Wear-Resistant Fe6AlCoCrNi Medium-Entropy Alloy Coating Made by Laser Cladding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the MEA Coating
2.2. Test Methods
3. Results
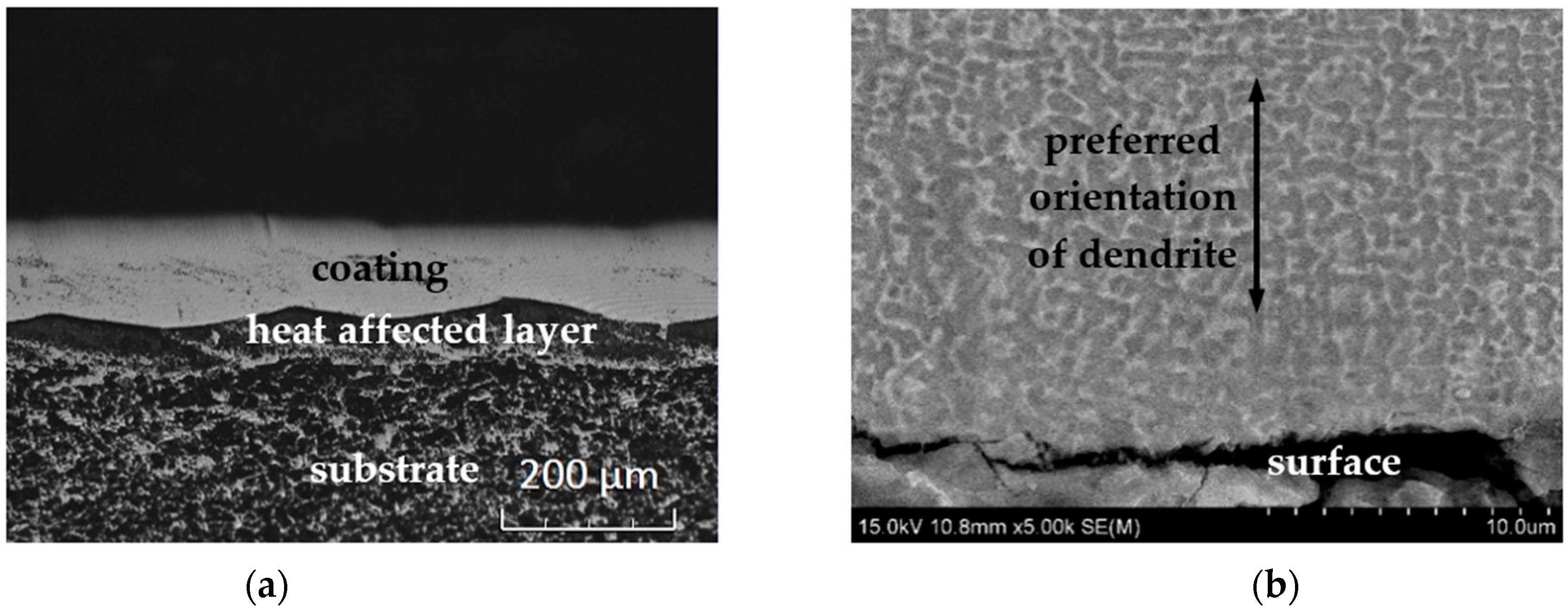
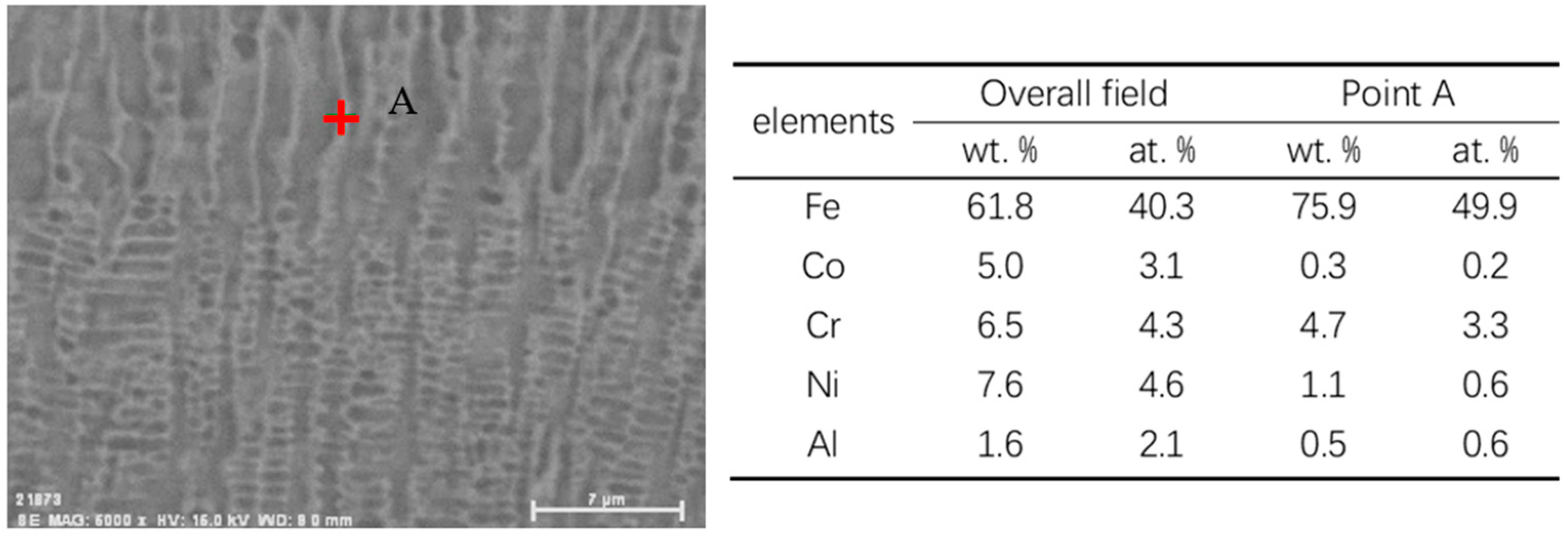
3.1. Morphology, Metallographic Structure, and Phase Composition
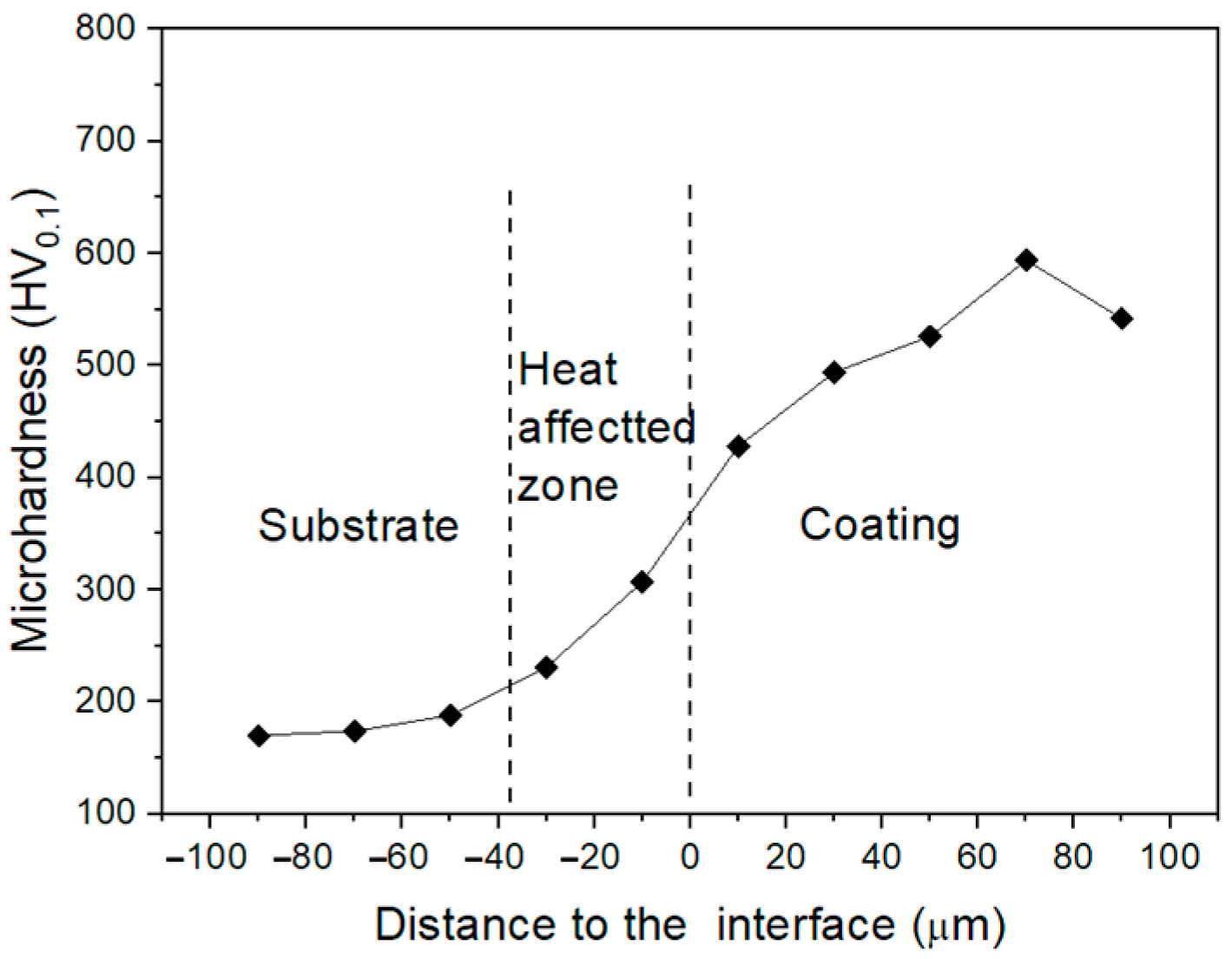
3.2. Hardness
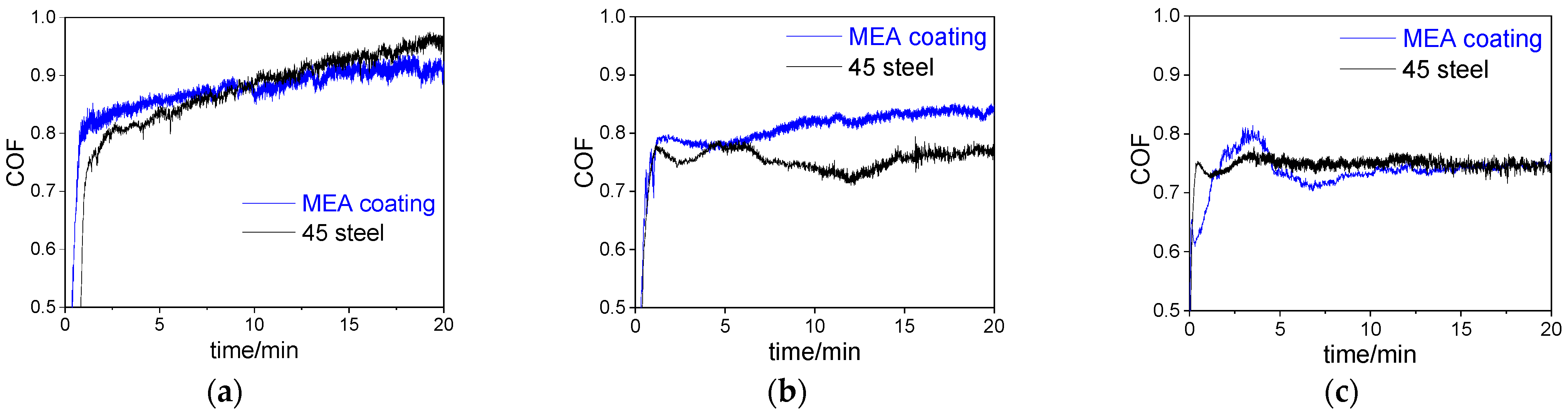
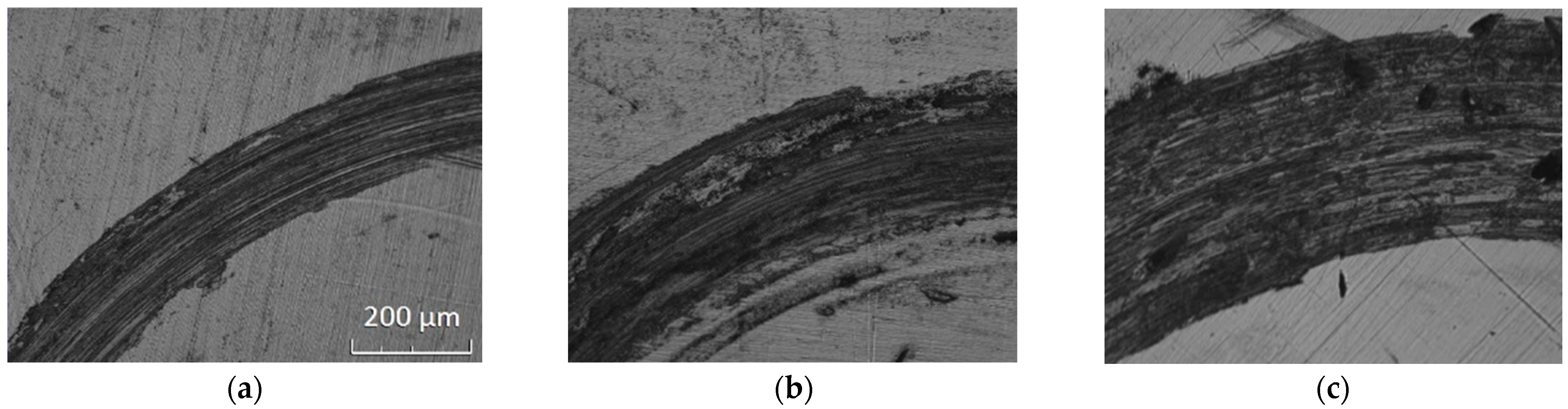
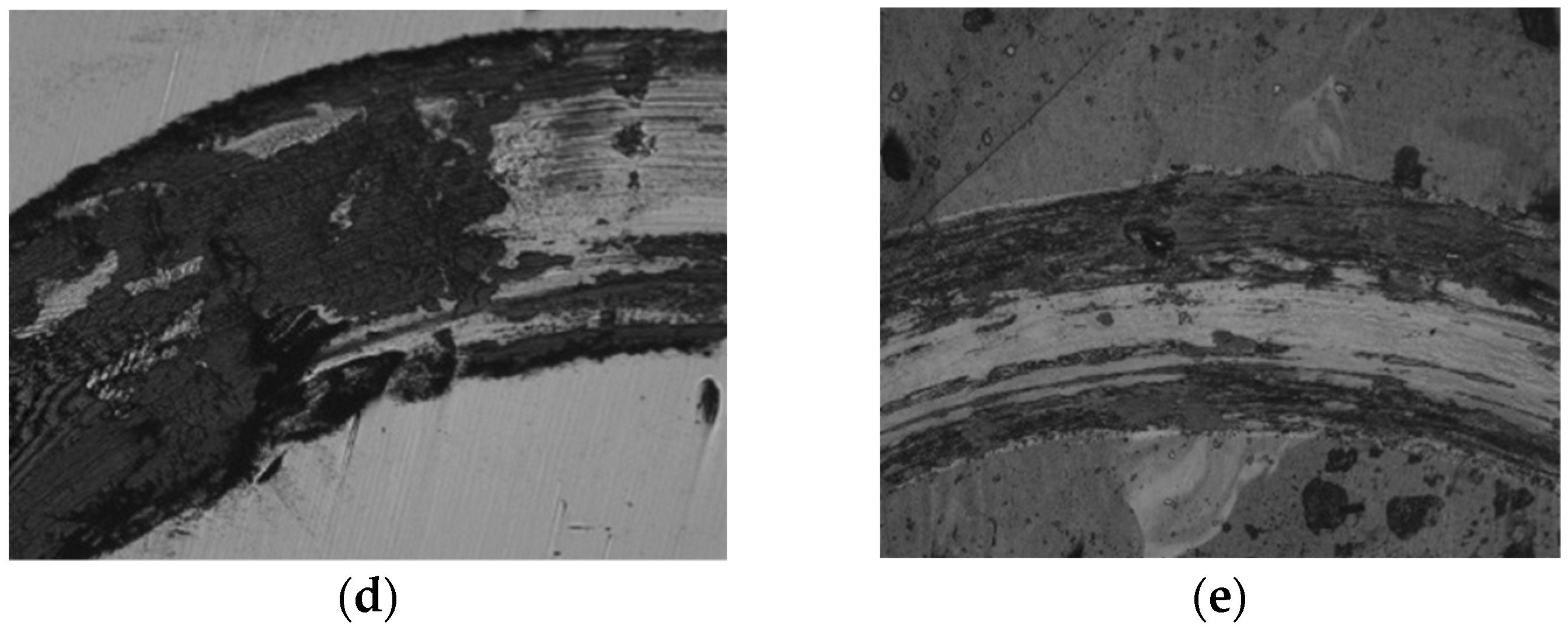
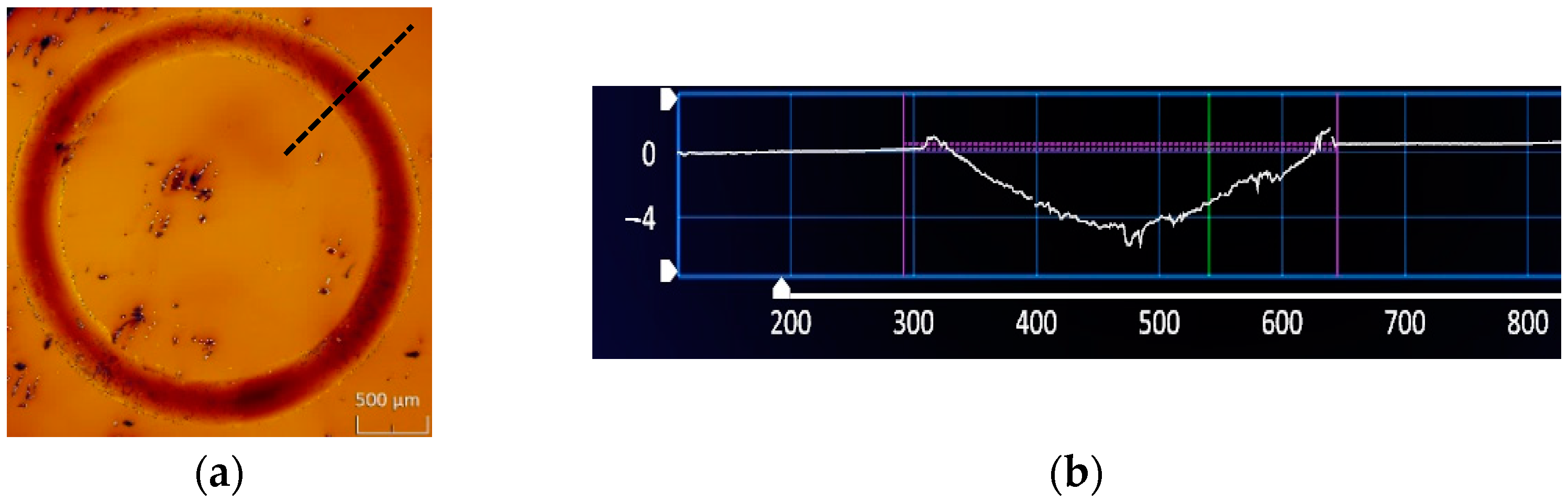
3.3. Friction and Wear Properties
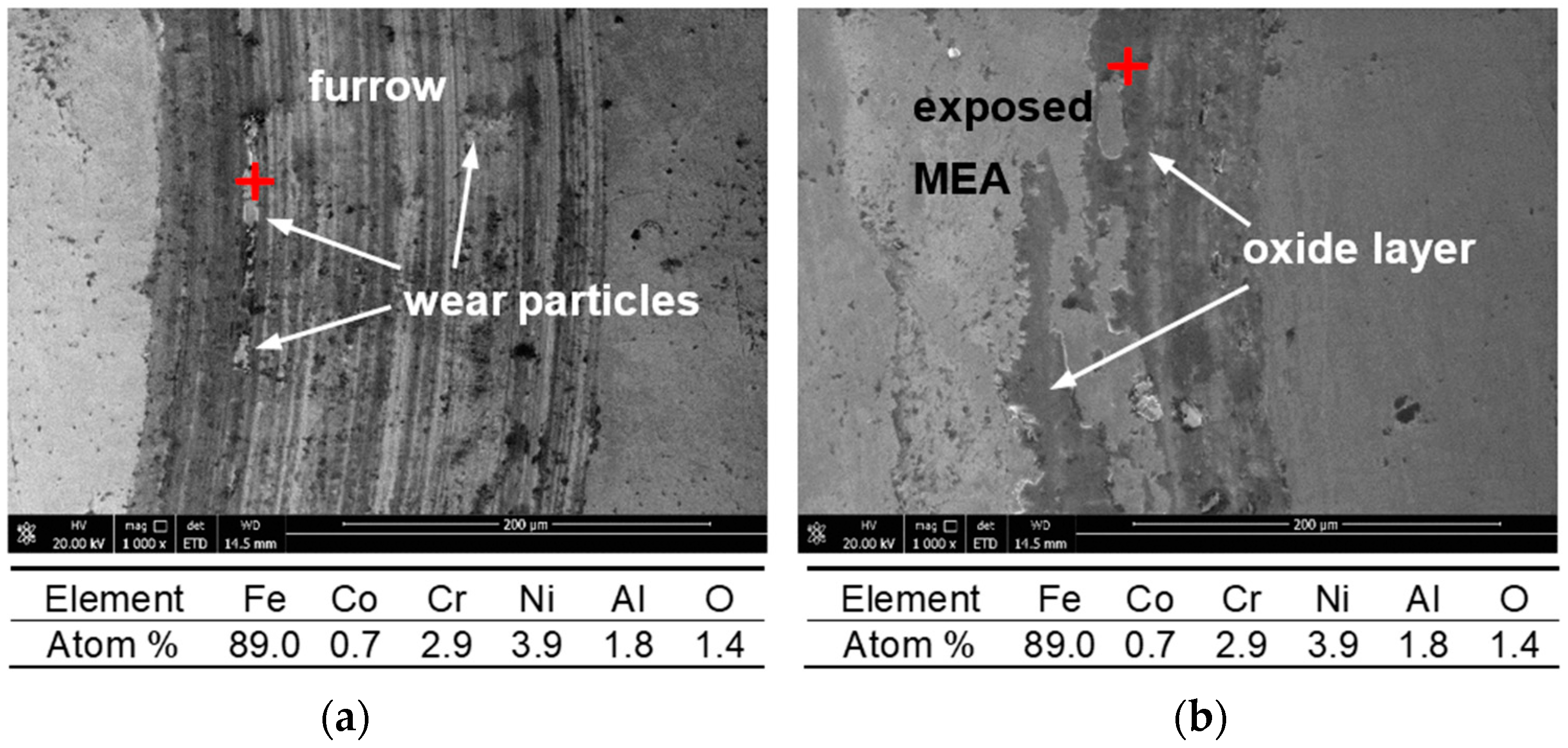
3.4. Further Analysis and Discussion
4. Conclusions
- (1)
- Analysis of the micro-structure and phases of the MEA coating indicated that the coating was mainly composed of FCC, in dendritic form, and BCC phases. The FCC phase had its preferred (2 0 0) crystal plane parallel to the coating surface.
- (2)
- At different temperatures and loads, the wear rate of the MEA coating was always much lower than that of the steel substrate. At the maximum testing temperature of 573 K, the coating presented the highest wear resistance, benefiting from the formed oxide scale, with its volume loss being only 1/14 that of the steel substrate.
- (3)
- The wear mechanism of MEA coating changed from abrasive wear to oxidative wear when the temperature increased from RT to 573 K. At 573 K, the COF of the coating sharply decreased because of the formation of oxide scale containing Al and Cr oxides. The oxide scale effectively reduced the COF and wear.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Yoosefan, F.; Ashraf, A.; Monir Vaghef, S.M. Characterization of Co–Cr–Fe–Mn–Ni High-Entropy Alloy Thin Films Synthesized by Pulse Electrodeposition: Part 2: Effect of Pulse Electrodeposition Parameters on the Wettability and Corrosion Resistance. Met. Mater. Int. 2021, 27, 106–117. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, J.; Manladan, S.M.; Geng, K.; Hu, S. Wear Resistance of FeCoCrNiMnAlx High-Entropy Alloy Coatings at High Temperature. Appl. Surf. Sci. 2020, 512, 145736. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, L.; Xu, L.; Prashanth, K.; Zhang, N.; Ma, X.; Jia, Y.; Xu, Y.; Jia, Y.; Wang, G. Frictional Wear and Corrosion Behavior of AlCoCrFeNi High-Entropy Alloy Coatings Synthesized by Atmospheric Plasma Spraying. Entropy 2020, 22, 740. [Google Scholar] [CrossRef]
- Zhang, G.J.; Tian, Q.W.; Yin, K.X.; Niu, S.Q.; Wu, M.H.; Wang, W.W.; Wang, Y.N.; Huang, J.C. Effect of Fe on Microstructure and Properties of AlCoCrFexNi (x =1.5, 2.5) High Entropy Alloy Coatings Prepared by Laser Cladding. Intermetallics 2020, 119, 106722. [Google Scholar] [CrossRef]
- Wang, W.; Qi, W.; Zhang, X.; Yang, X.; Xie, L.; Li, D.; Xiang, Y. Superior Corrosion Resistance-Dependent Laser Energy Density in (CoCrFeNi)95Nb5 High Entropy Alloy Coating Fabricated by Laser Cladding. Int. J. Miner. Met. Mater. 2021, 28, 888–897. [Google Scholar] [CrossRef]
- Fan, J.; Liu, X.; Pu, J.; Shi, Y. Anti-Friction Mechanism of VAlTiCrMo High-Entropy Alloy Coatings Through Tribo-Oxidation Inducing Layered Oxidic Surface. Tribol. Int. 2022, 171, 107523. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Jayalakshmi, S.; Singh, R.A.; Konovalov, S.; Wang, Y. Replacement of Ta with Equi-Atomic Radius Nb Atoms in CoCrFeNiTa High Entropy Alloys: Effect on Microstructure and Mechanical Properties. Mater. Lett. 2021, 297, 129966. [Google Scholar] [CrossRef]
- Kanyane, L.R.; Malatji, N.; Popoola, A.P.I.; Fayomi, O.S.I. Synthesis of Equi-Atomic Ti–Al–Mo–Si–Ni High Entropy Alloy via Spark Plasma Sintering Technique: Evolution of Microstructure, Wear, Corrosion and Oxidation Behaviour. Results Phys. 2019, 14, 102465. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Q.; Li, Y.; Wang, Z.; Li, J.; Wang, J. Phase Selection of BCC/B2 Phases for the Improvement of Tensile Behaviors in FeNiCrAl Medium Entropy Alloy. J. Alloys Compd. 2022, 916, 165382. [Google Scholar] [CrossRef]
- Bae, J.W.; Kim, H.S. Towards Ferrous Medium-Entropy Alloys with Low-Cost and High-Performance. Scr. Mater. 2020, 186, 169–173. [Google Scholar] [CrossRef]
- Song, J.; Chai, Z.; Zheng, J.; Wu, Q.; He, F.; Yang, Z.; Li, J.; Wang, J.; Yang, H.; Wang, Z. Design Fe-based Eutectic Medium-Entropy Alloys Fe2NiCrNbx. Acta Metall. Sin. Engl. Lett. 2021, 34, 1103–1108. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Zhang, L. Interdiffusion Behaviors Between NiCrFe alloy and Low-/Medium-/High-Entropy Alloys. J. Alloys Compd. 2022, 896, 162711. [Google Scholar] [CrossRef]
- Xue, B.; Lei, W.; Liu, X.; Chen, S. Effect of Medium Entropy Alloy Powder-Core Wire on Friction Wear and Corrosion Resistance of Arc Cladding Additive Layer. Mater. Res. Express 2020, 7, 076521. [Google Scholar] [CrossRef]
- Wu, M.; Chen, K.; Xu, Z.; Li, D.Y. Effect of Ti Addition on the Sliding Wear Behavior of AlCrFeCoNi High-Entropy Alloy. Wear 2020, 462, 203493. [Google Scholar] [CrossRef]
- Guo, W.; Ding, N.; Liu, G.; Jing, C.; Xu, H.; Liu, L.; Xu, N.; Wu, X.; He, J.; Zaïri, F. Microstructure Evolution of a Multi-Track AlCoCrFeNi High Entropy Alloy Coating Fabricated by Laser Cladding. Mater. Charact. 2022, 184, 111660. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, W.; Zhan, X.; Ding, L.; Wang, E. Optimization of Dilution Rate of Laser Cladding Repair Based on Deep Learning. Int. J. Adv. Manuf. Technol. 2020, 110, 1471–1484. [Google Scholar] [CrossRef]
- González-Barrio, H.; Calleja-Ochoa, A.; Norberto López de Lacalle, L.; Lamikiz, A. Hybrid manufacturing of complex components: Full methodology including laser metal deposition (LMD) module development, cladding geometry estimation and case study validation. Mech. Syst. Signal Process. 2022, 179, 109337. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, P.; Yu, Z.; Yan, H.; Wu, D.; Shi, H.; Chen, J.; Lu, Q.; Tian, Y.; Ma, S.; et al. Effect of Phase Transformation on Mechanical Properties of Al16.80Co20.74Cr20.49Fe21.28Ni20.70 High Entropy Alloy Coatings Processed by Laser Cladding. J. Alloys Compd. 2021, 862, 15856. [Google Scholar] [CrossRef]
- Hecht, U.; Gein, S.; Stryzhyboroda, O.; Eshed, E.; Osovski, S. The BCC-FCC Phase Transformation Pathways and Crystal Orientation Relationships in Dual Phase Materials from Al–(Co)–Cr–Fe–Ni Alloys. Front. Mater. 2020, 7, 287. [Google Scholar] [CrossRef]
- Liu, X.S.; Li, R.; Lu, Y.; Zhang, Y.F.; Yu, P.F.; Li, G. Spinodal Decomposition Induced Nanoprecipitates Strengthened CoCrNi-Base Medium Entropy Alloy. Mater. Sci. Eng. A 2021, 822, 141674. [Google Scholar] [CrossRef]
- Vida, Á.; Lábár, J.; Dankházi, Z.; Maksa, Z.; Molnár, D.; Varga, L.K.; Kalácska, S.; Windisch, M.; Huhn, G.; Chinh, N.Q. A Sequence of Phase Transformations and Phases in NiCoFeCrGa High Entropy Alloy. Materials 2021, 14, 1076. [Google Scholar] [CrossRef]
- Sim, R.K.; Xu, Z.; Wu, M.Y.; He, A.; Chen, D.L.; Li, D.Y. Microstructure, mechanical properties, corrosion and wear behavior of high-entropy alloy AlCoCrFeNix (x > 0) and medium-entropy alloy (x = 0). J. Mater. Sci. 2022, 57, 11949. [Google Scholar] [CrossRef]
- Feng, K.; Shao, T. The Evolution Mechanism of Tribo-Oxide Layer during High Temperature Dry Sliding Wear for Nickel-Based Superalloy. Wear 2021, 476, 203747. [Google Scholar] [CrossRef]



| Parameters | Laser Power (W) | Scan Rate (mm·min−1) | Spot Diameter (mm) | Overlap Rate | Preset Layer Thickness (µm) |
|---|---|---|---|---|---|
| Value | 200 | 240 | 0.6 | 50% | 300 |
| Group No. | Temperature (K) | Load (N) | COF of Coating (μ) | COF of 45 Steel (μ) |
|---|---|---|---|---|
| 1 | 293 | 1 | 0.891 | 0.905 |
| 2 | 293 | 5 | 0.821 | 0.753 |
| 3 | 293 | 10 | 0.736 | 0.749 |
| 4 | 373 | 10 | 0.788 | 0.795 |
| 5 | 573 | 10 | 0.348 | 0.735 |
| Group No. | Temperature (°C) | Load (N) | WMEAC (×10−3 mm3/N·m) | W45 (×10−3 mm3/N·m) |
|---|---|---|---|---|
| 1 | 20 | 1 | 1.039 | 3.948 |
| 2 | 20 | 5 | 2.439 | 18.376 |
| 3 | 20 | 10 | 8.344 | 57.248 |
| 4 | 100 | 10 | 36.48 | 112.74 |
| 5 | 300 | 10 | 9.74 | 133.356 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Pan, H.; Wu, M.; Wang, X.; Li, D. Wear-Resistant Fe6AlCoCrNi Medium-Entropy Alloy Coating Made by Laser Cladding. Metals 2022, 12, 1686. https://doi.org/10.3390/met12101686
Chen K, Pan H, Wu M, Wang X, Li D. Wear-Resistant Fe6AlCoCrNi Medium-Entropy Alloy Coating Made by Laser Cladding. Metals. 2022; 12(10):1686. https://doi.org/10.3390/met12101686
Chicago/Turabian StyleChen, Ke, Hongbo Pan, Mingyu Wu, Xianfa Wang, and Dongyang Li. 2022. "Wear-Resistant Fe6AlCoCrNi Medium-Entropy Alloy Coating Made by Laser Cladding" Metals 12, no. 10: 1686. https://doi.org/10.3390/met12101686
APA StyleChen, K., Pan, H., Wu, M., Wang, X., & Li, D. (2022). Wear-Resistant Fe6AlCoCrNi Medium-Entropy Alloy Coating Made by Laser Cladding. Metals, 12(10), 1686. https://doi.org/10.3390/met12101686








