The Role of Microparticles of β-TCP and Wollastonite in the Creation of Biocoatings on Mg0.8Ca Alloy
Abstract
:1. Introduction
2. Materials and Methods
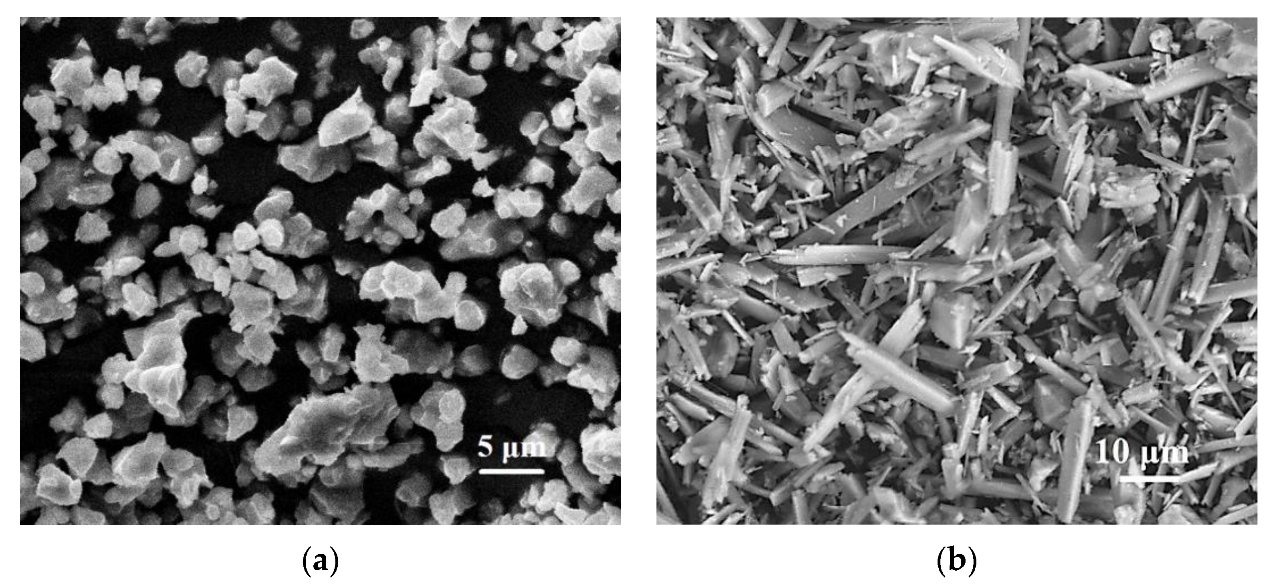
2.1. Sample Preparation and Characterization
2.2. Scratch Test
2.3. Electrochemical Studies
2.4. Biological Studies
3. Results
3.1. Dependences of the Coatings Formation, their Morphology, and Elemental Composition
3.2. Coatings Phase Composition
3.3. Mechanical Properties of the Coatings
3.4. Electrochemical Properties of the Coatings
3.5. Biological Study
3.5.1. Bioresorption
3.5.2. Immersion in the SBF Solution
3.5.3. MTT-Test
3.5.4. 3T3 Cells Research with the Help of the Flow Cytometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mat. Sci. Eng. R. 2015, 87, 1–5. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, G. Johansson, Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Shao, Y.; Zeng, R.C.; Li, S.Q.; Cui, L.Y.; Zou, Y.H.; Guan, S.K.; Zheng, Y.F. Advance in antibacterial magnesium alloys and surface coatings on magnesium alloys: A Review. Acta Metall. Sin. Engl. Lett. 2020, 33, 615–629. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Khlusov, I.A.; Mitrichenko, D.V.; Prosolov, A.B.; Nikolaeva, O.O.; Slepchenko, G.B.; Sharkeev, Y.P. Short review of the biomedical properties and application of magnesium alloys for bone tissue bioengineering. Bull. Sib. Med. 2019, 18, 274–286. [Google Scholar] [CrossRef]
- Chen, Y.T.; Hung, F.Y.; Syu, J.C. Biodegradable implantation material: Mechanical properties and surface corrosion mechanism of Mg-1Ca-0.5Zr Alloy. Metals 2019, 9, 857. [Google Scholar] [CrossRef]
- Putra, N.E.; Mirzaali, M.J.; Apachitei, I.; Zhou, J.; Zadpoor, A.A. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020, 109, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, N.; Nakao, Y.; Ishikawa, R.; Tsuchida, D.; Iijima, M. Degradation and biocompatibility of AZ31 magnesium alloy implants in vitro and in vivo: A micro-computed tomography study in rats. Materials 2020, 13, 473. [Google Scholar] [CrossRef]
- Ali, M.; Elsherif, M.; Salih, A.E.; Ul-Hamid, A.; Hussein, M.A.; Park, S.; Yetisen, A.K.; Butt, H. Surface modification and cytotoxicity of Mg-based bio-alloys: An overview of recent advances. J. Alloys Compd. 2020, 825, 154140. [Google Scholar] [CrossRef]
- Atrens, A.; Johnston, S.; Shi, Z.; Dargusch, M.S. Viewpoint–understanding Mg corrosion in the body for biodegradable medical implants. Scripta Mater. 2018, 154, 92–100. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Liu, Z.; Wang, Q. Advances in antibacterial functionalized coatings on Mg and its alloys for medical use—A Review. Coatings 2020, 10, 828. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Lastovka, V.V.; Belyavskaya, O.A.; Lychagin, D.V.; Schmidt, J.; Sharkeev, Y.P. Tailoring the surface morphology and the crystallinity state of Cu- and Zn-substituted hydroxyapatites on Ti and Mg-based alloys. Materials 2020, 13, 4449. [Google Scholar] [CrossRef] [PubMed]
- Cheon, K.H.; Park, C.; Kang, M.H.; Kang, I.G.; Lee, M.K.; Lee, H.; Kim, H.E.; Jung, H.D.; Jang, T.S. Construction of tantalum/poly(ether imide) coatings on magnesium implants with both corrosion protection and osseointegration properties. Bioact. Mater. 2021, 6, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Kaseem, M.; Ko, Y.G. Morphological modification and corrosion response of MgO and Mg3(PO4)2 composite formed on magnesium alloy. Compos. B Eng. 2019, 176, 107225. [Google Scholar] [CrossRef]
- Rahman, M.; Li, Y.; Wen, C. HA coating on Mg alloys for biomedical applications: A review. J. Magnes. Alloy. 2020, 8, 929–943. [Google Scholar] [CrossRef]
- Tian, Q.; Lin, J.; Rivera-Castaneda, L.; Tsanhani, A.; Dunn, Z.S.; Rodriguez, A.; Aslani, A.; Liu, H. Nano-to-submicron hydroxyapatite coatings for magnesium-based bioresorbable implants–deposition, characterization, degradation, mechanical properties, and cytocompatibility. Sci. Rep. 2019, 9, 810. [Google Scholar] [CrossRef]
- Albulescu, R.; Popa, A.C.; Enciu, A.M.; Albulescu, L.; Dudau, M.; Popescu, I.D.; Mihai, S.; Codrici, E.; Pop, S.; Lupu, A.R.; et al. Comprehensive in vitro testing of calcium phosphate-based bioceramics with orthopedic and dentistry applications. Materials 2019, 12, 3704. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Tolkacheva, T.V.; Sheikin, V.V.; Egorkin, V.S.; Mashtalyar, D.V.; Kazakbaeva, A.A.; Schmidt, J. Characterization of the Micro-Arc Coatings Containing β-Tricalcium Phosphate Particles on Mg-0.8Ca Alloy. Metals 2018, 8, 238. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Sharkeev, Y.P.; Tolkacheva, T.V.; Khimich, M.A.; Bakina, O.V.; Fomenko, A.N.; Kazakbaeva, A.A.; Fadeeva, I.V.; Egorkin, V.S.; Gnedenkov, S.V.; et al. Comparative Study of the structure, properties, and corrosion behavior of Sr-containing biocoatings on Mg0.8Ca. Materials 2020, 13, 1942. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Ugodchikova, A.V.; Uvarkin, P.V.; Chebodaeva, V.V.; Tolkacheva, T.V.; Schmidt, J.; Sharkeev, Y.P. Structural-morphological and adhesive properties of calcium-phosphate coatings formed on a magnesium alloy by micro-arc oxidation in an electrolyte containing disperse particles. Russ. Phys. J. 2021, 64, 830–837. [Google Scholar] [CrossRef]
- Chaharmahali, R.; Fattah-alhosseini, A.; Babaei, K. Surface characterization and corrosion behavior of calcium phosphate (Ca-P) base composite layer on Mg and its alloys using plasma electrolytic oxidation (PEO): A review. J. Magnes. Alloy. 2021, 9, 21–40. [Google Scholar] [CrossRef]
- Lazarev, D.M.; Farrakhov, R.G.; Mukaeva, V.R.; Kulyasova, O.B.; Parfenov, E.V.; Yerokhin, A.L. Growth kinetics and corrosion protection properties of plasma electrolytic oxidation coatings on biodegradable Mg−2% Sr alloy. Surf. Eng. Appl. Electrochem. 2020, 56, 83–92. [Google Scholar] [CrossRef]
- Yang, J.; Lu, X.; Blawert, C.; Di, S.; Zheludkevich, M.L. Microstructure and corrosion behavior of Ca/P coatings prepared on magnesium by plasma electrolytic oxidation. Surf. Coat. Techn. 2017, 319, 359–369. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Pillado, B.; Mohedano, M.; Arrabal, R.; Matykina, E. Calcium doped flash-PEO coatings for corrosion protection of Mg alloy. Metals 2020, 10, 916. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Echeverria, F.; Harmsen, M.C. Interaction of different cell types with magnesium modified by plasma electrolytic oxidation. Colloids Surf. B 2020, 193, 111153. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, C.; Wang, D.; Huang, D. Dissolution and precipitation behaviors of silicon-containing ceramic coating on Mg–Zn–Ca alloy in simulated body fluid. Colloids Surf. B 2014, 122, 746–751. [Google Scholar] [CrossRef]
- Palakurthy, S.; Reddy, K.V.G.; Samudrala, R.K.; Azeem, P.A. In vitro bioactivity and degradation behaviour of β-wollastonite derived from natural waste. Mat. Sci. Eng. C 2019, 98, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Hafezi, M.; Nezafati, N.; Heasarki, S.; Nadernezhad, A.; Ghazanfari, S.M.H.; Sepantafar, M. Bioinorganics in bioactive calcium silicate ceramics for bone tissue repair: Bioactivity and biological properties. J. Ceram. Sci. Techn. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Zakaria, M.Y.; Sulong, A.B.; Muhamad, N.; Raza, M.R.; Ramli, M.I. Incorporation of wollastonite bioactive ceramic with titanium for medical applications: An overview. Mat. Sci. Eng. C 2019, 97, 884–895. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Ugodchikova, A.V.; Tolkacheva, T.V.; Chebodaeva, V.V.; Cluklhov, I.A.; Khimich, M.A.; Bakina, O.V.; Lerner, M.I.; Egorkin, V.S.; Schmidt, J.; et al. Surface modification of Mg0.8Ca alloy via wollastonite micro-arc coatings: Significant improvement in corrosion resistance. Metals 2021, 11, 754. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, H.; Wu, C.; Chen, L.; Lin, X.; Naoki, K.; Chen, G.; Chang, J. Stimulatory effects of the ionic products from Ca–Mg–Si bioceramics on both osteogenesis and angiogenesis in vitro. Acta Biomater. 2013, 9, 8004–8014. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. Degradation, bioactivity, and cytocompatibility of diopside, akermanite, and bredigite ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 153–160. [Google Scholar] [CrossRef]
- Ni, S.; Chang, J.; Chou, L.; Zhai, W. Comparison of osteoblast-like cell responses to calcium silicate and tricalcium phosphate ceramics in vitro, Inc. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80B, 174–183. [Google Scholar] [CrossRef]
- Liu, Q.; Cen, L.; Yin, S.; Chen, L.; Liu, G.; Chang, J.; Cui, L. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and β-TCP ceramics. Biomaterials 2008, 29, 4792–4799. [Google Scholar] [CrossRef] [PubMed]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Jamil, M.; Elouahli, A.; Abida, F.; Khallok, H.; Gourri, E.; Kheribech, A.; Hatim, Z. Development of triphasic hydroxyapatite/(α and β)–tricalcium phosphate based composites by sintering powder of calcium–apatite in the presence of montmorillonite. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2489–2498. [Google Scholar] [CrossRef]
- Rosemann, P.; Schmidt, J.; Heyn, A. Short and long term degradation behaviour of Mg–1Ca magnesium alloys and protective coatings based on plasma-chemical oxidation and biodegradable polymercoating in synthetic body fluid. Mater. Corros. 2013, 64, 714–722. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Sharkeev, Y.P.; Komarova, E.G.; Khlusov, I.A.; Chebodaeva, V.V. Structure and properties of the wollastonite–calcium phosphate coatings deposited on titanium and titanium–niobium alloy using micro-arc oxidation method. Surf. Coat. Techn. 2016, 307, 1274–1283. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Tolkacheva, T.V.; Rau, J.V.; Buyko, E.E.; Ivanov, V.V.; Sheikin, V.V. Modification of titanium surface via Ag-, Sr- and Si-containing micro-arc calcium phosphate coating. Bioact. Mater. 2019, 4, 224–235. [Google Scholar] [CrossRef]
- Bragg, W.L.; Claringbull, G.F. Crystal Structure of Minerals; Cornell University Press: Ithaca, NY, USA, 1965; Volume 4. [Google Scholar]
- ASTM G59–97; Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. ASTM International: West Conshohocken, PA, USA, 2009.
- Shi, Z.; Atrens, A. An innovative specimen configuration for the study of Mg corrosion. Corros. Sci. 2011, 53, 226–246. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, J.M.; Wei, F.; Guo, J.; Guillem-Marti, J.; Lin, Z.; Ginebra, M.P.; Xiao, Y. The effect of biomimetic calcium deficient hydroxyapatite and sintered β-tricalcium phosphate on osteoimmune reaction and osteogenesis. Acta Biomater. 2019, 96, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Somemura, S.; Kumai, T.; Yatabe, K.; Sasaki, C.; Fujiya, H.; Niki, H.; Yudoh, K. Physiologic Mechanical Stress Directly Induces Bone Formation by Activating Glucose Transporter 1 (Glut 1) in Osteoblasts, Inducing Signaling via NAD+-Dependent Deacetylase (Sirtuin 1) and Runt-Related Transcription Factor 2 (Runx2). Int. J. Mol. Sci. 2021, 22, 9070. [Google Scholar] [CrossRef]
- Mortazavi, G.; Jiang, J.; Meletis, E.I. Investigation of the plasma electrolytic oxidation mechanism of titanium. Appl. Surf. Sci. 2019, 488, 370–382. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Babaei, K. Effect of particles addition to solution of plasma electrolytic oxidation (PEO) on the properties of PEO coatings formed on magnesium and its alloys: A review. J. Magnes. Alloy. 2020, 8, 799–818. [Google Scholar] [CrossRef]
- Lee, K.M.; Shin, K.R.; Namgung, S.; Yoo, B.; Shin, D.H. Electrochemical response of ZrO2-incorporated oxide layer on AZ91 Mg alloy processed by plasma electrolytic oxidation. Surf. Coat. Techn. 2011, 205, 3779–3784. [Google Scholar] [CrossRef]
- Osborn, E.F.; Muan, A.; Levin, E.M.; Robbins, C.R.; McMurdie, H.F. Phase Diagrams for Ceramists. J. Am. Ceram. Soc. 1978, 496, 257. [Google Scholar]
- Dorozhkin, S.V. Multiphasic calcium orthophosphate (CaPO4) bioceramics and their biomedical applications. Ceram. Int. 2016, 42, 6529–6554. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl. Surf. Sci. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Khan, R.H.U.; Yerokhin, A.L.; Li, X.; Dong, H.; Matthews, A. Influence of current density and electrolyte concentration on DC PEO titania coatings. Surf. Eng. 2014, 30, 102–108. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Synthetic amorphous calcium phosphates (ACPs): Preparation, structure, properties, and biomedical applications. R. Soc. Chem. Biomater. Sci. 2021, 9, 7748–7798. [Google Scholar] [CrossRef]
- Polunin, A.V.; Cheretaeva, A.O.; Borgardt, E.D.; Rastegaev, I.A.; Krishtal, M.M.; Katsman, A.V.; Yasnikov, I.S. Improvement of oxide layers formed by plasma electrolytic oxidation on cast Al–Si alloy by incorporating TiC nanoparticles. Surf. Coat. Technol. 2021, 423, 127603. [Google Scholar] [CrossRef]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Guo, Y.Q.; Xiang, N.; Xiong, Y.; Hu, Q.; Song, R.G. A study on microstructure and corrosion resistance of ZrO2-containingPEO coatings formed on AZ31 Mg alloy in phosphate-based electrolyte. Appl. Surf. Sci. 2015, 357, 1463–1471. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, B.U.; Yoon, S.I.; Lee, E.S.; Yoo, B.; Shin, D.H. Evaluation of plasma temperature during plasma oxidation processing of AZ91 Mg alloy through analysis of the melting behavior of incorporated particles. Electrochim. Acta 2012, 67, 6–11. [Google Scholar] [CrossRef]
- Lu, X.; Sah, S.P.; Scharnagl, N.; Störmer, M.; Starykevich, M.; Mohedano, M.; Blawert, C.; Zheludkevich, M.L.; Kainer, K.U. Degradation behavior of PEO coating on AM50 magnesium alloy produced from electrolytes with clay particle addition. Surf. Coat. Technol. 2015, 269, 155–169. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Warchoł, F.; Oleshko, O.; Husak, Y.; Kazek-Kęsik, A.; Korniienko, V.; Deineka, V.; Sowa, M.; Maciej, A.; Michalska, J.; et al. Effects of the sources of calcium and phosphorus on the structural and functional properties of ceramic coatings on titanium dental implants produced by plasma electrolytic oxidation. Mater. Sci. Eng. C 2021, 119, 111607. [Google Scholar] [CrossRef]




















| Compound | The Content of Compounds in the Electrolyte, g/L | ||
|---|---|---|---|
| I | II | III | |
| CaSiO3 | - | 30–60 | 20–40 |
| β-Ca3(PO4)2 | 30–70 | - | 20–40 |
| Na2HPO4·12H2O | 30–50 | - | - |
| NaOH | 5 | 5 | 5 |
| Na2SiO3 | - | 10 | 10 |
| NaF | 3 | 3 | 3 |
| Coating symbol | TC | WS | TW |
| Elements, at.% | Marked Zones | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |
| O Kα | 50.2 | 62.0 | 63.9 | 63.6 | 57.2 | 59.8 | 58.2 |
| Mg Kα | 6.6 | 17.2 | 0.7 | 11.1 | 1.8 | 2.0 | 20.5 |
| P Kα | 20.3 | 15.2 | - | - | 16.3 | 3.3 | 4.4 |
| Ca Kα | 22.9 | 5.6 | 16.7 | 6.4 | 22.2 | 19.7 | 5.7 |
| Si Kα | - | - | 18.7 | 18.9 | 2.5 | 15.2 | 11.2 |
| The Coatings | Critical Load, Lc, N | ||
|---|---|---|---|
| 350 V | 450 V | 500 V | |
| TC | 11.4 ± 0.9 | 17.0 ± 0.2 | 19.2 ± 0.3 |
| WS | 10.1 ± 0.4 | 12.9 ± 0.2 | 16.4 ± 0.2 |
| TW | 10.7 ± 0.8 | 16.4 ± 1.0 | 17.8 ± 0.4 |
| Sample (Applied Voltage, V) | Ec, V (vs. Ag/AgCl) | ic, A cm−2 | Rp, Ω cm2 |
|---|---|---|---|
| Mg0.8Ca | −1.46 | 1.3 × 10−4 | 3.1 × 102 |
| TC (350) | −1.65 | 1.8 × 10−6 | 2.0 × 104 |
| TC (500) | −1.58 | 7.2 × 10−7 | 4.6 × 104 |
| WS (350) | −1.44 | 4.8 × 10−7 | 5.2 × 104 |
| WS (500) | −1.41 | 1.8 × 10−7 | 1.2 × 105 |
| TW (350) | −1.41 | 1.1 × 10−5 | 1.9 × 103 |
| TW (500) | −1.44 | 7.0 × 10−6 | 3.5 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedelnikova, M.; Bakina, O.; Ugodchikova, A.; Tolkacheva, T.; Khimich, M.; Uvarkin, P.; Kashin, A.; Miller, A.; Egorkin, V.; Schmidt, J.; et al. The Role of Microparticles of β-TCP and Wollastonite in the Creation of Biocoatings on Mg0.8Ca Alloy. Metals 2022, 12, 1647. https://doi.org/10.3390/met12101647
Sedelnikova M, Bakina O, Ugodchikova A, Tolkacheva T, Khimich M, Uvarkin P, Kashin A, Miller A, Egorkin V, Schmidt J, et al. The Role of Microparticles of β-TCP and Wollastonite in the Creation of Biocoatings on Mg0.8Ca Alloy. Metals. 2022; 12(10):1647. https://doi.org/10.3390/met12101647
Chicago/Turabian StyleSedelnikova, Mariya, Olga Bakina, Anna Ugodchikova, Tatiana Tolkacheva, Margarita Khimich, Pavel Uvarkin, Alexander Kashin, Andrey Miller, Vladimir Egorkin, Juergen Schmidt, and et al. 2022. "The Role of Microparticles of β-TCP and Wollastonite in the Creation of Biocoatings on Mg0.8Ca Alloy" Metals 12, no. 10: 1647. https://doi.org/10.3390/met12101647
APA StyleSedelnikova, M., Bakina, O., Ugodchikova, A., Tolkacheva, T., Khimich, M., Uvarkin, P., Kashin, A., Miller, A., Egorkin, V., Schmidt, J., & Sharkeev, Y. (2022). The Role of Microparticles of β-TCP and Wollastonite in the Creation of Biocoatings on Mg0.8Ca Alloy. Metals, 12(10), 1647. https://doi.org/10.3390/met12101647








