Study of the Microstructure, Corrosion and Optical Properties of Anodized Aluminum for Solar Heating Applications
Abstract
1. Introduction
2. Experimental Apparatus and Equipment
3. Experimental Procedure
3.1. Microstructure Study
3.2. Solar Heating System
3.3. Corrosion Resistance Study
4. Results and Discussion
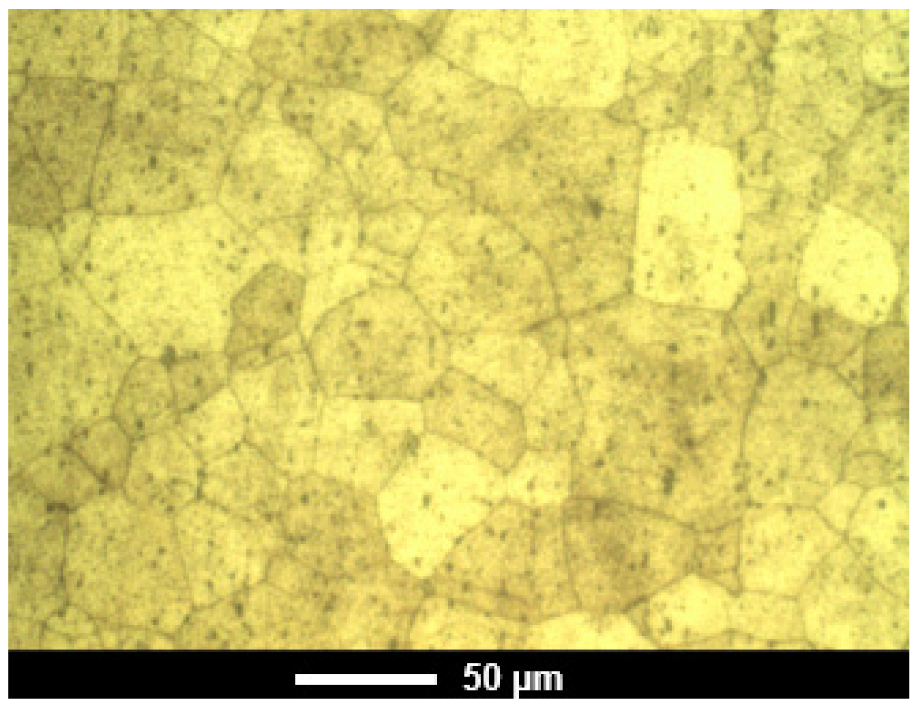
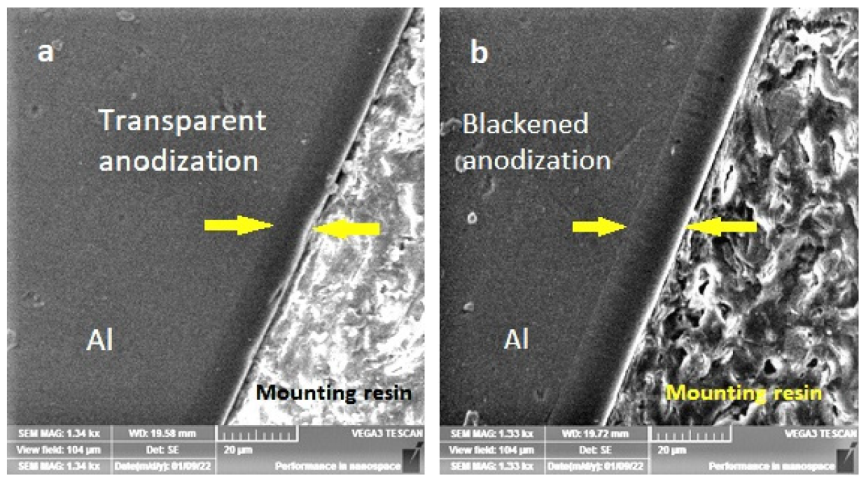
4.1. Microstructure
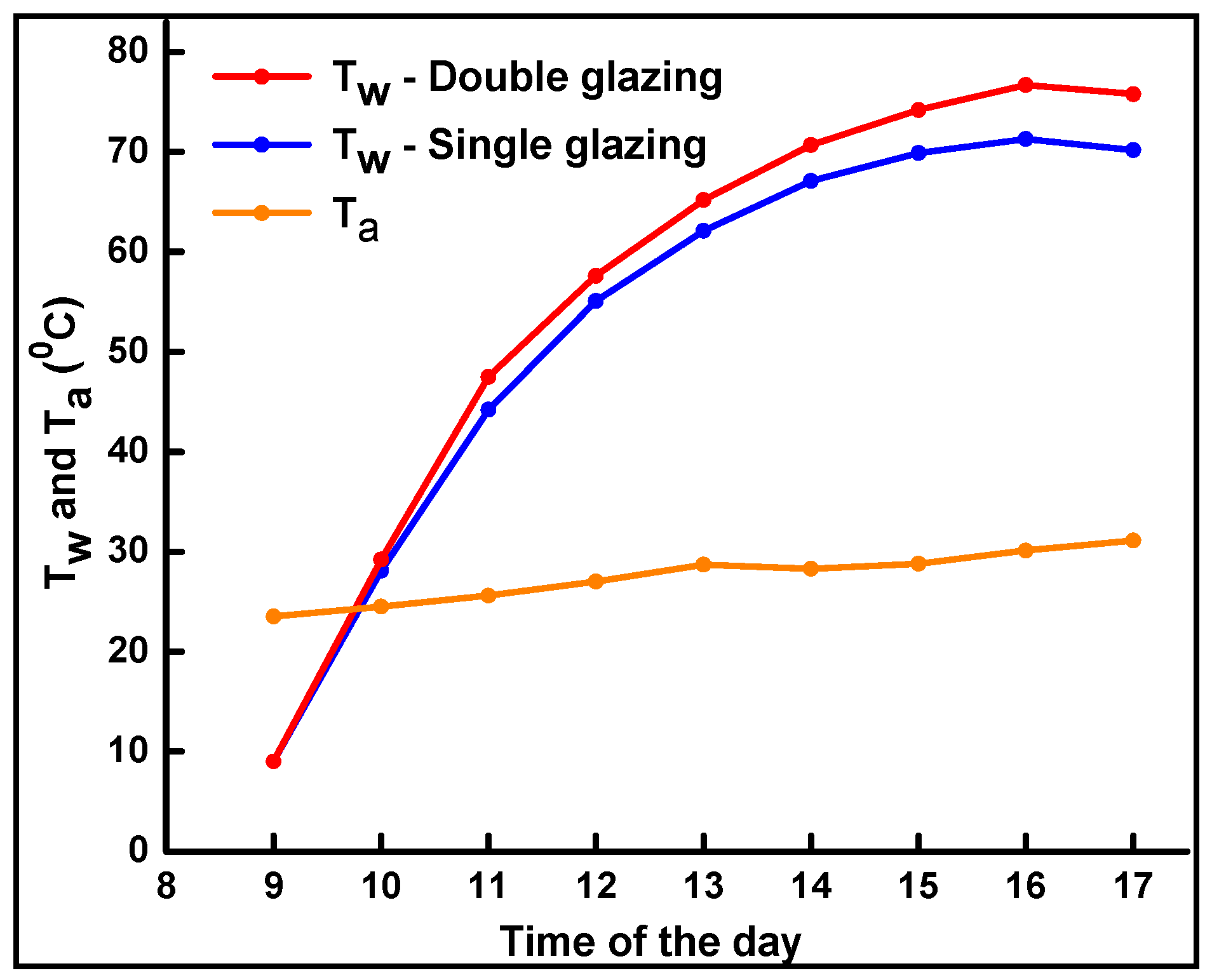
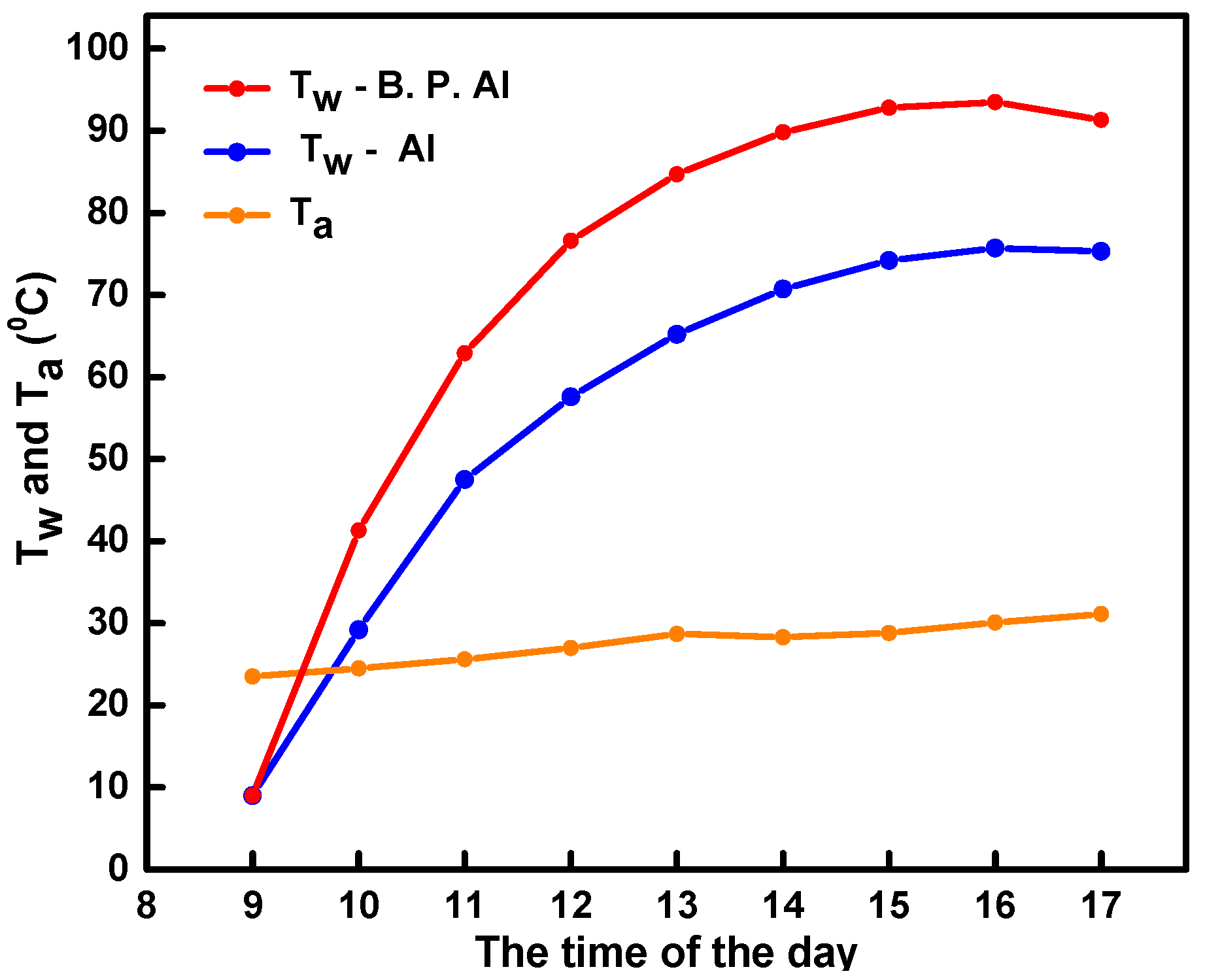
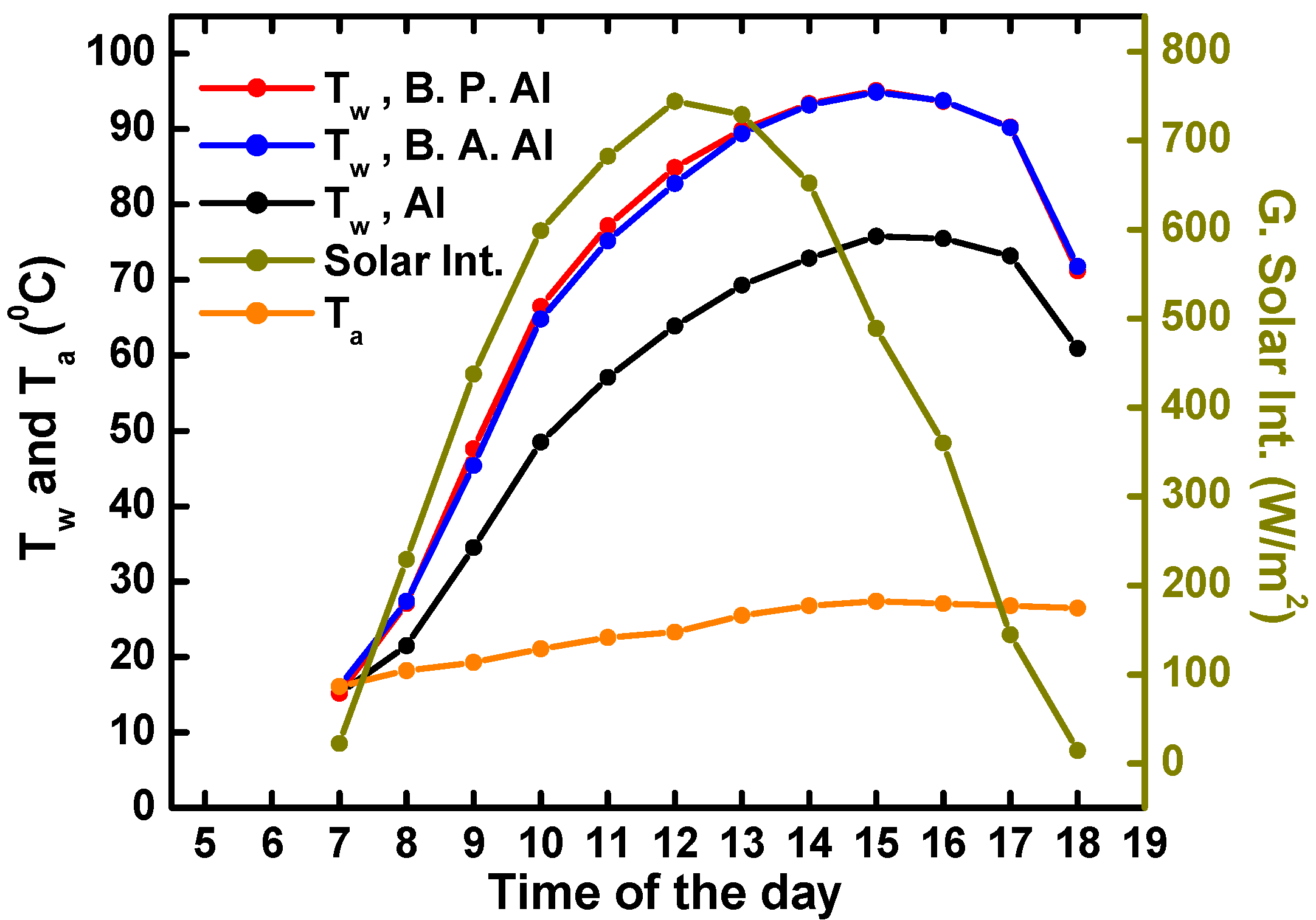
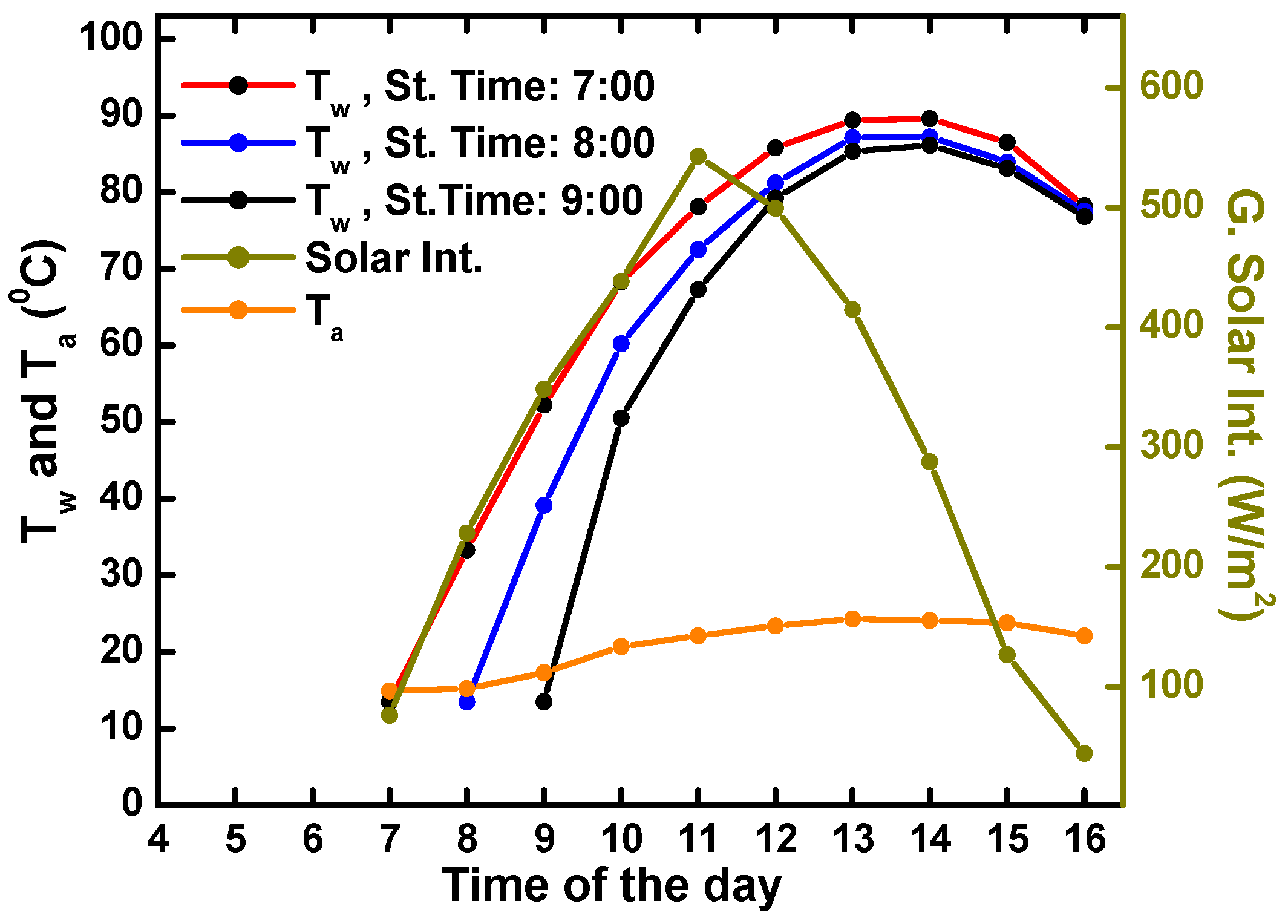
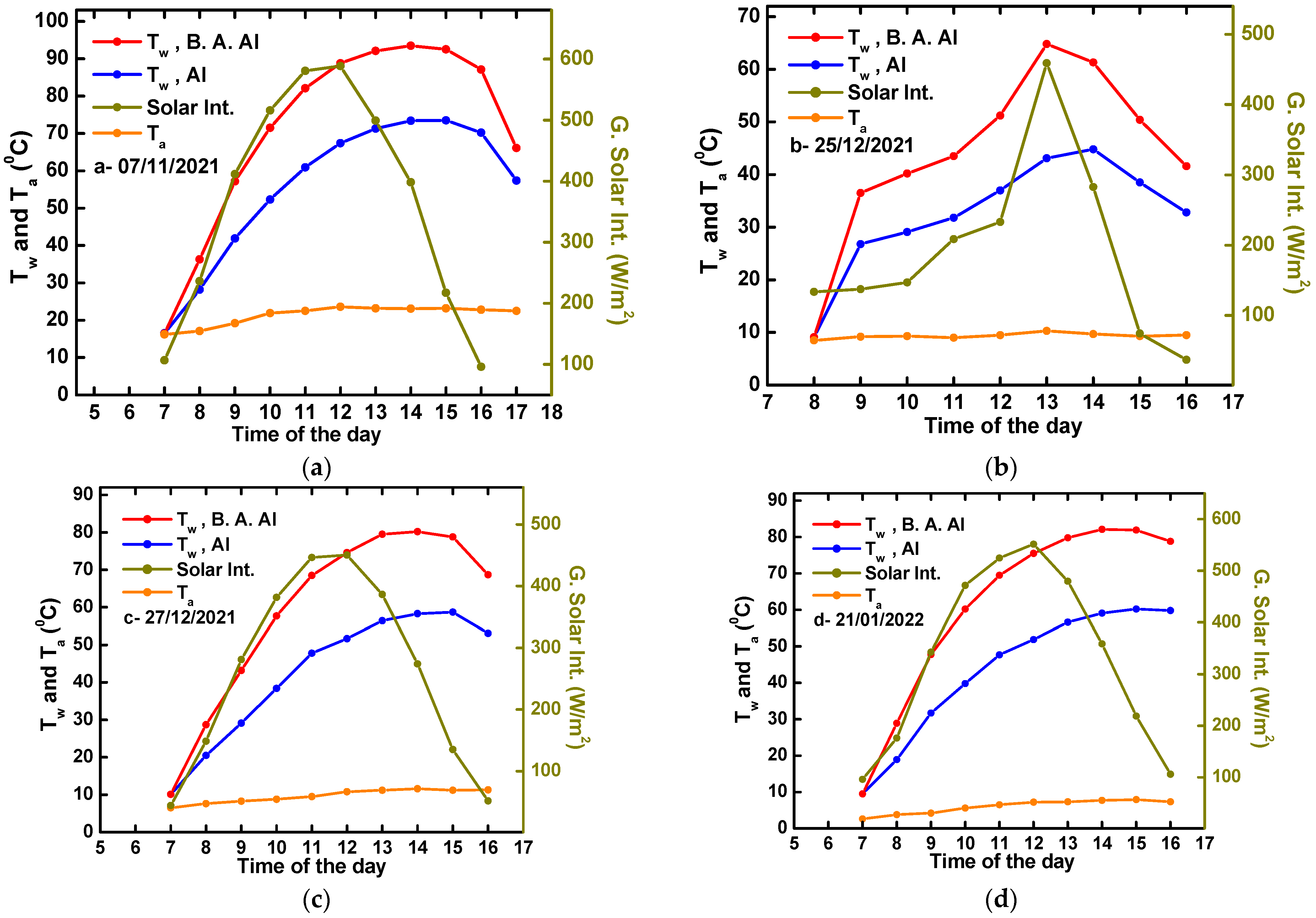
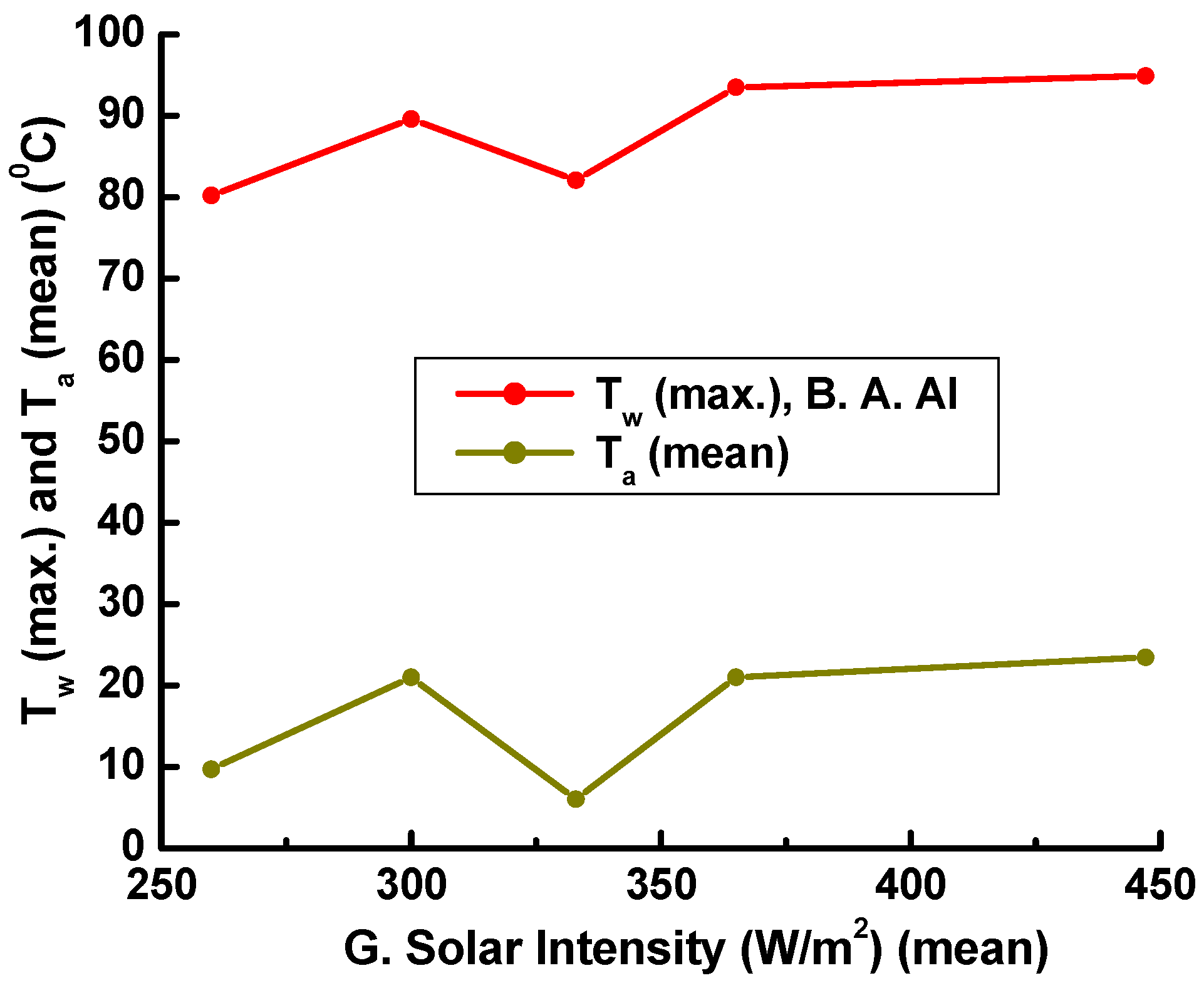
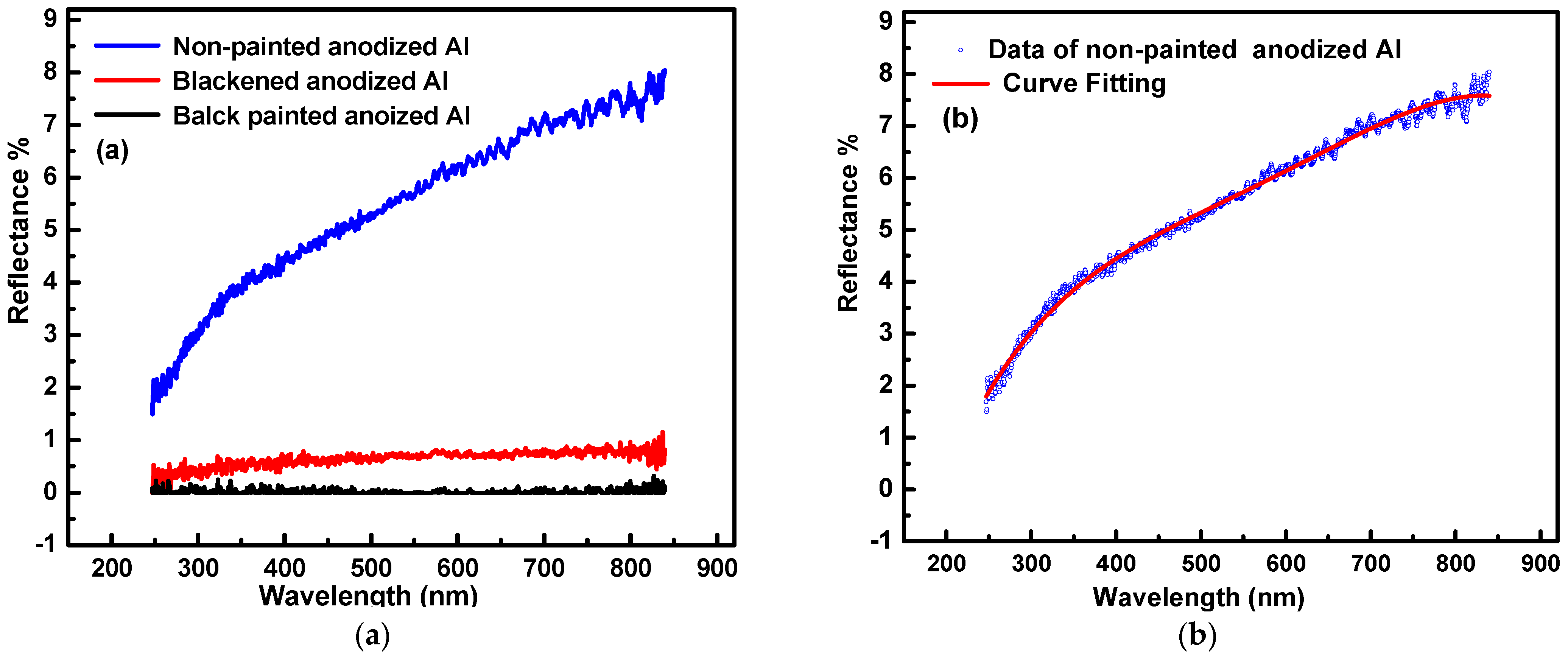
4.2. Solar Water Heating
4.3. Corrosion Resistance Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moosa, I.S.; Kazem, H.A. Review of Basic Renewable Energy in GCC Countries: Current Status and Future Vision. Int. J. Comput. Appl. Sci. 2019, 6, 397–406. [Google Scholar]
- Moosa, I.S.; Kazem, H.A.; Al-Iessi, L.M.R. Production of Hydrogen via Renewable Energy and Investigation of Water Molecular Changes During Electrolysis Process. J. Renew. Energy Environ. 2021, 8, 19–28. [Google Scholar] [CrossRef]
- Jamar, A.; Majid, Z.; Azmi, W.; Norhafana, M.; Razak, A. A review of water heating system for solar energy applications. Int. Commun. Heat Mass Transf. 2016, 76, 178–187. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Khalid, M.; Vaka, M.; Walvekar, R.; Numan, A.; Rasheed, A.K.; Mubarak, N.M. Recent progress in solar water heaters and solar collectors: A comprehensive review. Therm. Sci. Eng. Prog. 2021, 25, 100981. [Google Scholar] [CrossRef]
- Dehghan, M.; Pfeiffer, C.F.; Rakhshani, E.; Bakhshi-Jafarabadi, R. A Review on Techno-Economic Assessment of Solar Water Heating Systems in the Middle East. Energies 2021, 14, 4944. [Google Scholar] [CrossRef]
- AlShamaileh, E. Testing of a new solar coating for solar water heating applications. Sol. Energy 2010, 84, 1637–1643. [Google Scholar] [CrossRef]
- Moosa, I.S.; Maqableh, B.B. Temperature Difference with Respect to Exposure Time for Black Paint and Galena Powder-Black Paint Composite Selective Surfaces. In Transition Towards 100% Renewable Energy, Proceedings of the World Renewable Energy Congress XVI, Murdoch University, Perth, Western Australia, 5–9 February 2017; Sayigh, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; Chapter 13; pp. 139–147. ISBN 978-3-319-69844-1. [Google Scholar]
- Kafle, B.; Lamichhane, R.; Basnet, S. Dependence of Optical and Thermal Properties on Substrate of Solar Thermal Collectors. IOP Conf. Ser. Earth Environ. Sci. 2017, 73, 012015. [Google Scholar] [CrossRef]
- Nunes, R.A.X.; Costa, V.C.; Sade, W.; Araújo, F.R.; Silva, G.M. Selective Surfaces of Black Chromium for Use in Solar Absorbers. Mater. Res. 2018, 21, e20170556. [Google Scholar] [CrossRef]
- Al-Kayiem, H.H.; Ismaeel, A.A.; Baheta, A.T.; Aurybi, M.A. Performance enhancement of solar vortex power generator by Al2O3-in-black paint coating. J. Clean. Prod. 2021, 316, 128303. [Google Scholar] [CrossRef]
- Filli, F.; Gebray, P.; Kebedom, A. Comparative Study of Antireflection Coating Materials for Solar Thermal Collectors. Momona Ethiop. J. Sci. (MEJS) 2018, 10, 1–14. [Google Scholar] [CrossRef]
- El Nady, J.; Kashyout, A.; Ebrahim, S.; Soliman, M. Nanoparticles Ni electroplating and black paint for solar collector applications. Alex. Eng. J. 2016, 55, 723–729. [Google Scholar] [CrossRef]
- Simalango, J.; Ambarita, H.; Napitupulu, F.; Sihombing, H.V. Testing of commercial black painted for flat plate solar collector applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1122, 012077. [Google Scholar] [CrossRef]
- Hussein, H.; Shaffei, M.; Khatab, N.; Awad, A.; Shabaan, N.; Shalaby, M.E. A comparative study for selecting more efficient black selective coating in solar water heating system. Egypt. J. Chem. 2021, 64, 3–4. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bhojraj, H.; Kaila, V.K.; Narayanamurthy, H. Anodizing and Inorganic Black Coloring of Aluminum Alloys for Space Applications. Met. Finish. 1997, 95, 14–20. [Google Scholar] [CrossRef]
- Goueffon, Y.; Arurault, L.; Mabru, C.; Tonon, C.; Guigue, P. Black anodic coatings for space applications: Study of the process parameters, characteristics and mechanical properties. J. Mater. Processing Technol. 2009, 209, 5145–5151. [Google Scholar] [CrossRef]
- Logori, D.; Pezzato, L.; Settimi, A.G.; Hanoz, D.; Dabalà, M. Microstructural and Corrosion Properties of Burnished 6060 Aluminum Alloy. Appl. Sci. 2021, 11, 4460. [Google Scholar] [CrossRef]
- García, L.; Dietz, C.; Criado, A.J.; Martínez, J.A. Colour Metallography of Cast Aluminium Alloys. Pract. Metallogr. 2014, 51, 514–529. [Google Scholar] [CrossRef]
- Kalogirou, S. The potential of solar industrial process heat applications. Appl. Energy 2003, 76, 337–361. [Google Scholar] [CrossRef]
- Natishan, P.M.; Grady, W.E.O. Chloride Ion Interactions with Oxide-Covered Aluminum Leading to Pitting Corrosion: A Review. J. Electrochem. Soc. 2014, 161, C421–C432. Available online: https://iopscience.iop.org/article/10.1149/2.1011409jes/pdf (accessed on 10 August 2022). [CrossRef]
- Serway, R.A.; Vuille, C. College Physics, 9th ed.; Serway, R.A., Vuille, C., Eds.; Brooks/Cole 20 Channel Center Street: Boston, MA, USA, 2012; p. 376. [Google Scholar]




| Parameters | Value | Standard Error |
|---|---|---|
| Residual Sum of Squares | 24.25198 | - |
| R-Square | 0.99439 | - |
| Intercept | −14.12885 | 0.33066 |
| B1 | 0.11748 | 0.00275 |
| B2 | −2.88577 × 10−4 | 8.17811 × 10−6 |
| B3 | 3.35354 × 10−7 | 1.03572 × 10−8 |
| B4 | −1.44851 × 10−10 | 4.73667 × 10−12 |
| System | Surface Denotation | Ecorr (i = 0) mV | Icorr (mA/cm²) | Rp (kohm·cm²) | Ba (mV) | Bc (mV) | Corrosion Rate (µm/Y) |
|---|---|---|---|---|---|---|---|
| Backside of blackened anodized Al | - | −856.0 | 0.3703 | 42.60 | 30.2 | −375.7 | 4.029 |
| Blackened anodized Al | B. A. Al | −979.9 | 0.0807 | 65.01 | 47.4 | −99.0 | 0.878 |
| Non-painted anodized Al | Al | −761.4 | 0.1567 | 180.41 | 22.1 | −211.5 | 1.705 |
| Backside of non-painted anodized Al | - | −831.0 | 0.3921 | 54.79 | 24.2 | −470.3 | 4.266 |
| Matte black painted Al | B. P. Al | −775.7 | 0.0959 | 135.93 | 16.3 | −120.6 | 1.044 |
| Backside of matte black painted Al | - | −828.5 | 0.2795 | 34.87 | 27.1 | −531.8 | 3.041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlShamaileh, E.; Altwaiq, A.M.; Esaifan, M.; Al-Fayyad, H.; Shraideh, Z.; Moosa, I.S.; Hamadneh, I. Study of the Microstructure, Corrosion and Optical Properties of Anodized Aluminum for Solar Heating Applications. Metals 2022, 12, 1635. https://doi.org/10.3390/met12101635
AlShamaileh E, Altwaiq AM, Esaifan M, Al-Fayyad H, Shraideh Z, Moosa IS, Hamadneh I. Study of the Microstructure, Corrosion and Optical Properties of Anodized Aluminum for Solar Heating Applications. Metals. 2022; 12(10):1635. https://doi.org/10.3390/met12101635
Chicago/Turabian StyleAlShamaileh, Ehab, Abdelmnim M. Altwaiq, Muayad Esaifan, Heba Al-Fayyad, Ziad Shraideh, Iessa Sabbe Moosa, and Imad Hamadneh. 2022. "Study of the Microstructure, Corrosion and Optical Properties of Anodized Aluminum for Solar Heating Applications" Metals 12, no. 10: 1635. https://doi.org/10.3390/met12101635
APA StyleAlShamaileh, E., Altwaiq, A. M., Esaifan, M., Al-Fayyad, H., Shraideh, Z., Moosa, I. S., & Hamadneh, I. (2022). Study of the Microstructure, Corrosion and Optical Properties of Anodized Aluminum for Solar Heating Applications. Metals, 12(10), 1635. https://doi.org/10.3390/met12101635








