Structure, Microstructure, Hyperfine, Mechanical and Magnetic Behavior of Selective Laser Melted Fe92.4Si3.1B4.5 Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selective Laser Melting
2.2. Characterization
3. Results
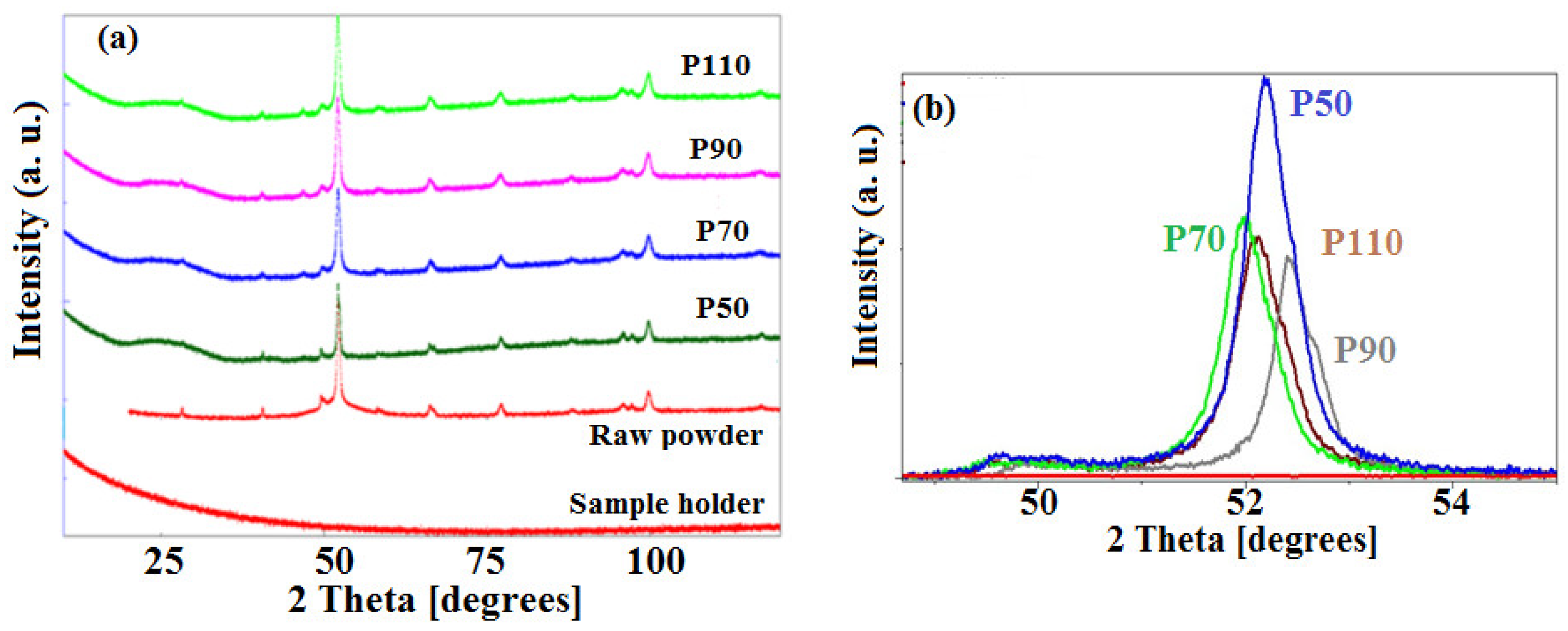
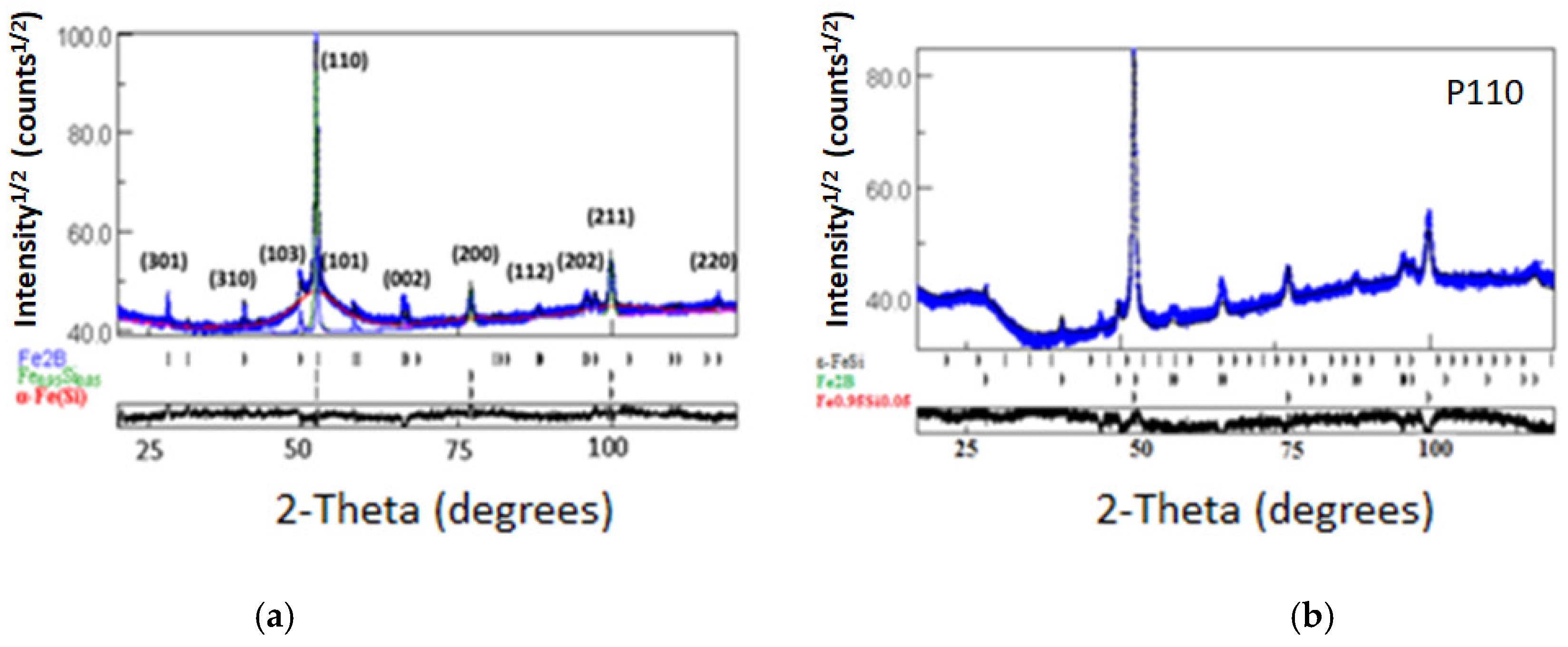
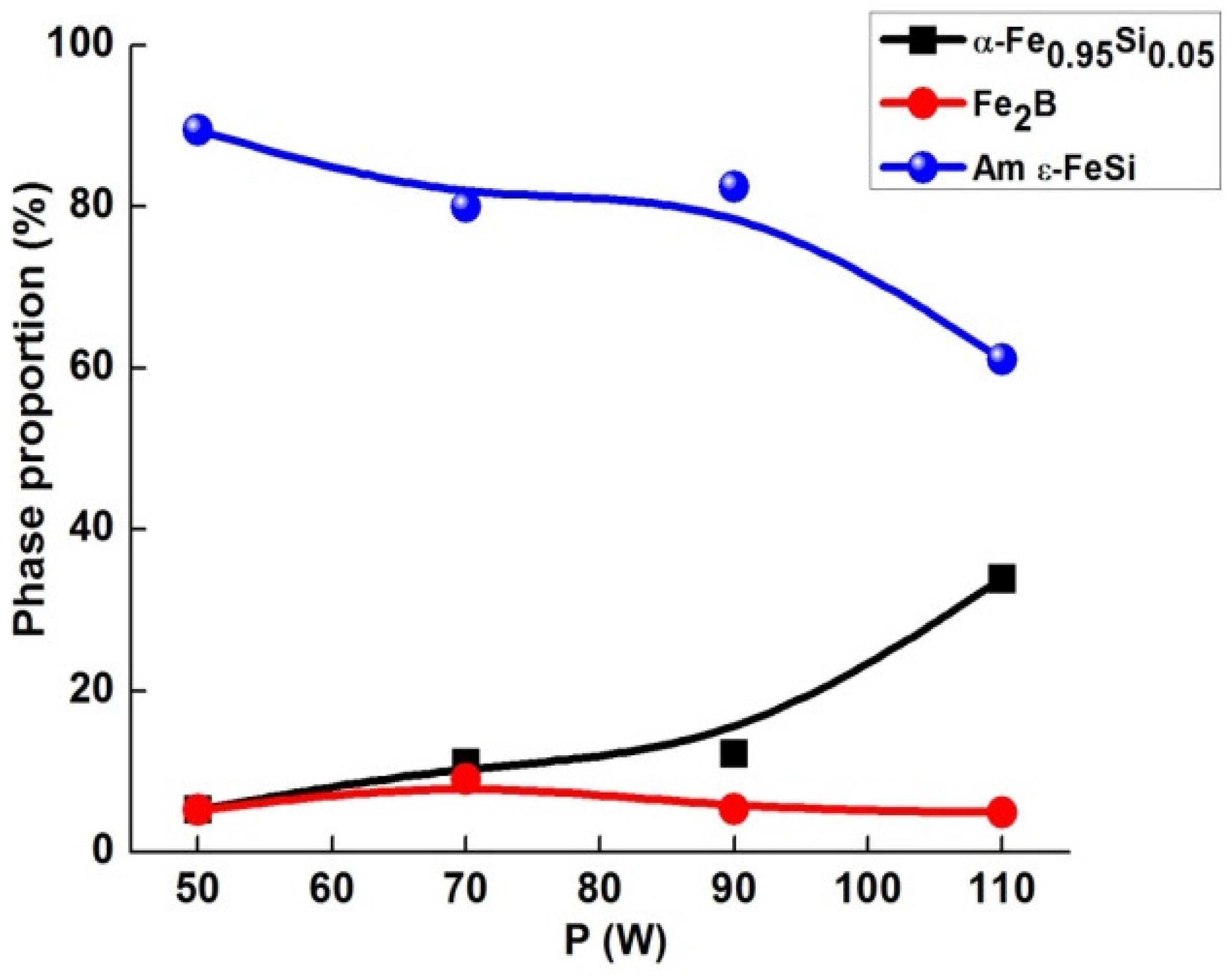
3.1. Structure and Microstructure
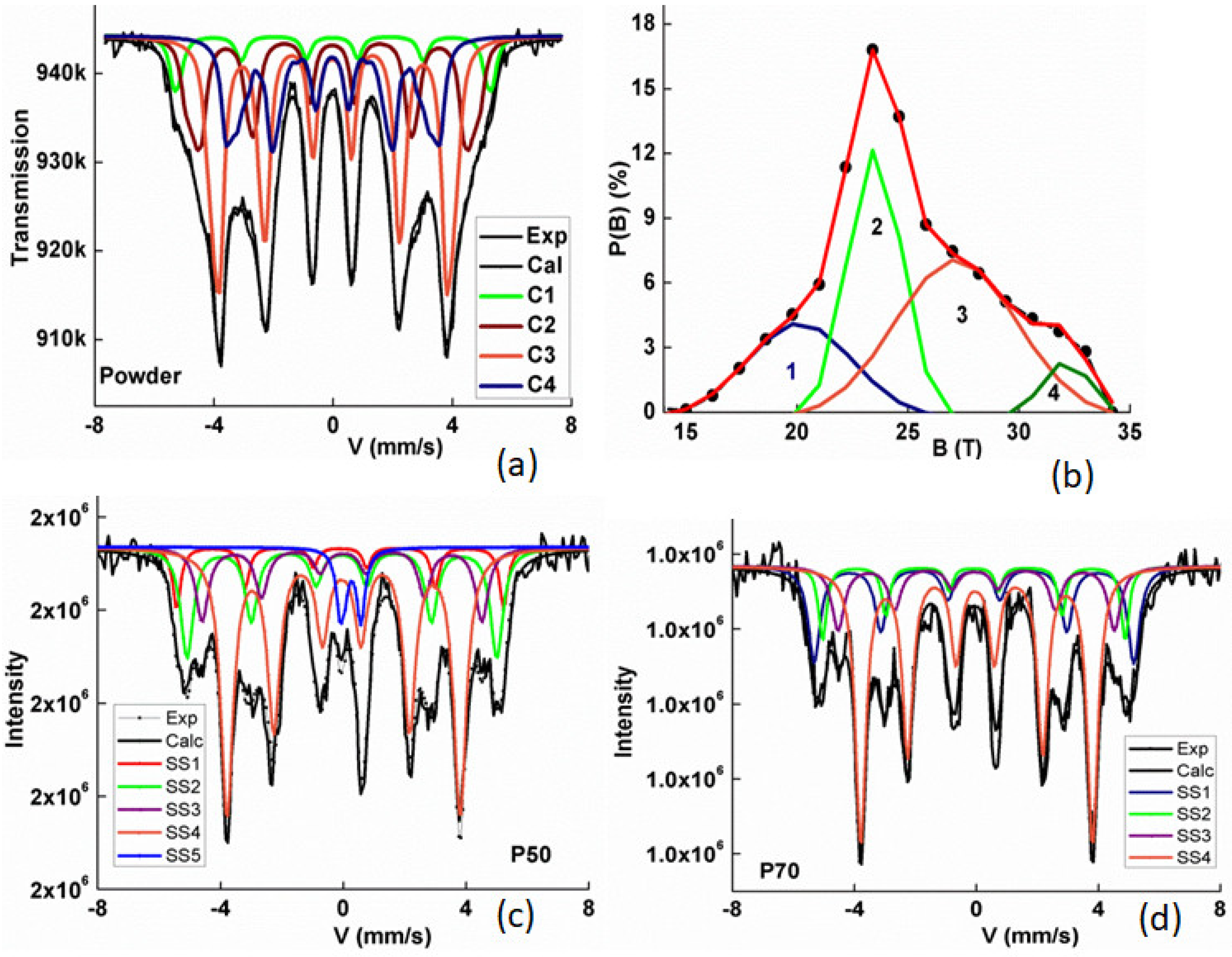
3.2. Mössbauer Spectrometry Results
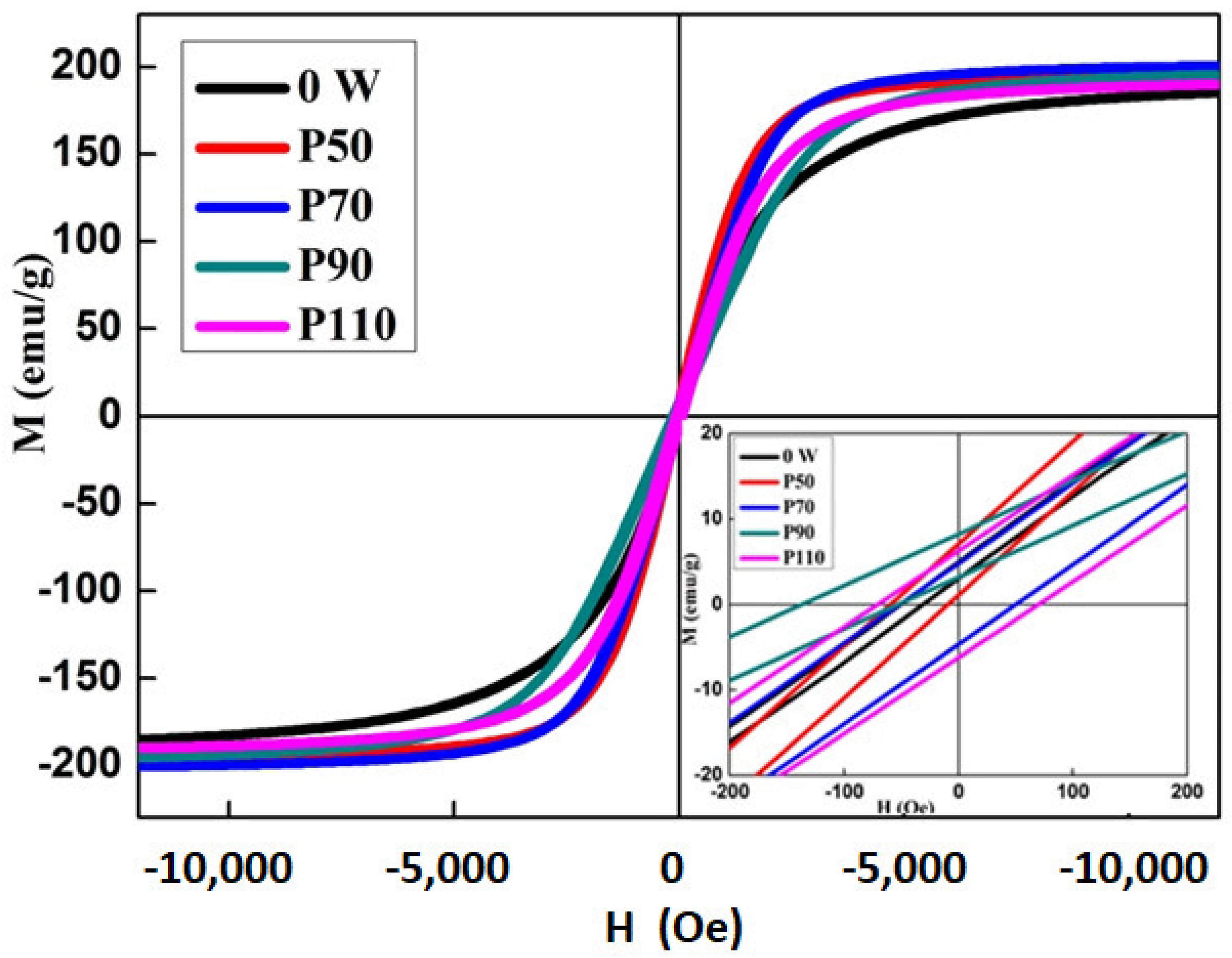
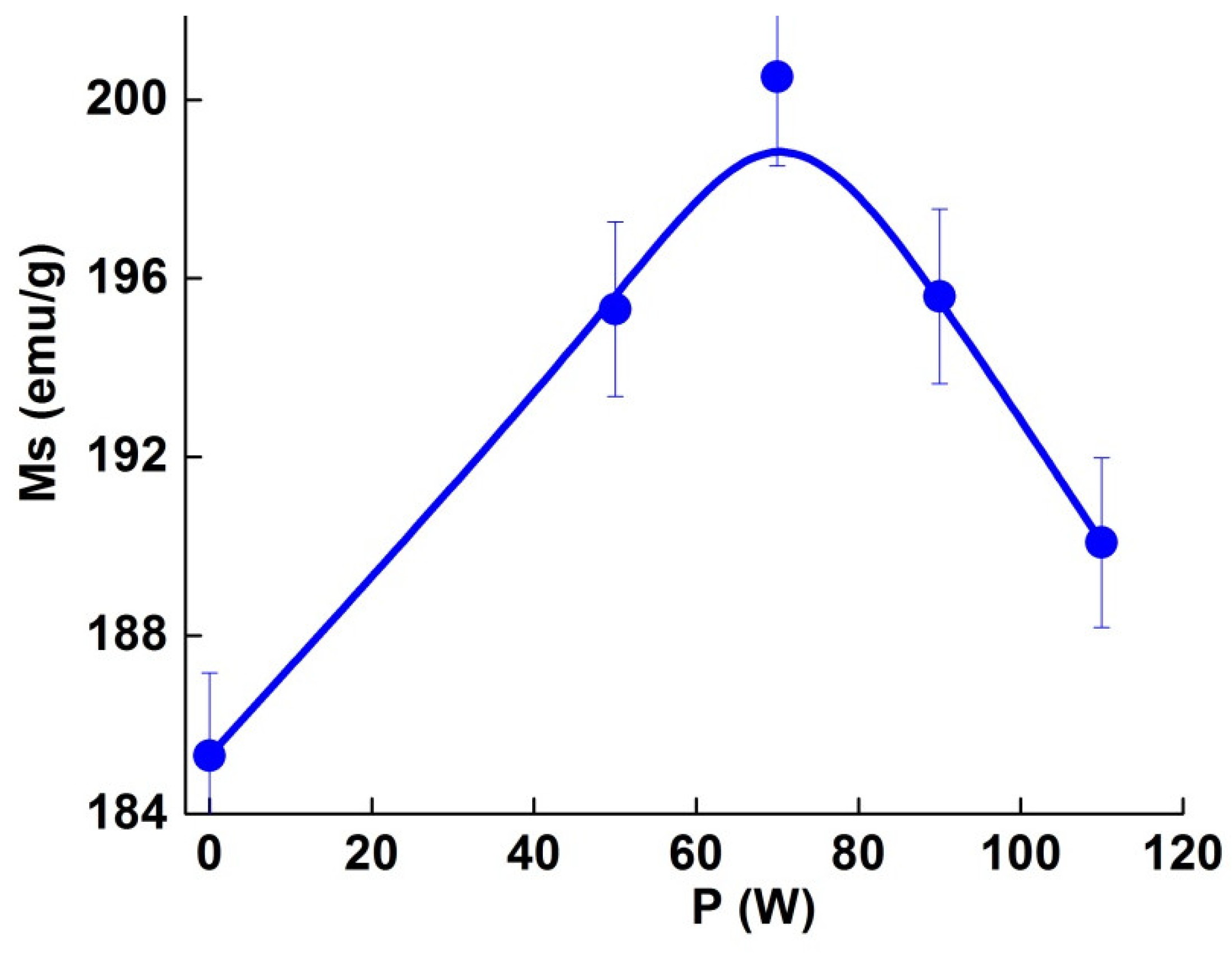
3.3. Magnetic Properties
3.4. Morphology Changes
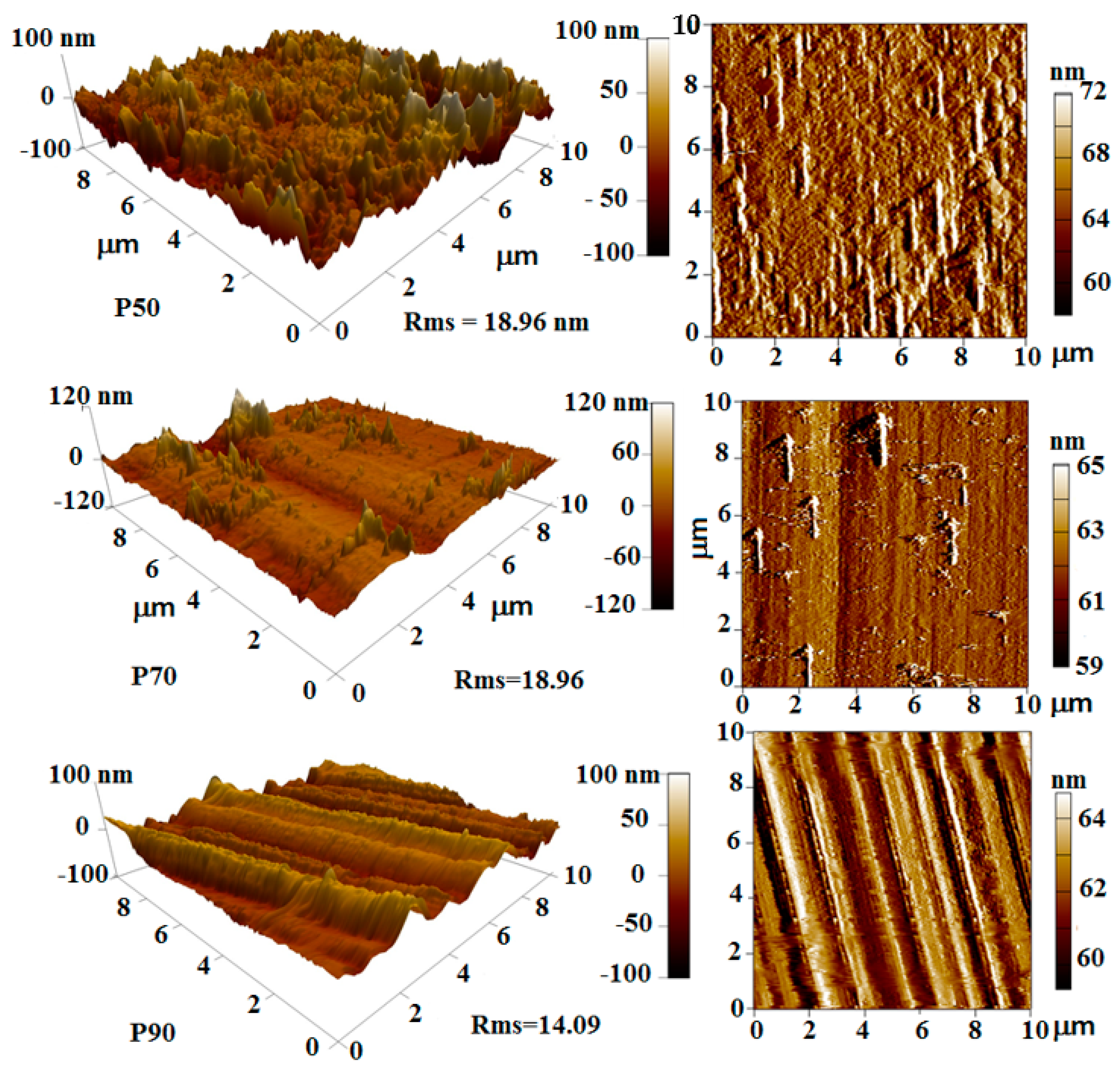
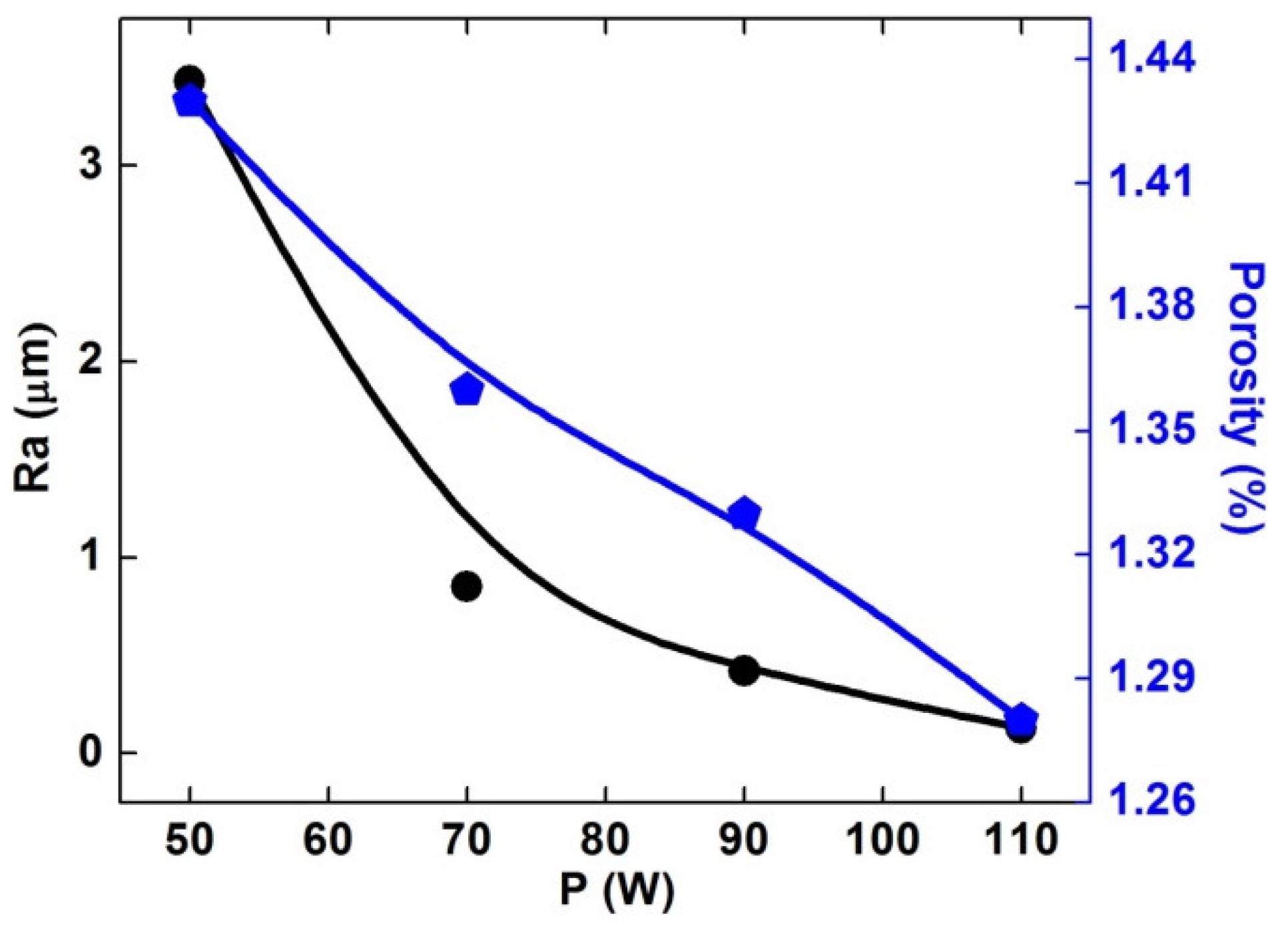
3.5. Surface Roughness
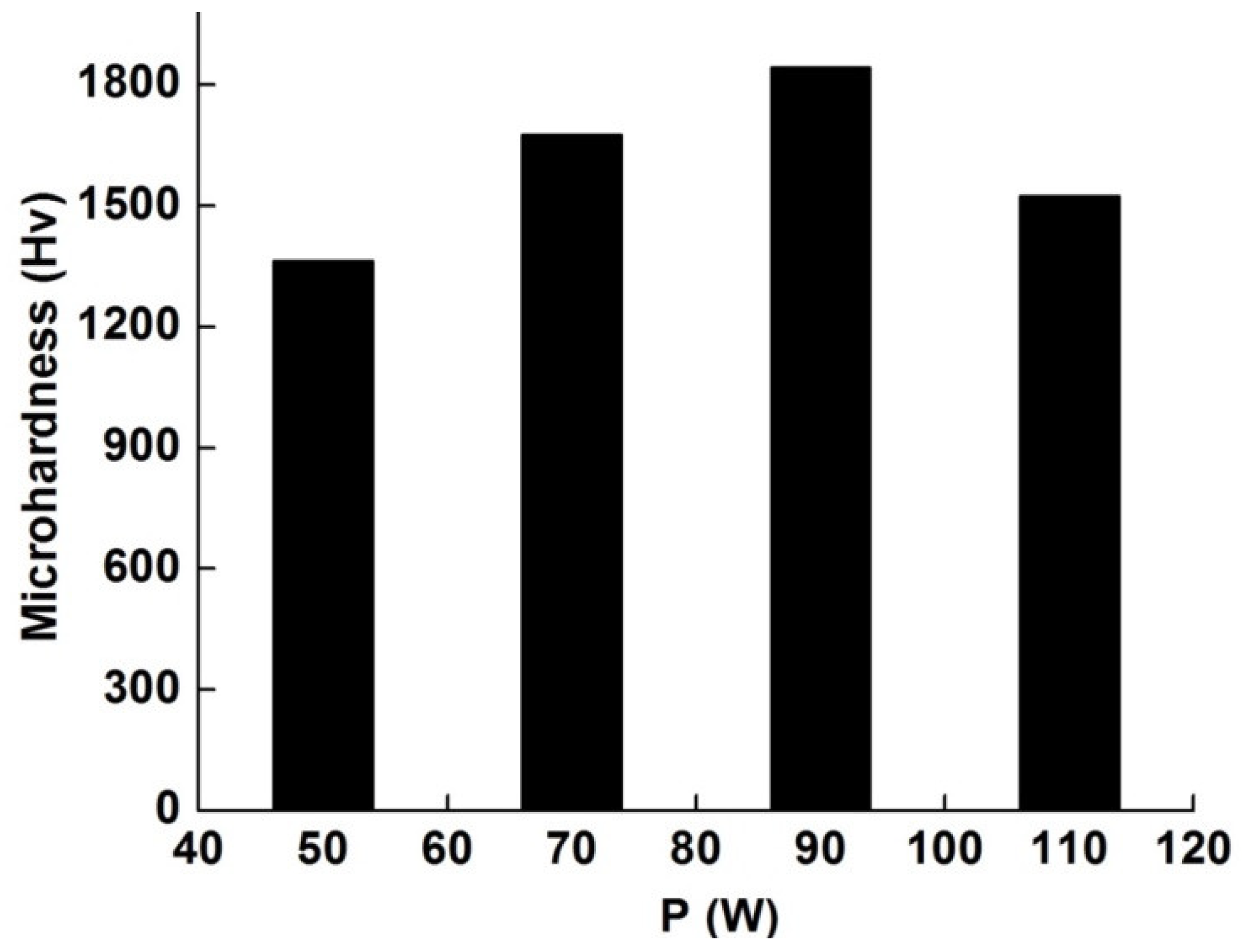
3.6. Microhardness
4. Conclusions
- -
- A disordered α-FeSi type phase was induced by SLM technology.
- -
- The α-Fe0.95Si0.05 and Fe2B boride phases were preserved from the feedstock powder with small variations in the lattice parameters.
- -
- The crystallite size of both α-Fe0.95Si0.05 and Fe2B boride decreased with increasing laser power.
- -
- The Mössbauer spectrometry results confirmed the presence of Fe2B and a disordered FeSi-type structure.
- -
- Almost completely dense samples could be obtained and their roughness and porosity decreased with increasing laser power.
- -
- The selectively laser melted specimens showed high microhardness values (ranged between 1364 and 1843 Hv), and soft magnetic behavior with Ms = 188–201 emu/g. Higher Ms and microhardness values could be obtained for the P90 sample.
- -
- An EB has been observed in the hysteresis loops of the P50 and P90 samples at room temperature.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahare, Y. Irreversible structural relaxation in FeSiB amorphous alloys. Mater. Sci. Eng. 1997, A231, 128–133. [Google Scholar] [CrossRef]
- Yue, S.; Zhang, H.; Cheng, R.; Wang, A.; Dong, Y.; He, A.; Liu, C.T. Magnetic and thermal stabilities of FeSiB eutectic amorphous alloys: Compositional effects. J. Alloys Compds. 2019, 776, 833–838. [Google Scholar] [CrossRef]
- Xu, D.D.; Zhou, B.L.; Wang, Q.Q.; Zhou, J.; Yang, W.M.; Yuan, C.C.; Shen, B.L. Effects of Cr addition on thermal stability, soft magnetic properties and corrosion resistance of FeSiB amorphous alloys. Corr. Sci. 2018, 138, 20–27. [Google Scholar] [CrossRef]
- Luborsky, F.E.; Becker, J.J.; Walter, J.L.; Liebermann, H.H. Formation and magnetic properties of Fe–B–Si amorphous alloys. IEEE Trans. Magn. 1979, 31, 146–1149. [Google Scholar] [CrossRef]
- Inoue, A.; Komuro, M.; Masumoto, T. Fe-Si-B amorphous alloys with high silicon concentration. J. Mater. Sci. 1984, 19, 4125–4132. [Google Scholar] [CrossRef]
- Zhang, H.; Mei, X.; Wang, Y.; Wang, Z.; Wang, Y. Resistance to H+ induced irradiation damage in metallic glass Fe80Si7.43B12.57. J. Nuclear Mater. 2015, 456, 344–350. [Google Scholar] [CrossRef]
- Zhou, J.; Kong, D. Immersion corrosion and electrochemical performances of laser cladded FeSiB, FeSiBCr and FeSiBCrMo coatings in 3.5 wt% NaCl solution. Surf. Coat. Technol. 2020, 383, 125229. [Google Scholar] [CrossRef]
- Tokunaga, T.; Ohtani, H.; Hasebe, M. Thermodynamic evaluation of the phase equilibria and glass-forming ability of the Fe–Si–B system. Calphad 2004, 28, 354–362. [Google Scholar] [CrossRef]
- Zeng, C.; Xu, Y.; Liu, R.; Zhao, H.; Zhu, Z.; Jia, Z. Effect of oriented magnetic field on the solidified structure and soft magnetic properties of Fe78Si13B9 alloy. Res. Phys. 2021, 28, 104584. [Google Scholar] [CrossRef]
- Alleg, S.; Ibrir, M.; Fenineche, N.E.; Azzaza, S.; Suñol, J.J. Magnetic and structural characterization of the mechanically alloyed Fe75Si15B10 powders. J. Alloys Compds. 2010, 494, 109–115. [Google Scholar] [CrossRef]
- Murty, B.S.; Ranganathan, S. Novel materials synthesis by mechanical alloying/milling. Int. Mater. Rev. 1998, 43, 101–141. [Google Scholar] [CrossRef]
- Quispe Marcatoma, J.; Peña Rodrígueze, V.A.; Baggio-Saitovitch, M. FeSiB amorphous alloy prepared by mechanosynthesis. Hyperfine Int. 2001, 134, 207–212. [Google Scholar] [CrossRef]
- Takahara, Y.; Matsuda, H. Effect of cold rolling on the physical properties and the structure of Fe79B16Si15 amorphous alloy. Trans. JIM 1987, 28, 535. [Google Scholar] [CrossRef]
- Coïsson, M.; Celegato, F.; Olivetti, E.; Tiberto, P.; Vinai, F.; Baricco, M. Stripe domains and spin reorientation transition in Fe78B13Si9 thin films produced by rf sputtering. J. Appl. Phys. 2008, 104, 033902. [Google Scholar] [CrossRef]
- Alleg, S.; Ibrir, M.; Fenineche, N.E.; Suñol, J.J. Microstructure and magnetic properties of HVOF thermally sprayed Fe75Si15B10 coatings. Surf. Coat. Technol. 2010, 205, 281–286. [Google Scholar] [CrossRef]
- Dong, S.; Song, B.; Zhang, X.; Deng, C.; Fenineche, N.E.; Hansz, B.; Liao, H.; Coddet, C. Fabrication of FeSiB magnetic coatings with improved saturation magnetization by plasma spray and dry-ice blasting. J. Alloys Compds. 2014, 584, 254–260. [Google Scholar] [CrossRef]
- Sypień, A.; Kusiński, J.; Kusiński, G.J.; Nelson, E.C. TEM study of the FeSiB amorphous alloy nanocrystallized by means of Nd: YAG pulsed laser heating. Mat. Chem. Phys. 2003, 81, 390–392. [Google Scholar] [CrossRef]
- Katakam, S.; Hwang, J.Y.; Vora, H.; Harimkar, S.P.; Banerjee, R.; Dahotre, N.B. Laser-induced thermal and spatial nanocrystallization of amorphous Fe–Si–B alloy. J. Scr. Mater. 2012, 66, 538–554. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F. 3D Printing and Additive Manufacturing: Principles and Applications, 4th ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2014. [Google Scholar]
- Dong, W.; Han, B.; Hui, J.; Yan, M. Bending behavior and fracture surface characters for FeSiB amorphous ribbons in different free volume state. Appl. Phys. A 2020, 126, 670. [Google Scholar] [CrossRef]
- Nagalingam, A.P.; Vohra, M.S.; Kapur, P.; Yen, S.H. Effect of cut-off, evaluation length, and measurement area in profile and areal surface texture characterization of as-built metal additive manufactured components. Appl. Sci. 2021, 11, 5089. [Google Scholar] [CrossRef]
- Zhang, B.; Coddet, C. Selective laser melting of iron powder: Observation of melting mechanism and densification behavior via point-track-surface-part research. J. Manuf. Sci. Eng. 2016, 138, 051001. [Google Scholar] [CrossRef]
- Agarwala, M.; Bourell, D.; Beaman, J.; Marcus, H.; Barlow, J. Direct selective laser sintering of metals. Rap. Prototyp. J. 1995, 1, 26–36. [Google Scholar] [CrossRef]
- Simchi, A.; Pohl, H. Effects of laser sintering processing parameters on the microstructure and densification of iron powder. Mater. Sci. Eng. 2003, A359, 119–128. [Google Scholar] [CrossRef]
- Alleg, S.; Drablia, R.; Fenineche, N.E. Effect of the laser scan rate on the microstructure, magnetic properties and microhardness of selective laser melted FeSiB. J. Supercond. Nov. Magn. 2018, 31, 3565–3577. [Google Scholar] [CrossRef]
- Lutterotti, L. MAUD CPD Newsletter (IUCr) 24 (2000). Available online: http://maud.radiographema.eu/ (accessed on 30 May 2022).
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. Acta Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Varret, F.; Teillet, J. Unpublished Mosfit Program; Le Mans Université: Le Mans, France, 1976. [Google Scholar]
- Vocadlo, L.; Knight, K.S.; Price, G.D.; Wood, I.G. Thermal expansion and crystal structure of FeSi between 4 and 1173 K determined by time-of-flight neutron powder diffraction. Phys. Chem. Min. 2002, 29, 132–139. [Google Scholar] [CrossRef]
- Stoica, M. Glass-forming ability of [(Fe0.5Co0.5)0.75B0.2Si0.05]96Nb4 alloy. In Fe-Based Bulk Metallic Glasses; MatWerk Springer: Wiesbaden, Germany, 2017; pp. 53–78. [Google Scholar] [CrossRef]
- de Boer, F.R.; Boom, R.; Mattens, W.C.M.; Miedema, A.R.; Niessen, A.K. Cohesion in Metals; North-Holland: Amsterdam, The Netherlands, 1988; p. 276. [Google Scholar]
- Poletti, M.G.; Battezzati, L. Assessment of the ternary Fe–Si–B phase diagram. Calphad 2013, 43, 40–47. [Google Scholar] [CrossRef]
- Imafuku, M.; Sato, S.; Koshiba, H.; Matsubara, E.; Inoue, A. Structural variation of Fe-Nb-B metallic glasses during crystallization process. Scripta Mater. 2001, 44, 2369. [Google Scholar] [CrossRef]
- Souilah, S.; Alleg, S.; Djebbari, C.; Suñol, J.J. Magnetic and microstructural properties of the mechanically alloyed Fe57Co21Nb7B15 powder mixture. Mater. Chem. Phys. 2012, 132, 766–772. [Google Scholar] [CrossRef]
- Liu, F.; Yao, K.; Ding, H.Y. Fe-based glassy alloys with high iron content and high saturation magnetization. Intermetallics 2011, 19, 1674–1677. [Google Scholar] [CrossRef]
- Iga, A.; Tawara, Y.; Yanase, A. Magnetocrystalline anisotropy of Fe2B. J. Phys. Soc. Jpn. 1966, 21, 404. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Deng, S.; Liao, H.; Coddet, C. Microstructure and tensile properties of iron parts fabricated by selective laser melting Comp. J. Opt. Las. Technol. 2014, 56, 451–460. [Google Scholar] [CrossRef]
- Aronsson, B.; Engström, I. X-ray Investigations on Me-Si-B Systems (Me=Mn, Fe, Co). Acta Chem. Scand. 1960, 14, 1403–1413. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Li, Z.G.; Li, R.F.; Li, M.; Feng, K.; Wu, Y.X.; Wada, T.; Kato, H. High power diode laser cladding of Fe–Co–B–Si–C–Nb amorphous coating: Layered microstructure and properties. Surf. Coat. Technol. 2013, 235, 699–705. [Google Scholar] [CrossRef]
- Li, R.F.; Li, Z.G.; Huang, J.; Zhu, Y. Dilution effect on the formation of amorphous phase in the laser cladded Ni–Fe–B–Si–Nb coatings after laser remelting process. App. Surf. Sci. 2012, 258, 7956–7961. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, H.; Yao, C.; Li, Z.; Yu, Z.; Xu, P. Synthesis of Fe–Ni–B–Si–Nb amorphous and crystalline composite coatings by laser cladding and remelting. Surf. Coat. Technol. 2011, 206, 1229–1236. [Google Scholar] [CrossRef]
- Li, R.; Li, Z.; Huang, J.; Zhang, P.; Zhu, Y. Effect of Ni-to-Fe ratio on structure and properties of Ni–Fe–B–Si–Nb coatings fabricated by laser processing. Appl. Surf. Sci. 2011, 257, 3554–3557. [Google Scholar] [CrossRef]

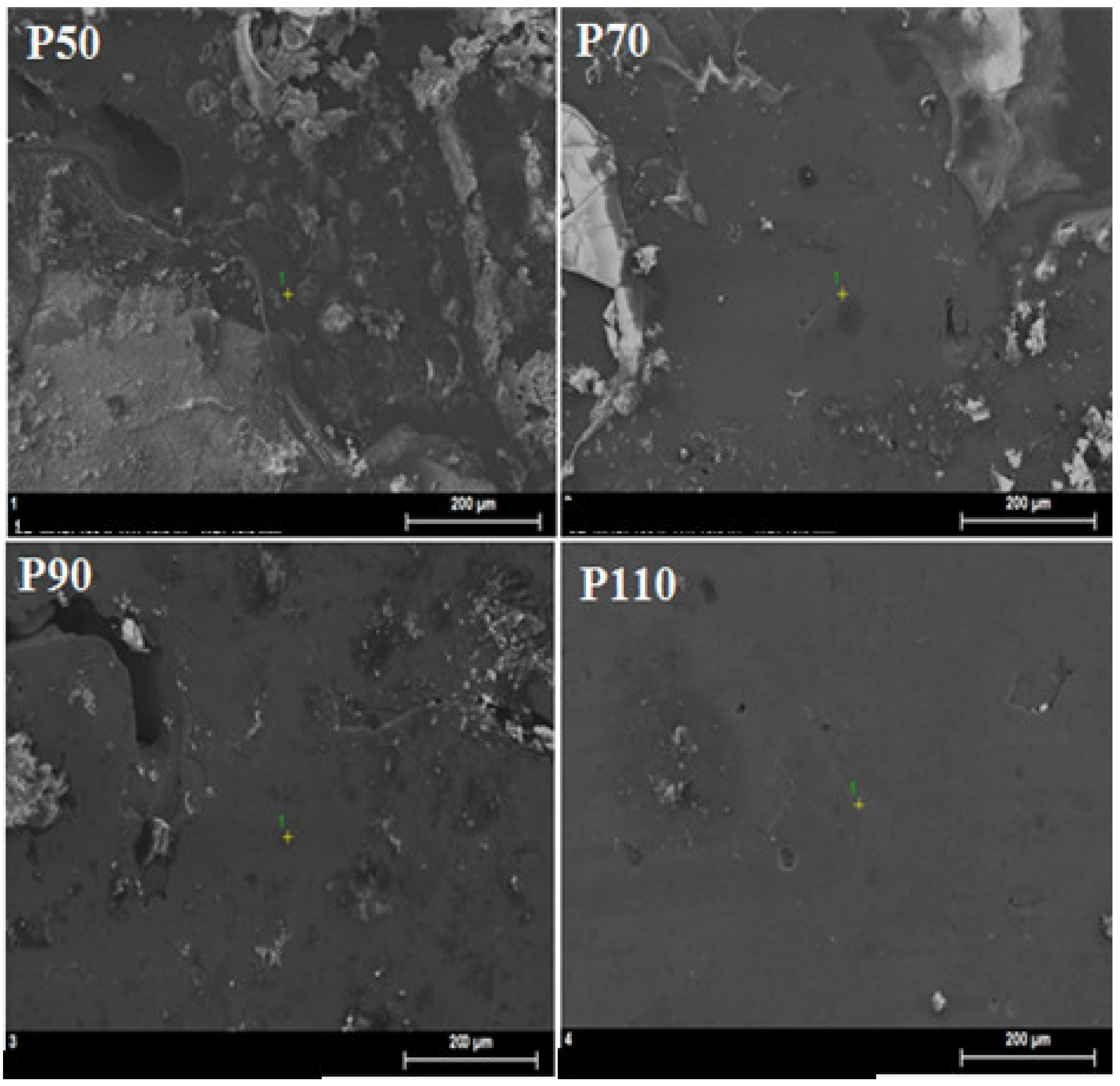
| Point | Element (at.%) ±0.1 | ||
|---|---|---|---|
| Fe | Si | B | |
| 1 | 88.0 | 9.6 | 2.4 |
| 2 | 84.9 | 12.3 | 2.8 |
| 3 | 87.4 | 10.4 | 2.0 |
| 4 | 91.8 | 3.5 | 4.7 |
| 5 | 91.3 | 4.0 | 4.7 |
| 6 | 90.3 | 3.9 | 5.8 |
| 7 | 89.7 | 3.8 | 6.5 |
| Mean value | 90.4 | 6.8 | 4.1 |
| Sample | Phases | a (nm) ±10−4 | Δa (%) | c (nm) ±10−4 | Δc (%) | <L> (nm) ±5 |
|---|---|---|---|---|---|---|
| Raw powder | Amorphous type | ---- | ---- | ---- | ---- | ---- |
| α-Fe0.95Si0.05 Fe2B | 0.2857 0.5108 | 0.035 −0.039 | ---- 0.4229 | ---- −0.47 | 102 90 | |
P50 | ε-FeSi type α-Fe0.95Si0.05 | 0.6549 0.2856 | 43.93 0 | ---- ---- | ---- ---- | 43 66 |
| Fe2B | 0.5106 | −0.078 | 0.4230 | −0.44 | 60 | |
| ε-FeSi type | 0.6405 | 40.76 | ---- | ---- | 36 | |
| P70 | α-Fe0.95Si0.05 | 0.2856 | 0 | ---- | ---- | 41 |
| Fe2B | 0.5105 | −0.097 | 0.4232 | −0.40 | 58 | |
| P90 | ε-FeSi type α-Fe0.95Si0.05 Fe2B | 0.6515 0.2862 0.5124 | 43.18 0.21 0.27 | ---- ---- 0.4241 | ---- ---- −0.18 | 30 40 61 |
| P110 | ε-FeSi type α-Fe0.95Si0.05 Fe2B | 0.6280 0.2864 0.5125 | 38.02 0.28 0.29 | ---- ---- 0.4243 | ---- ---- −0.14 | 37 45 62 |
| Sample | Atom | x | y | z | Occupancy |
|---|---|---|---|---|---|
| P50 | Fe | 0.736 | 0.736 | 0.760 | 0.62 |
| Si | −0.056 | 0.055 | −0.055 | 1.0 | |
| P70 | Fe | 0.736 | 0.736 | 0.735 | 1.0 |
| Si | −0.056 | 0.056 | 0.056 | 0.2 | |
| P90 | Fe | 0.736 | 0.737 | 0.736 | 0.97 |
| Si | 0.058 | 0.056 | 0.056 | 0.84 | |
| P110 | Fe | 0.736 | 0.736 | 0.760 | 0.62 |
| Si | −0.056 | 0.055 | −0.055 | 1.0 |
| Sample | Phase | Site | Bhf ±0.5 | IS ±0.01 | 2ε or Δ ±0.01 | % ±0.02 | Area ±2 | <Bhf> | <IS> |
|---|---|---|---|---|---|---|---|---|---|
| Powder | HFD | ---- | ---- | 0.02 | 0.36 | 100 | 24.7 | 0.11 | |
| FeSi | SS1 | 32.5 | 0.03 | 0 | 0.36 | 4 | |||
| SS2 | 30.5 | 0.07 | −0.03 | 0.53 | 11 | ||||
| SS3 | 27.4 | 0.09 | −0.03 | 0.50 | 19 | 21.0 | 0.09 | ||
| Fe2B | SS4 | 23.9 | 0.12 | 0.01 | 0.55 | 47 | |||
| HFD | ---- | 0.05 | −0.02 | 0.30 | 19 | ||||
| P50 | FeSi Fe2B | SS1 | 32.9 | 0.04 | 0 | 0.32 | 8 | ||
| SS2 | 31.1 | 0.08 | 0.01 | 0.49 | 21 | ||||
| SS3 | 28.1 | 0.11 | −0.03 | 0.50 | 14 | 25.4 | 0.11 | ||
| SS5 | 0 | 0.38 | 0.60 | 0.33 | 5 | ||||
| SS4 | 23.4 | 0.11 | 0.06 | 0.49 | 52 | ||||
| P70 | FeSi Fe2B | SS1 | 32.4 | 0.05 | 0 | 0.27 | 21 | ||
| SS2 | 30.6 | 0.05 | −0.00 | 0.32 | 11 | 26.7 | 0.09 | ||
| SS3 | 28.0 | 0.11 | 0.04 | 0.50 | 14 | ||||
| SS4 | 23.4 | 0.12 | 0.04 | 0.42 | 54 |
| Sample | Element (at.%) ± 0.5 | ||
|---|---|---|---|
| Fe | Si | B | |
| P50 | 93.40 | 3.3 | 3.3 |
| P70 | 83.10 | 5.6 | 11.4 |
| P90 | 81.80 | 5.2 | 13.0 |
| P110 | 78.85 | 4.9 | 16.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drablia, R.; Alleg, S.; Fenineche, N.-E.; Escoda, L.; Suñol, J.-J.; Greneche, J.-M. Structure, Microstructure, Hyperfine, Mechanical and Magnetic Behavior of Selective Laser Melted Fe92.4Si3.1B4.5 Alloy. Metals 2022, 12, 1620. https://doi.org/10.3390/met12101620
Drablia R, Alleg S, Fenineche N-E, Escoda L, Suñol J-J, Greneche J-M. Structure, Microstructure, Hyperfine, Mechanical and Magnetic Behavior of Selective Laser Melted Fe92.4Si3.1B4.5 Alloy. Metals. 2022; 12(10):1620. https://doi.org/10.3390/met12101620
Chicago/Turabian StyleDrablia, Rima, Safia Alleg, Nour-Eddine Fenineche, Lluisa Escoda, Joan-Josep Suñol, and Jean-Marc Greneche. 2022. "Structure, Microstructure, Hyperfine, Mechanical and Magnetic Behavior of Selective Laser Melted Fe92.4Si3.1B4.5 Alloy" Metals 12, no. 10: 1620. https://doi.org/10.3390/met12101620
APA StyleDrablia, R., Alleg, S., Fenineche, N.-E., Escoda, L., Suñol, J.-J., & Greneche, J.-M. (2022). Structure, Microstructure, Hyperfine, Mechanical and Magnetic Behavior of Selective Laser Melted Fe92.4Si3.1B4.5 Alloy. Metals, 12(10), 1620. https://doi.org/10.3390/met12101620









