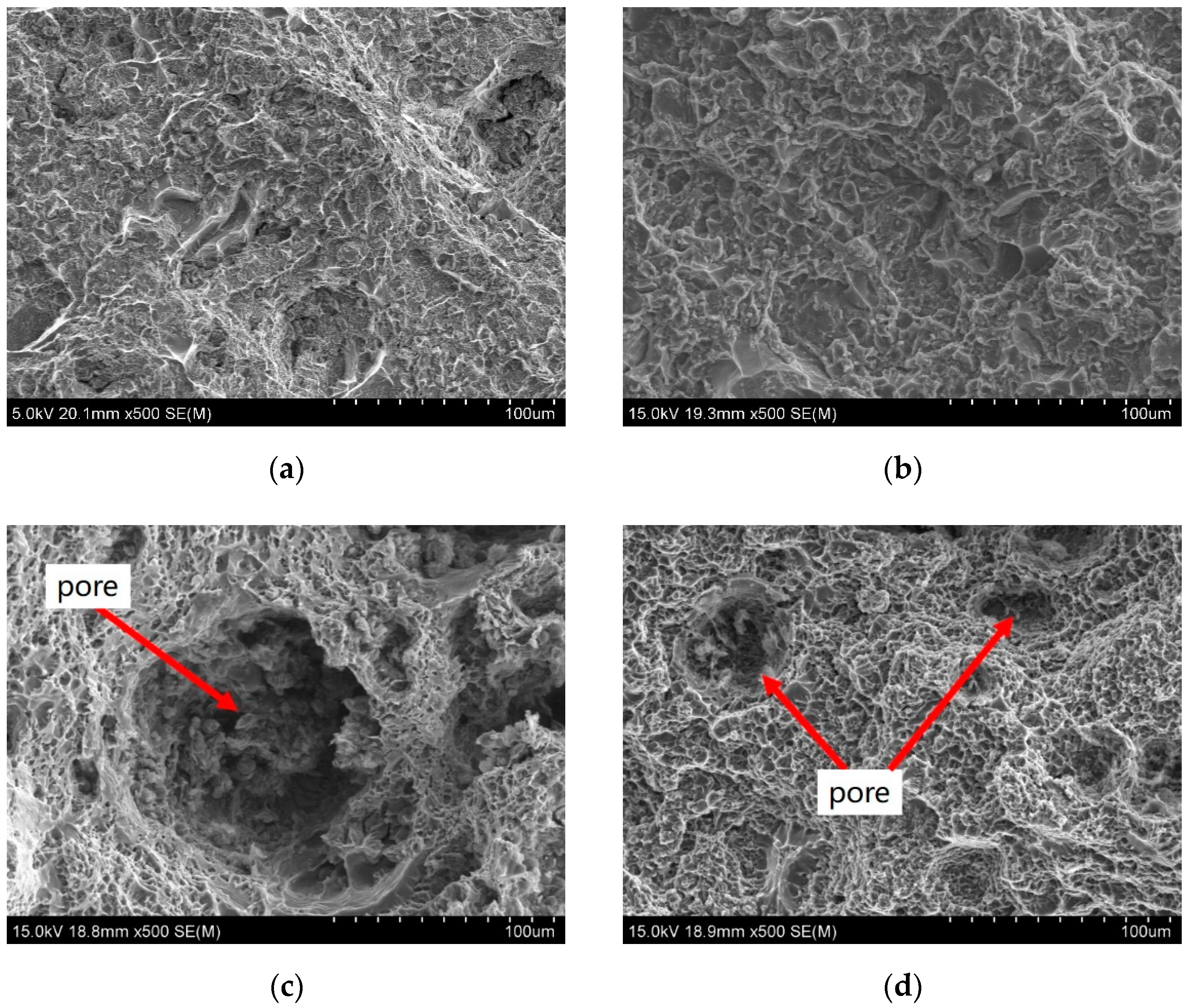
3.4.2. Fracture Analysis
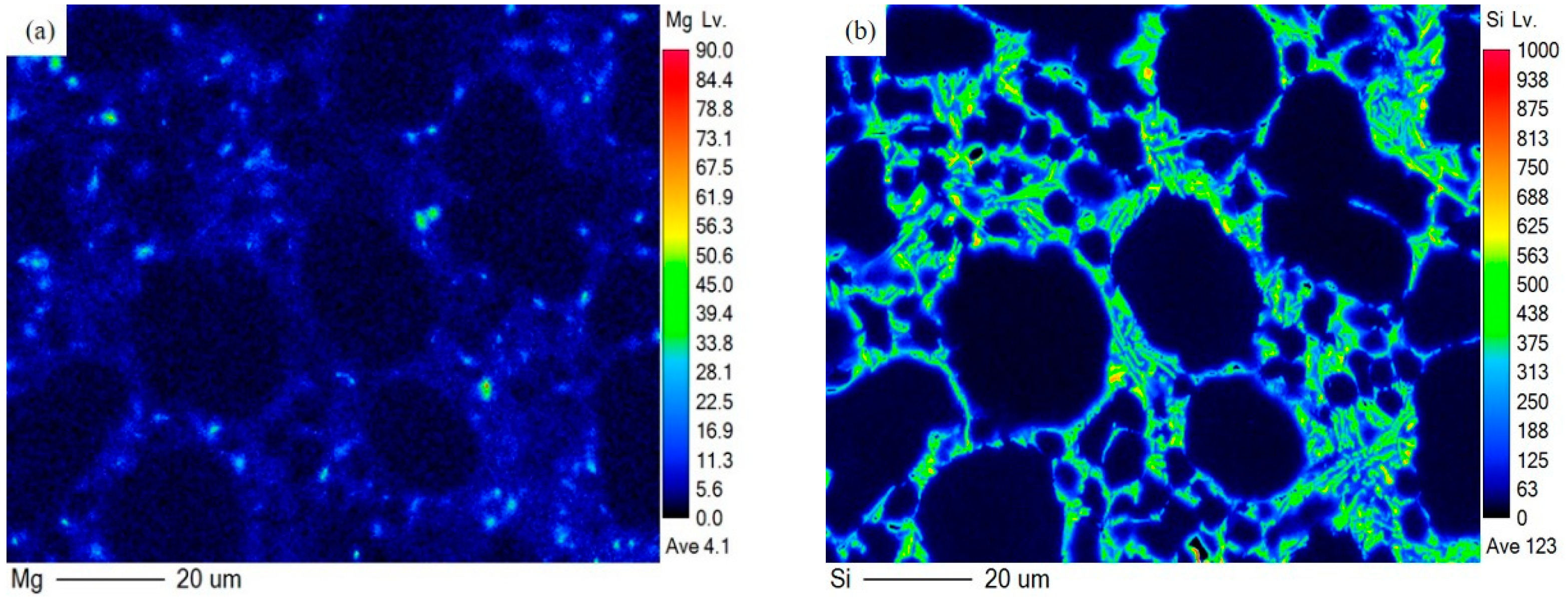
3.4.3. Elements Distribution
4. Discussion
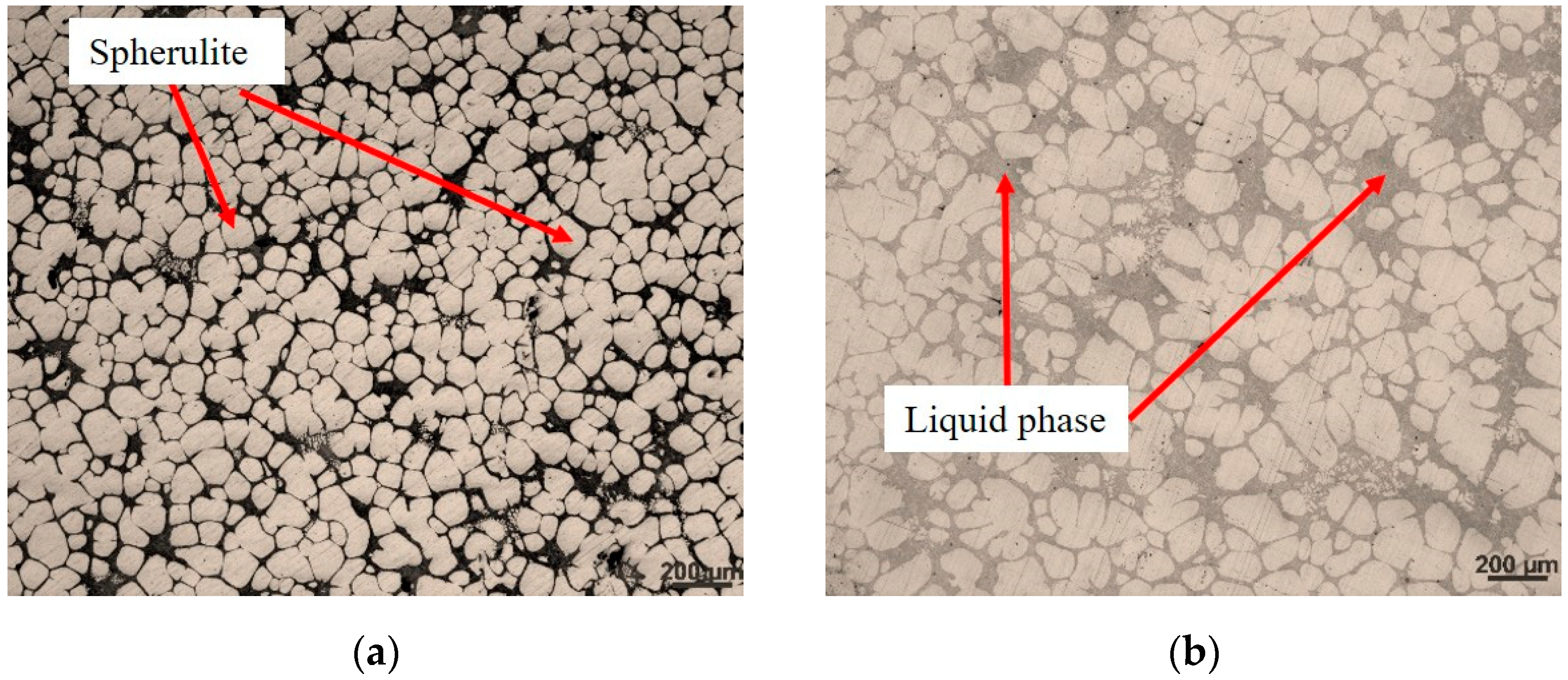
4.1. Formation of Non-Dendritic α-Al Grains
4.2. Influence of Holding Time on Semi-Solid Microstructure
4.3. Effect of Medium Alloy Addition on Semi-Solid Microstructure
4.4. Mechanical Properties of Traditional Die Casting and Semi-Solid Die-Casting
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, M.F.; Kang, Y.L.; Zhou, B. A forced convection stirring process for Rheo-HPDC aluminum and magnesium alloys. J. Mater. Process. Technol. 2016, 234, 353–367. [Google Scholar] [CrossRef]
- Kang, Y.L.; Li, J.Y.; LI, G.N. Preparation and Rheological Die-Casting of 7075 Aluminum Alloy Semisolid Slurry. J. Netshape Form. Eng. 2020, 12, 74–80. [Google Scholar]
- Qi, M.F.; Kang, Y.L.; Zhu, G.M. Microstructure and properties of rheo-HPDC Al-8Si alloy prepared by air-cooled stirring rod process. T Nonferrous Metal. Soc. 2017, 27, 1939–1946. [Google Scholar] [CrossRef]
- Xue, L.W.; Zhou, W.Q.; Piao, Y.N. Progress in effects of squeeze casting process parameters and heat treatment on microstructure and properties of Al-Si alloy. Spec. Cast. Nonferrous Alloy. 2021, 41, 842–848. [Google Scholar]
- Zhou, B.; Qiu, Z.Y.; Chen, K.P.; Xu, C.; Wang, Z.Y. Microstructure, properties, and numerical simulation of semi-solid aluminum alloy under planetary stirring process. Materials 2022, 15, 3009. [Google Scholar] [CrossRef]
- Liao, F.J.; Wang, L.D.; Zhu, D.Y. Preparation of aluminum alloy semi-solid slurry by internal cooling process. Spec. Cast. Nonferrous Alloy. 2017, 37, 1208–1211. [Google Scholar]
- Zhu, Y.L.; Xu, X.L.; Zhao, J.W. Effect on microstructure and corrosion resistance of semi-solid slurry of 7A04 aluminum alloy by electromagnetic stirring. Mater. Res. Express. 2021, 8, 16506–16517. [Google Scholar] [CrossRef]
- Hong, X.; Liu, Z.; Zhang, K.W. Effects of current mutation on electromagnetic field and solidified microstructure in semi-solid A356 aluminum alloy under electromagnetic. Spec. Cast. Nonferrous Alloys 2021, 41, 1393–1399. [Google Scholar]
- Shabestari, S.G.; Honarmand, M.; Saghafian, H. Microstructural evolution of A380 aluminum alloy produced by gas-induced semi-solid technique (GISS). Adv. Mater. Processing Technol. 2015, 1, 155–163. [Google Scholar] [CrossRef]
- Wannasin, J.; Martinez, R.; Flemings, M. Grain refinement of an aluminum alloy by introducing gas bubbles during solidification. Scr. Mater. 2006, 55, 115–118. [Google Scholar] [CrossRef]
- Li, M.; Yang, W.L.; Zhang, Y. Microstructure and mechanical properties of AZ91D magnesium alloy rheo-diecasting prepared by self-inoculation method. Trans. Mater. Heat Treat. 2020, 41, 41–48. [Google Scholar]
- Jain, A.; Ratke, L.; Sharma, A. Non-dendritic structural changes in Al-7Si alloy cast through rapid slurry formation (RSF) process. Trans. Indian Inst. Met. 2012, 65, 545–551. [Google Scholar] [CrossRef]
- Granath, O.; Wessén, M.; Cao, H. Determining effect of slurry process parameters on semi-solid A356 alloy microstructures produced by RheoMetal process. Int. J. Cast Met. Res. 2013, 21, 349–356. [Google Scholar] [CrossRef]
- Das, P.; Samanta, S.K.; Das, R. Optimization of degree of sphericity of primary phase during cooling slope casting of A356 Al alloy: Taguchi method and regression analysis. Measurement 2014, 55, 605–615. [Google Scholar] [CrossRef]
- Geng, X.X.; Mao, W.M.; Li, N.Y. Effect of serpentine channel preparation method on semi-solid slurry of 6061 aluminum alloy. Spec. Cast. Nonferrous Alloys 2021, 41, 900–904. [Google Scholar]
- Wang, M.; Liu, X.B.; Guo, H.M. Preparation of semi-solid slurries of A356 alloy by helical curve duct. Spec. Cast. Nonferrous Alloys 2015, 35, 941–944. [Google Scholar]
- Li, G.; Qu, W.Y.; Luo, M. Semi-solid processing of aluminum and magnesium alloys: Status, opportunity, and challenge in China. T Nonferrous Metal. Soc. 2021, 31, 3255–3280. [Google Scholar] [CrossRef]
- Luo, S.J.; Keung, W.C.; Kang, Y.L. Theory and application research development of semi-solid forming in China. T Nonferrous Metal. Soc. 2010, 20, 1805–1814. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Y.L.; Wang, L.D.; Li, Q.J. Study on Preparation of semi-solid aluminum-silicon alloy slurries. Hot Work. Technol. 2013, 42, 14–17. [Google Scholar]
- Li, J.Q.; Zhang, L.; Dong, X.P. Study on Microstructure of Semi-Solid Magnesium Alloy Manufactured by Gas Bubbles Stirring. Adv. Mater. Res. 2010, 129–131, 728–732. [Google Scholar] [CrossRef]
- Honarmand, M.; Salehi, M.; Shabestari, S.G. Impact strength and structural refinement of A380 aluminum alloy produced through gas-induced semi-solid process and Sr addition. T Nonferrous Metal. Soc. 2022, 32, 1405–1415. [Google Scholar] [CrossRef]
- Zou, Y.; Dong, X.P.; Zhang, J.; Fan, Z.T.; Wang, A.H. A new casting technique of agitating semi-solid magnesium alloy using a gas blowing method. Foundry 2008, 57, 215–218. [Google Scholar]
- He, F.; Zhuang, L.Z.; He, G.Y. A356 aluminum alloy for automobile wheel hubs-research process and influence of alloying elements on its microstructure and properties. Foundry 2021, 70, 431–437. [Google Scholar]
- Li, J.J.; Zhang, X.T. Optimization of different pressure casting process of automobile bracket. Hot Work. Technol. 2021, 50, 71–74. [Google Scholar]
- Wang, Z.L.; Zhu, X.B.; Wang, G.Y. Analysis and countermeasure of pinhole defects in A356 cylinder head casting. Anhui Polytech. Univ. 2019, 34, 38–43. [Google Scholar]
- Ran, G.; Zhou, J.E.; Wang, Y.F. Microstructure and tensile properties of cast A356 aluminum alloy. Heat Treat. Met. 2007, 32, 13–18. [Google Scholar]
- Zabler, S.; Rueda, A.; Rack, A.; Riesemeier, H.; Zaslansky, P.; Manke, I.; Garcia-Moreno, F.; Banhart, J. Coarsening of grain-refined semi-solid Al-Ge32 alloy:X-ray microtomography and in situ radiography. Acta Mater. 2007, 55, 5045–5055. [Google Scholar] [CrossRef]
- Dong, J.; Lu, G.M.; Ren, X.F. Discussion on the formation mechanism of nondendritic semi-solid microstructures during liquidus casting. Acta Metall. Sin. 2002, 38, 203–207. [Google Scholar]
- Ji, S.; Fan, Z.; Bevis, M.J. Semi-solid processing of engineering alloys by a twin-screw rheomoulding process. Mater. Sci. Eng. A 2001, 299, 210–217. [Google Scholar] [CrossRef]
- Pilling, J.; Hellawell, A. Mechanical deformation of dendrites by fluid flow. Metall. Mater. Trans. A 1996, 27, 229–232. [Google Scholar] [CrossRef]
- Kirkwood, D.H. Semisolid metal processing. Int. Mater. 2013, 39, 174–189. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.D. Microstructural evolution of A356 aluminum alloy during continuous cooling, isothermal holding and furnace cooling. Trans. Mater. Heat Treat. 2020, 41, 23–31. [Google Scholar]
- Fan, Z.; Ji, S.; Liu, G.J. Development of the rheo-diecasting process for Mg-alloys. Mater. Sci. Forum 2005, 488–489, 405–412. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, X.P.; Li, J.Q. Microstructure evolution of Al-Si semi-solid slurry by gas bubble stirring method. J. Cent. South Univ. 2011, 18, 1789–1794. [Google Scholar] [CrossRef]
- Nafisi, S.; Ghomashchi, R. Effect of stirring on solidification pattern and alloy distribution during semi-solid-metal casting. Mater. Sci. Eng. A 2006, 437, 388–395. [Google Scholar] [CrossRef]
- Voorhees, P.W.; Glicksman, M.E. Ostwald ripening during liquid phase sintering—Effect of volume fraction on coarsening kinetics. Metall. Mater. Trans. A 1984, 15, 1081–1088. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.D.; Bi, G.L. Effects of melt treatment temperature and isothermal holding parameter on water-quenched microstructures of A356 aluminum alloy semisolid slurry. T Nonferrous Metal. Soc. 2018, 28, 393–403. [Google Scholar] [CrossRef]
- Deepak, K.S.; Mihira, A.; Mandai, A.; Chakraborty, M. Coarsening kinetics of semi-solid A356–5 wt% TiB2in situ composite. T rans. Nonferrous Metal. Soc. India 2015, 6, 1075–1080. [Google Scholar]
- Jiang, J.F.; Wang, Y.; Xiai, G.F. Influence of modification, refinement and heat treatment on mechanical properties of A356 Al-alloy components prepared by squeeze casting. J. Mater. Res. 2020, 34, 881–891. [Google Scholar]
- Guzowski, M.M.; Sigworth, G.K.; Sentner, D.A. The role of boron in the grain. Metall. Mater. Trans. A 1987, 5, 603–619. [Google Scholar] [CrossRef]
- Murty, B.S.; Kori, S.A.; Chakraborty, M. Grain refinement of aluminum and its alloys by heterogeneous nucleation and alloying. Int. Mater. Rev. 2013, 47, 3–29. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xu, Q.Y.; Huang, T.Y. Development of research on grain refiners for aluminum alloys. Mater. Rep. 2006, 20, 57–61. [Google Scholar]
- Mohanty, P.S.; Samuel, F.H.; Gruzleski, J.E. Studies on addition of inclusions to molten aluminum using a novel technique. Metall. Mater. Trans. B 1995, 26, 103–109. [Google Scholar] [CrossRef]
- Schumacher, P.; Greer, A.L.; Worth, J. New studies of nucleation mechanisms in aluminum alloys: Implications for grain refinement practice. J. Mater. Sci. Technol. 1998, 14, 394–404. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y. Grain refining mechanism in the Al/Al-Ti-B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Cheng, L.; Lu, H.X.; Zhu, Q. Evolution of microstructure and mechanical properties of semi-solid squeeze cast A356.2 aluminum alloy during heat treatment. Solid State Phenom. 2019, 285, 139–145. [Google Scholar] [CrossRef]




| Si | Cu | Mg | Mn | Fe | Ti | Al |
|---|---|---|---|---|---|---|
| 6.84 | 0.0261 | 0.492 | 0.0086 | 0.0271 | 0.0371 | 92.5 |
| Process Parameters | Varied Value | Fixed Value | Remark |
|---|---|---|---|
| Holding time/s | 85 160 220 270 | Ventilation temperature 625 °C, revolution 90 r/min, rotation 150 r/min, ventilation time 4 min, flow 0.35 m3/h, water cooling | Medium alloy addition 1.0 wt%, |
| Medium alloy addition/wt% | 0.5 1.0 1.5 2.0 | Holding time 85 s |
| Forming Process | State | Yield Strength/MPa | Tensile Strength/MPa | Elongation/% |
|---|---|---|---|---|
| liquid die-casting | as-cast | 129 ± 6 | 242 ± 8 | 11.2 ± 0.1 |
| semi-solid die-casting | 136 ± 5 | 266 ± 8 | 11.6 ± 0.2 | |
| liquid die-casting | T6 | 210 ± 7 | 238 ± 7 | 1.6 ± 0.3 |
| semi-solid die-casting | 240 ± 6 | 307 ± 7 | 8.8 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, G.; Xiang, L.; Li, R.; Xu, W.; Lu, Y.; Pesci, R. Effects of Process Parameters on Microstructure and Mechanical Properties of Semi-Solid Al-7Si-0.5Mg Aluminum Alloy by Gas Induced Semi-Solid Process. Metals 2022, 12, 1600. https://doi.org/10.3390/met12101600
Gu G, Xiang L, Li R, Xu W, Lu Y, Pesci R. Effects of Process Parameters on Microstructure and Mechanical Properties of Semi-Solid Al-7Si-0.5Mg Aluminum Alloy by Gas Induced Semi-Solid Process. Metals. 2022; 12(10):1600. https://doi.org/10.3390/met12101600
Chicago/Turabian StyleGu, Guochao, Lixin Xiang, Ruifen Li, Wenhua Xu, Yupeng Lu, and Raphaël Pesci. 2022. "Effects of Process Parameters on Microstructure and Mechanical Properties of Semi-Solid Al-7Si-0.5Mg Aluminum Alloy by Gas Induced Semi-Solid Process" Metals 12, no. 10: 1600. https://doi.org/10.3390/met12101600
APA StyleGu, G., Xiang, L., Li, R., Xu, W., Lu, Y., & Pesci, R. (2022). Effects of Process Parameters on Microstructure and Mechanical Properties of Semi-Solid Al-7Si-0.5Mg Aluminum Alloy by Gas Induced Semi-Solid Process. Metals, 12(10), 1600. https://doi.org/10.3390/met12101600






