Paste-Injection of Low-Density Barium Hexaferrite Magnets with Soft Magnetic Iron Phase
Abstract
:1. Introduction
2. Materials and Methods
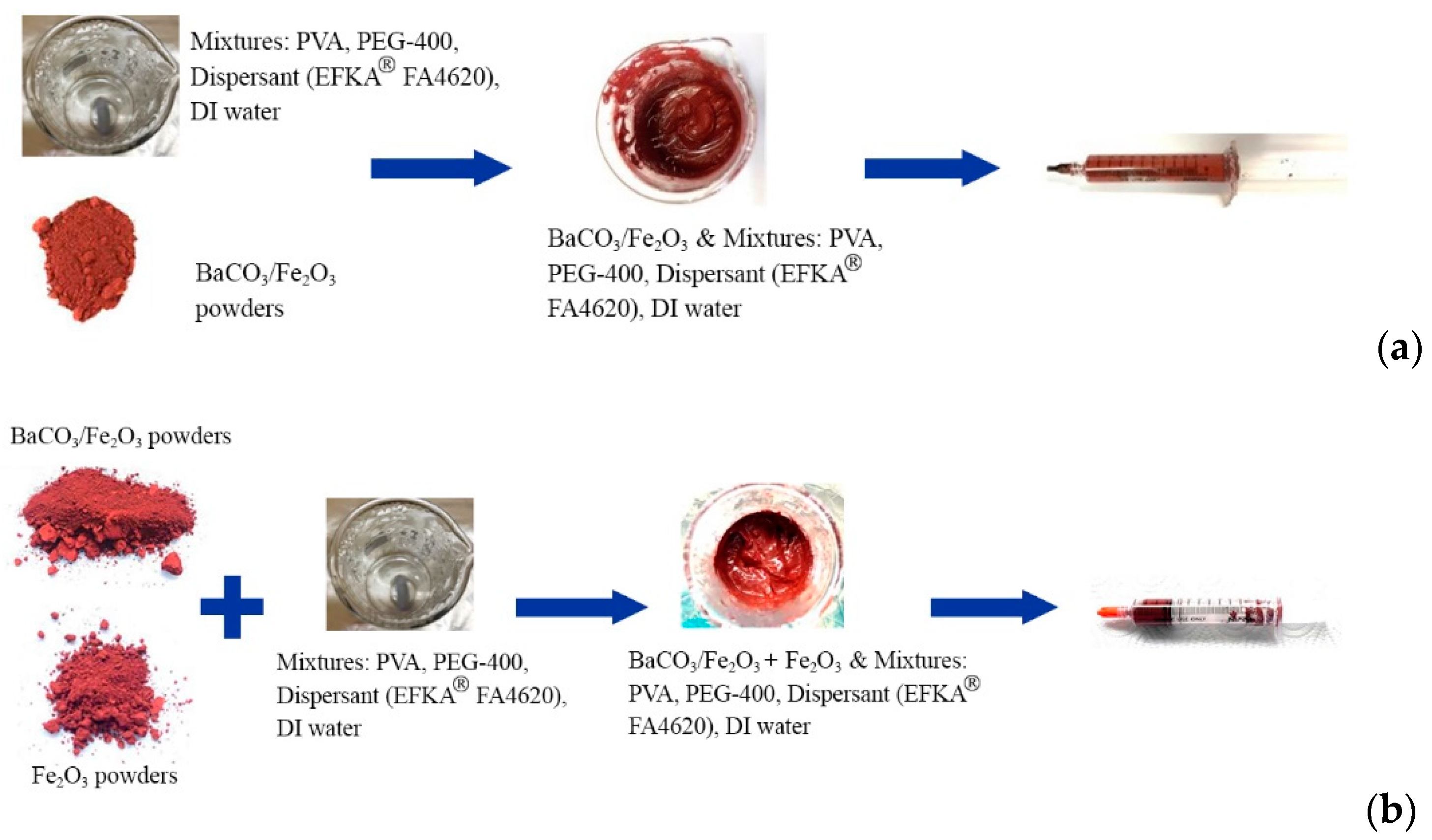
2.1. Manufacturing of Hard/Soft Magnetic Composites
2.2. Characterization of Hard/Soft Magnetic Composites
3. Results and Discussion
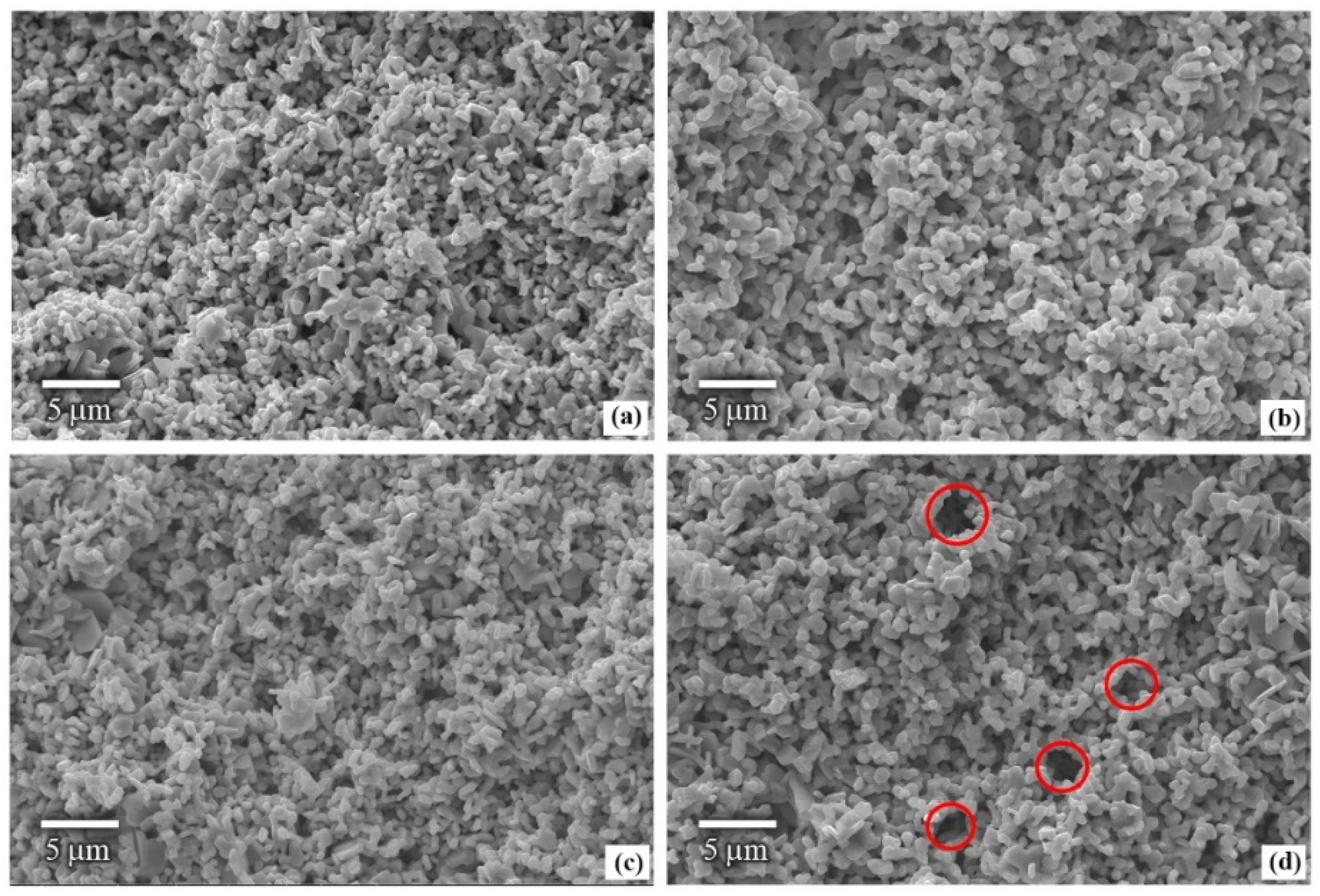
3.1. Density and Morphology of Hard/Soft Magnetic Composites
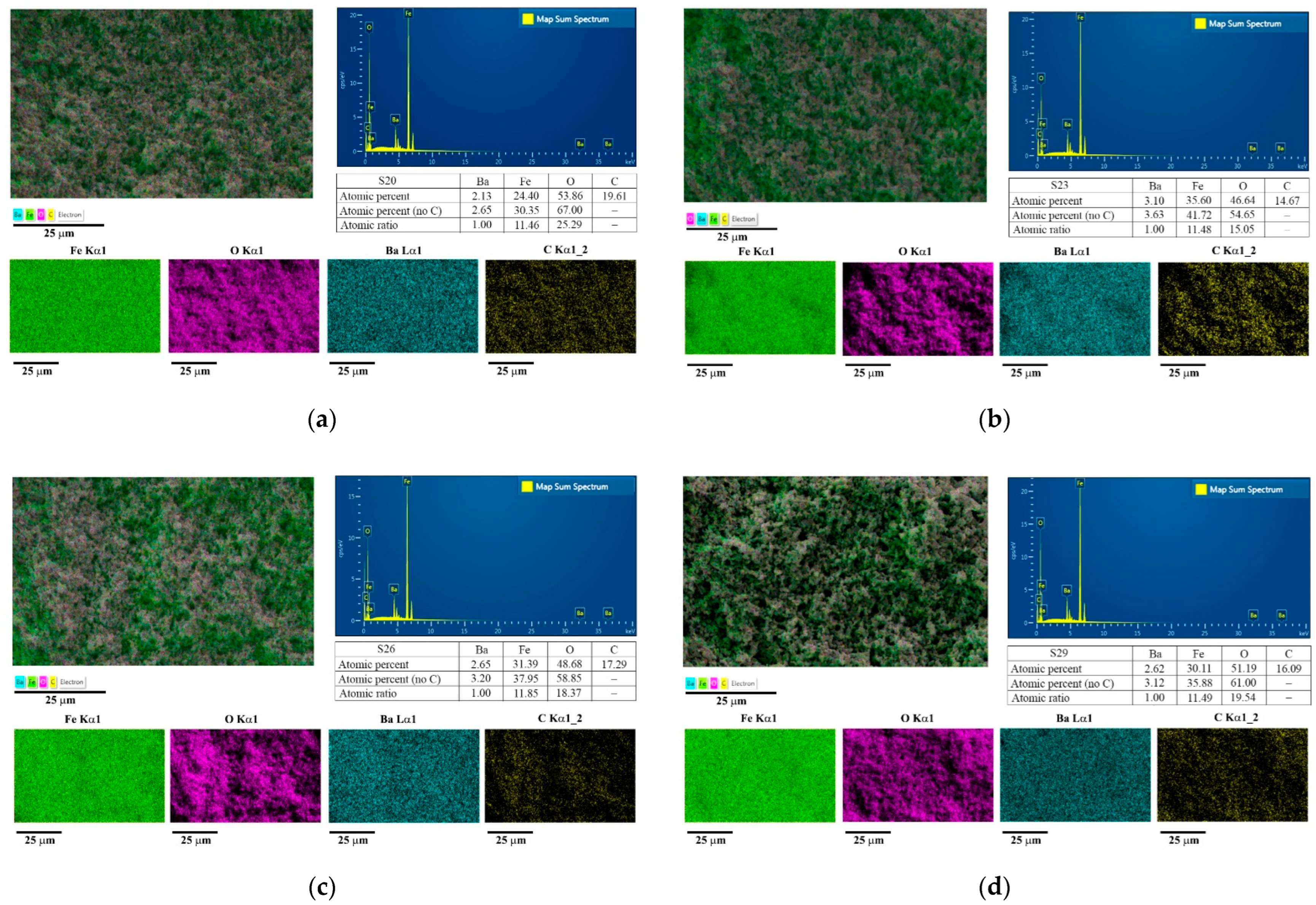
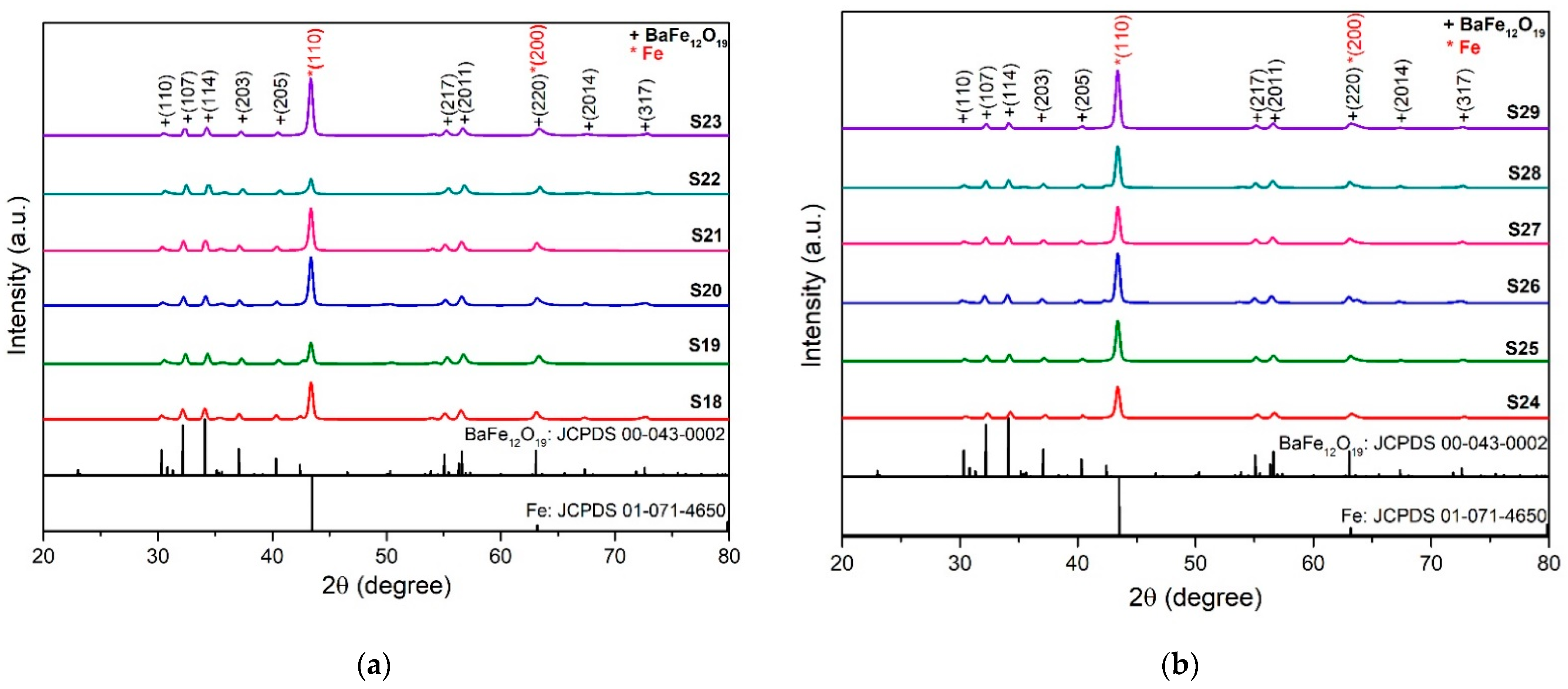
3.2. Phase and Elemental Composition of Hard/Soft Magnetic Composites
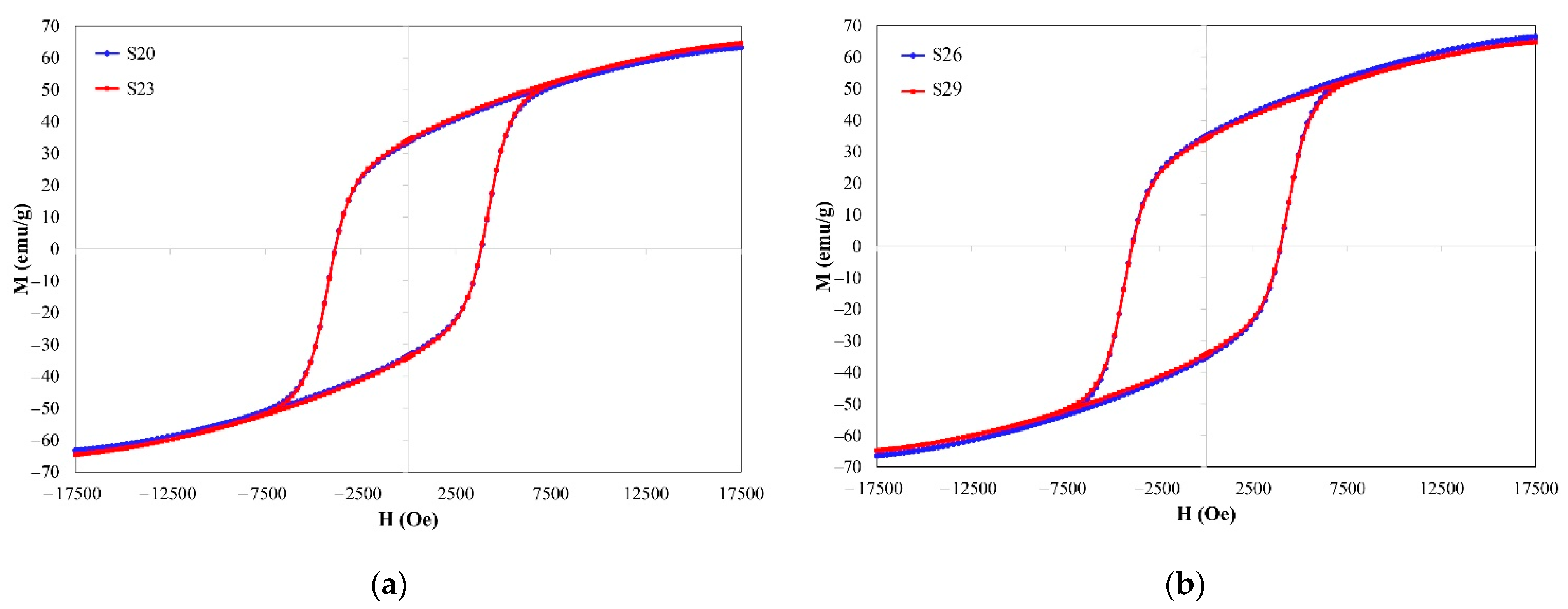
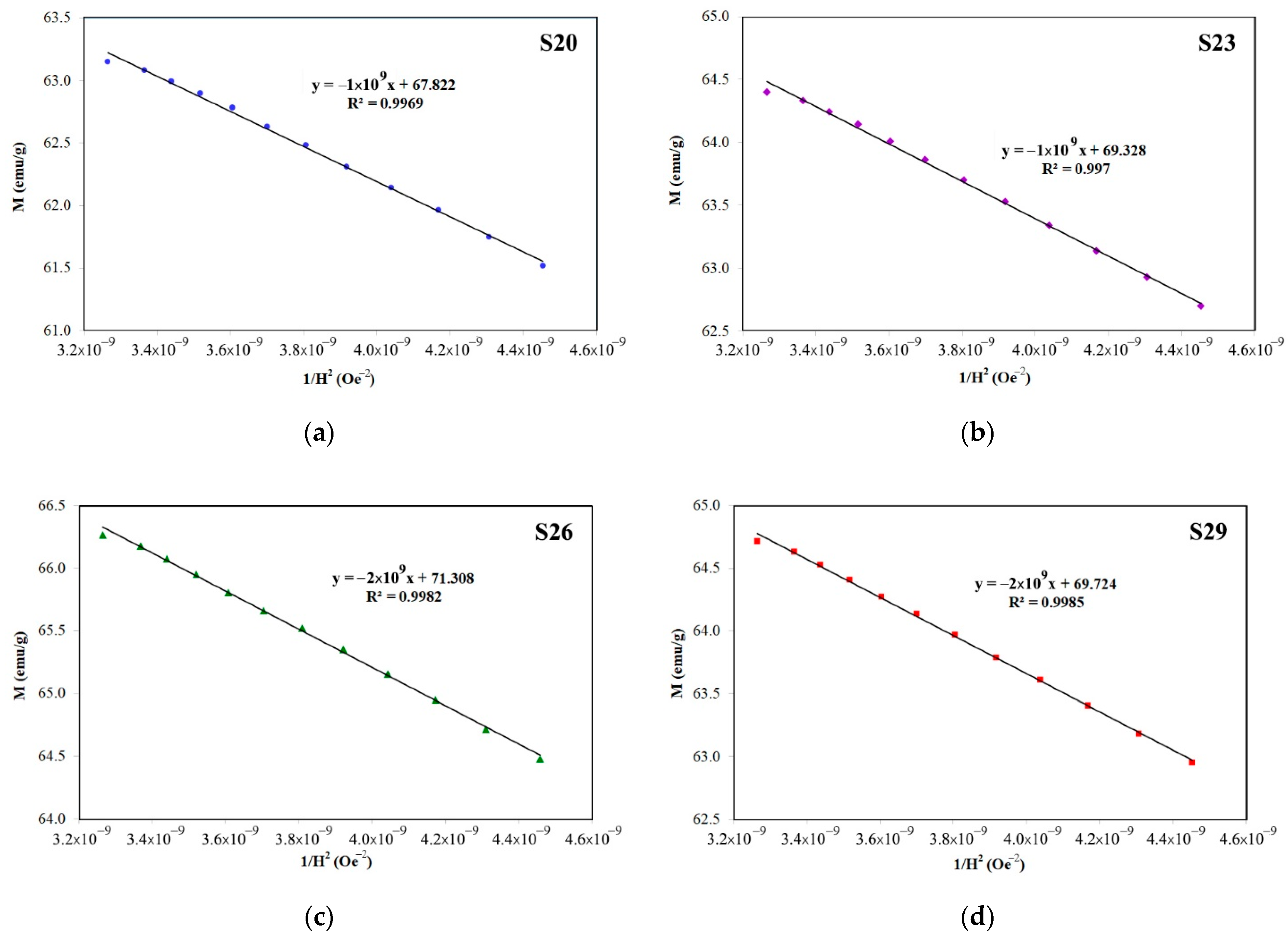
3.3. Magnetic Properties of Hard/Soft Magnetic Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Post, B.; Kunc, V.; Elliott, A.M.; Paranthaman, M.P. Additive manufacturing of near-net-shape bonded magnets: Prospects and challenges. Scr. Mater. 2017, 135, 100–104. [Google Scholar] [CrossRef]
- Périgo, E.A.; Jacimovic, J.; Ferré, F.G.; Scherf, L.M. Additive manufacturing of magnetic materials. Addit. Manuf. 2019, 30, 100870. [Google Scholar] [CrossRef]
- Chaudhary, V.; Mantri, S.A.; Ramanujan, R.V.; Banerjee, R. Additive manufacturing of magnetic materials. Prog. Mater. Sci. 2020, 114, 100688. [Google Scholar] [CrossRef]
- Merazzo, K.J.; Lima, A.C.; Rincón-Iglesias, M.; Fernandes, L.C.; Pereira, N.; Lanceros-Mendez, S.; Martins, P. Magnetic materials: A journey from finding north to an exciting printed future. Mater. Horiz. 2021, 8, 2654–2684. [Google Scholar] [CrossRef]
- Wei, X.; Jin, M.-L.; Yang, H.; Wang, X.-X.; Long, Y.-Z.; Chen, Z. Advances in 3D printing of magnetic materials: Fabrication, properties, and their applications. J. Adv. Ceram. 2022, 11, 665–701. [Google Scholar] [CrossRef]
- White, E.; Rinko, E.; Prost, T.; Horn, T.; Ledford, C.; Rock, C.; Anderson, I. Processing of alnico magnets by additive manufacturing. Appl. Sci. 2019, 9, 4843. [Google Scholar] [CrossRef] [Green Version]
- Rottmann, P.F.; Polonsky, A.T.; Francis, T.; Emigh, M.G.; Krispin, M.; Rieger, G.; Echlin, M.P.; Levi, C.G.; Pollock, T.M. TriBeam tomography and microstructure evolution in additively manufactured alnico magnets. Mater. Today 2021, 49, 23–34. [Google Scholar] [CrossRef]
- Palmero, E.M.; Rial, J.; de Vicente, J.; Camarero, J.; Skårman, B.; Vidarsson, H.; Larsson, P.O.; Bollero, A. Development of permanent magnet MnAlC/polymer composites and flexible filament for bonding and 3D-printing technologies. Sci. Technol. Adv. Mater. 2018, 19, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Palmero, E.M.; Casaleiz, D.; de Vicente, J.; Skårman, B.; Vidarsson, H.; Larsson, P.O.; Bollero, A. Effect of particle size distribution on obtaining novel MnAlC-based permanent magnet composites and flexible filaments for 3D-printing. Addit. Manuf. 2020, 33, 101179. [Google Scholar] [CrossRef]
- De Julián Fernández, C.; Sangregorio, C.; de la Figuera, J.; Belec, B.; Makovec, D.; Quesada, A. Progress and prospects of hard hexaferrites for permanent magnet applications. J. Phys. D Appl. Phys. 2021, 54, 153001. [Google Scholar] [CrossRef]
- Granados-Miralles, C.; Jenuš, P. On the potential of hard ferrite ceramics for permanent magnet technology: A review on sintering strategies. J. Phys. D Appl. Phys. 2021, 54, 303001. [Google Scholar] [CrossRef]
- Hanemann, T.; Syperek, D.; Nötzel, D. 3D printing of ABS barium ferrite composites. Materials 2020, 13, 1481. [Google Scholar] [CrossRef] [Green Version]
- Palmero, E.M.; Casaleiz, D.; Jiménez, N.A.; Rial, J.; de Vicente, J.; Nieto, A.; Altimira, R.; Bollero, A. Magnetic-polymer composites for bonding and 3D printing of permanent magnets. IEEE Trans. Magn. 2019, 55, 2101004. [Google Scholar] [CrossRef]
- Peng, E.; Wei, X.; Herng, T.S.; Garbe, U.; Yub, D.; Ding, J. Ferrite-based soft and hard magnetic structures by extrusion free-forming. RSC Adv. 2017, 7, 27128–27138. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Liu, Y.; Zhao, D.; Mao, X.; Jiang, W.; Ge, S.S. Net-shaped barium and strontium ferrites by 3D printing with enhanced magnetic performance from milled powders. J. Magn. Magn. Mater. 2020, 493, 165664. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, X.; Guo, Z.; Volinsky, A.A. 3D gel printing of Sr ferrite parts. Ceram. Int. 2018, 44, 22370–22377. [Google Scholar] [CrossRef]
- Thongsamrit, W.; Jantaratana, P.; Charoensuk, T.; Sirisathitkul, C. Enhanced coercivity of low-density barium hexaferrite magnets from paste-injection molding. Magnetochemistry 2022, 8, 46. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, X.; Guo, Z.; Ye, S.; Sui, Y.; Volinsky, A.A. 3D printing of NdFeB bonded magnets with SrFe12O19 addition. J. Alloys Compd. 2019, 779, 900–907. [Google Scholar] [CrossRef]
- Kneller, E.F.; Hawig, R. The exchange-spring magnet: A new material principle for permanent magnets. IEEE Trans. Magn. 1991, 27, 3588–3600. [Google Scholar] [CrossRef]
- Sam, S.A.; Balan, A.P.; Kaipamangalath, A.; Varma, M.R.; Nair, R.R.; Thomas, S. Nanocomposite permanent magnets based on SrFe12O19-Fe3O4. J. Supercond. Novel Magn. 2021, 34, 3333–3344. [Google Scholar] [CrossRef]
- Greculeasa, S.G.; Comanescu, C.; Iacob, N.; Kuncser, A. Exchange coupled nanocomposites: Magnetoplumbite Sr ferrite and magnetite. Rom. J. Phys. 2022, 67, 606. [Google Scholar]
- Tavakolinia, F.; Yousefi, M.; Afghahi, S.S.S.; Baghshahi, S.; Samadi, S. Synthesis of novel hard/soft ferrite composites particles with improved magnetic properties and exchange coupling. Proc. Appl. Ceram. 2018, 12, 248–256. [Google Scholar] [CrossRef]
- Xia, J.; Wu, X.; Huang, Y.; Wu, W.; Liang, J.; Li, Q. Enhancements of saturation magnetization and coercivity in Ni0.5Zn0.5Fe2O4/SrFe12O19 composite powders by exchange-coupling mechanism. J. Mater. Sci. Mater. Electron. 2019, 30, 11682–11693. [Google Scholar] [CrossRef]
- Jenuš, P.; Ucakar, A.; Repše, S.; Sangregorio, C.; Petrecca, M.; Albino, M.; Cabassi, R.; de Julian Fernandez, C.; Belec, B. Magnetic performance of SrFe12O19–Zn0.2Fe2.8O4 hybrid magnets prepared by spark plasma sintering. J. Phys. D Appl. Phys. 2021, 54, 204002. [Google Scholar] [CrossRef]
- Martínez García, R.; Bilovola, V.; Ferrari, S.; de la Presa, P.; Marín, P.; Pagnola, M. Structural and magnetic properties of a BaFe12O19/NiFe2O4 nanostructured composite depending on different particle size ratios. J. Magn Magn. Mater. 2022, 547, 168934. [Google Scholar] [CrossRef]
- Maltoni, P.; Sarkar, T.; Varvaro, G.; Barucca, G.; Ivanov, S.A.; Peddis, D.; Mathieu, R. Towards bi-magnetic nanocomposites as permanent magnets through the optimization of the synthesis and magnetic properties of SrFe12O19 nanocrystallites. J. Phys. D Appl. Phys. 2021, 54, 124004. [Google Scholar] [CrossRef]
- Dhabekar, K.; Kant, K.M. Magnetic and dielectric response of CoFe2O4-SrFe12O19 nanocomposites. J. Supercond. Nov. Magn. 2021, 34, 907–912. [Google Scholar] [CrossRef]
- Davarpanah, A.M.; Rahdar, A.; Dastnae, M.A.; Zeybek, O.; Beyzaei, H. (1−x)BaFe12O19/xCoFe2O4 hard/soft magnetic nanocomposites: Synthesis, physical characterization, and antibacterial activities study. J. Mol. Struct. 2019, 1175, 445–449. [Google Scholar] [CrossRef]
- Polley, K.; Alam, T.; Bera, J. Synthesis and characterization of BaFe12O19-CoFe2O4 ferrite composite for high-frequency antenna application. J. Aust. Ceram. Soc. 2020, 56, 1179–1186. [Google Scholar] [CrossRef]
- Rifai, L.; Fattouh, F.; Habanjar, K.; Yaacoub, N.; Awad, R. Exchange spring behaviour in BaFe12O19/CoFe2O4 magnetic nanocomposites. J. Alloys Compd. 2021, 868, 159072. [Google Scholar] [CrossRef]
- Manglam, M.K.; Kar, M. Tuning of reduced remanent and (BH)max by exchange spring phenomenon in ferrimagnetic composite. J. Magn. Magn. Mater. 2022, 560, 169569. [Google Scholar] [CrossRef]
- Hooda, N.; Sharma, R.; Hooda, A.; Khasa, S. Structural refinement, dielectric and spin exchange magnetic analysis of (1−x) BaFe12O19-(x)CoFe2O4 composites. Phys. B Condens. Matter 2022, 643, 414191. [Google Scholar] [CrossRef]
- Pubby, K.; Sharma, P.; Narang, S.B. Structural, magnetic, dielectric, microwave absorption, and optical characterization of Ni0.1Co0.9(MnZr)xFe2−2xO4/BaySr1−yFe12O19 nanocomposites. J. Mater. Sci. Mater. Electron. 2020, 31, 599–609. [Google Scholar] [CrossRef]
- Algarou, N.A.; Slimani, Y.; Almessiere, M.A.; Sadaqat, A.; Trukhanov, A.V.; Gondal, M.A.; Hakeem, A.S.; Trukhanov, S.V.; Vakhitov, M.G.; Klygach, D.S.; et al. Functional Sr0.5Ba0.5Sm0.02Fe11.98O4/x(Ni0.8Zn0.2Fe2O4) hard–soft ferrite nanocomposites: Structure, magnetic and microwave properties. Nanomaterials 2020, 10, 2134. [Google Scholar] [CrossRef] [PubMed]
- Hirian, R.; Bolinger, A.; Isnard, O.; Pop, V. Influence of high anisotropy phase on the properties of hard–soft magnetic nanocomposite powders obtained by mechanical milling. Powder Metall. 2018, 61, 369–373. [Google Scholar] [CrossRef]
- Xu, X.; Hong, Y.-K.; Park, J.; Lee, W.; Lane, A.M. Ex situ synthesis of magnetically exchange coupled SrFe12O19/Fe-Co composites. AIP Adv. 2016, 6, 056026. [Google Scholar] [CrossRef] [Green Version]
- Volodchenkov, A.D.; Kodera, Y.; Garay, J.E. Nanoscale integration of oxides and metals in bulk 3D composites: Leveraging SrFe12O19/Co interfaces for magnetic exchange coupling. J. Mater. Sci. 2019, 54, 8276–8288. [Google Scholar] [CrossRef] [Green Version]
- Molaei, M.J.; Ataie, A.; Raygan, S.; Picken, S.J.; Mendes, E.; Tichelaar, F.D. Synthesis and characterization of BaFe12O19/Fe3O4 and BaFe12O19/Fe/Fe3O4 magnetic nano-composites. Powder Technol. 2012, 221, 292–295. [Google Scholar] [CrossRef]
- Charoensuk, T.; Thongsamrit, W.; Ruttanapun, C.; Jantaratana, P.; Sirisathitkul, C. Loading effect of sol-gel derived barium hexaferrite on magnetic polymer composites. Nanomaterials 2021, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Devi, E.C.; Soibam, I. Law of approach to saturation in Mn–Zn ferrite nanoparticles. J. Supercond. Nov. Magn. 2019, 32, 1293–1298. [Google Scholar] [CrossRef]
- Hu, B.; Su, Z.; Bennett, S.; Chen, Y.; Harris, V.G. Epitaxial growth of 100-μm thick M-type hexaferrite crystals on wide bandgap semiconductor GaN/Al2O3 substrates. J. Appl. Phys. 2014, 115, 17A513. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Pub. Co.: Boston, MA, USA, 1956. [Google Scholar]
| Formulation/Samples | BaCO3/Fe2O3 (g) | Additional Fe2O3 (g) | PVA (mg) | PEG-400 (mg) | EFKA®4620 (mg) | DI Water (mL) | Density of Magnets (g/cm3) |
|---|---|---|---|---|---|---|---|
| I/S18–S20 | 15 | - | 287.0 | 150 | 40 | 4.6 | 2.34 ± 0.03 |
| II/S21–S23 | 15 | - | 143.5 | 75 | 40 | 4.6 | 2.28 ± 0.05 |
| III/S24–S26 | 14.85 | 0.15 | 574 | 300 | 40 | 4.6 | 2.15 ± 0.02 |
| IV/S27–S29 | 14.25 | 0.75 | 574 | 300 | 40 | 4.6 | 2.11 ± 0.06 |
| Samples | Synthesis Composition | Magnetic Properties | ||||
|---|---|---|---|---|---|---|
| Hc (Oe) | Mr (emu/g) | Ms (emu/g) | Mr/Ms | (BH)max (MGOe) | ||
| S18–S20 | BaCO3 + Fe2O3 (287 mg PVA, 150 mg PEG) | 3857 ± 14 | 33.10 ± 0.32 | 67.21 ± 0.54 | 0.492 | 0.215 ± 0.008 |
| S21–S23 | BaCO3 + Fe2O3 (143.5 mg PVA, 75 mg PEG) | 3854 ± 30 | 33.63 ± 0.54 | 68.34 ± 1.23 | 0.492 | 0.210 ± 0.007 |
| S24–S26 | BaCO3 + Fe2O3:Fe2O3 (99:1) (574 mg PVA, 300 mg PEG) | 3942 ± 13 | 34.85 ± 0.31 | 70.85 ± 0.40 | 0.492 | 0.202 ± 0.004 |
| S27–S29 | BaCO3 + Fe2O3:Fe2O3 (95:5) (574 mg PVA, 300 mg PEG) | 3945 ± 10 | 33.59 ± 0.59 | 68.57 ± 1.01 | 0.490 | 0.182 ± 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongsamrit, W.; Jantaratana, P.; Charoensuk, T.; Sirisathitkul, C. Paste-Injection of Low-Density Barium Hexaferrite Magnets with Soft Magnetic Iron Phase. Metals 2022, 12, 1659. https://doi.org/10.3390/met12101659
Thongsamrit W, Jantaratana P, Charoensuk T, Sirisathitkul C. Paste-Injection of Low-Density Barium Hexaferrite Magnets with Soft Magnetic Iron Phase. Metals. 2022; 12(10):1659. https://doi.org/10.3390/met12101659
Chicago/Turabian StyleThongsamrit, Wannisa, Pongsakorn Jantaratana, Thanida Charoensuk, and Chitnarong Sirisathitkul. 2022. "Paste-Injection of Low-Density Barium Hexaferrite Magnets with Soft Magnetic Iron Phase" Metals 12, no. 10: 1659. https://doi.org/10.3390/met12101659
APA StyleThongsamrit, W., Jantaratana, P., Charoensuk, T., & Sirisathitkul, C. (2022). Paste-Injection of Low-Density Barium Hexaferrite Magnets with Soft Magnetic Iron Phase. Metals, 12(10), 1659. https://doi.org/10.3390/met12101659






