Modelling of Single-Gas Adsorption Isotherms
Abstract
:1. Introduction
2. Modelling
- At low pressure, it corresponds to Henry’s law, according to which the amount of dissolved gas in a liquid is proportional to its partial pressure above the liquid;
- At infinite pressure, the adsorbed amount reaches its maximum value.
2.1. Langmuir Isotherm
- The surface is homogeneous;
- The adsorption energy is constant over all adsorption sites;
- The adsorption on surface is localised;
- Rach site can accommodate only one molecule or atom.
2.2. Freundlich Isotherm
2.3. Sips (Langmuir–Freundlich) Isotherm
2.4. Toth Isotherm
2.5. Jovanovic Isotherm
2.6. UNILAN Isotherm
2.7. O’Brien and Myers (OBMR) Isotherm
2.8. Potential Theory Isotherm
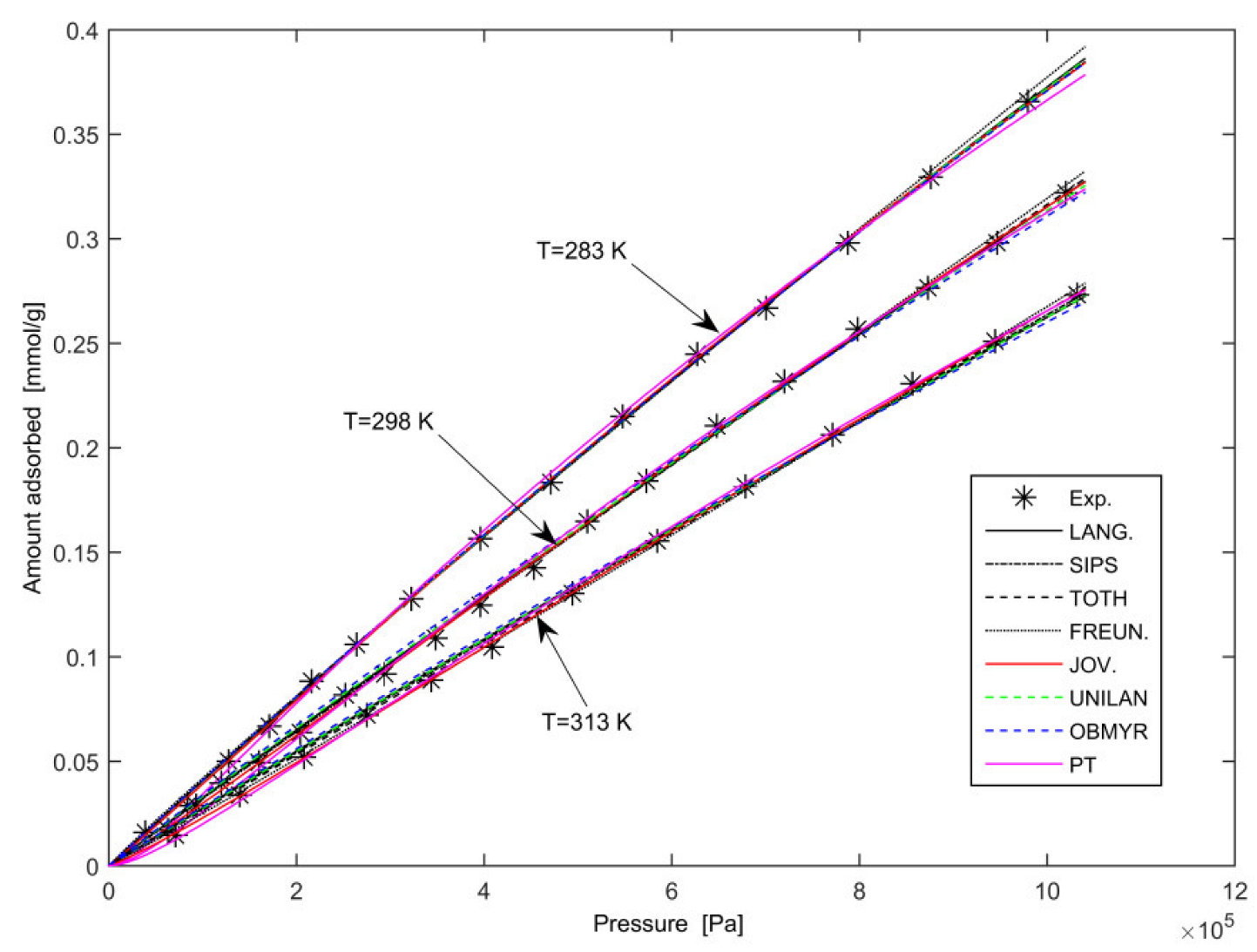
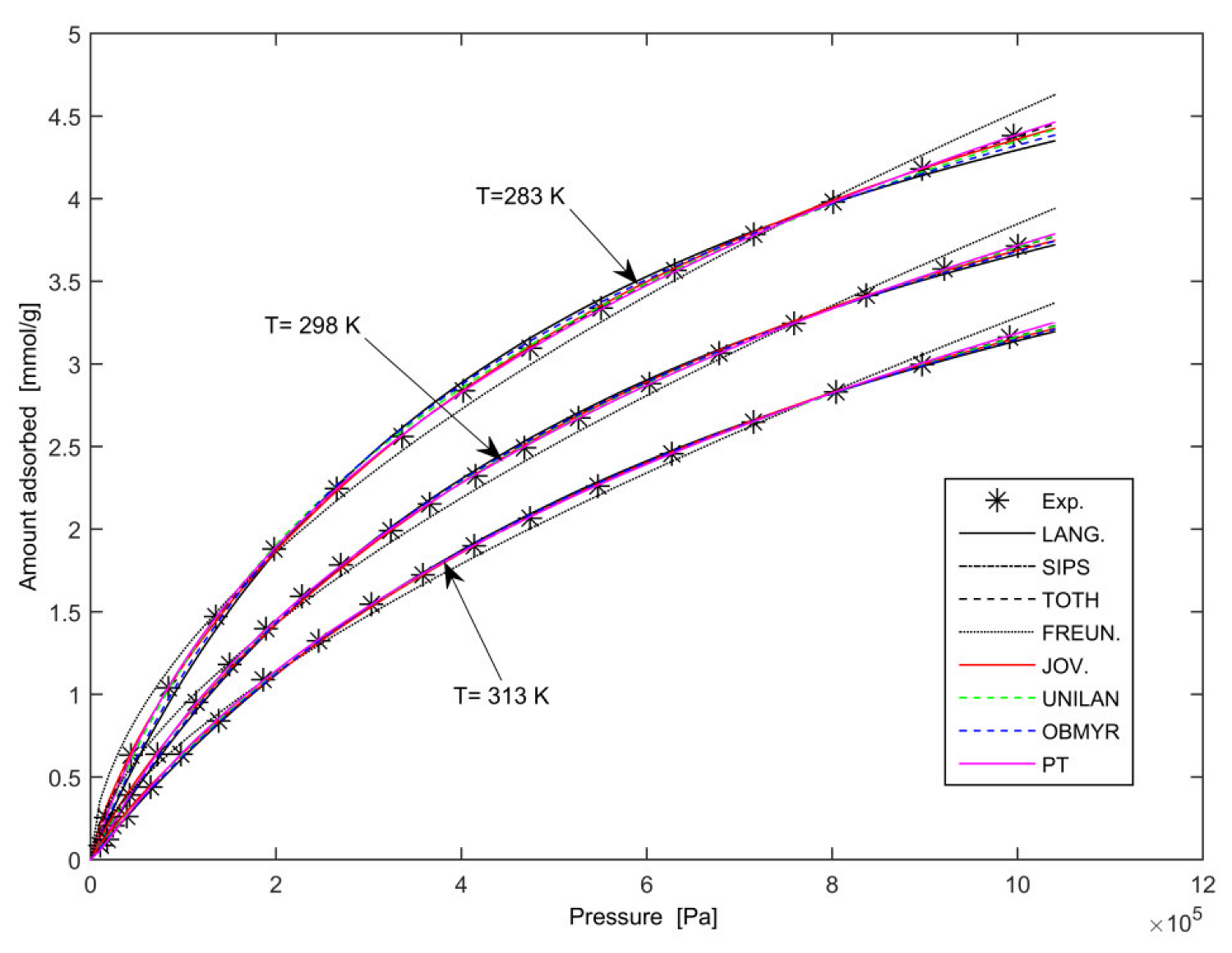
3. Modelling and Discussion
3.1. Estimation of Useful Physical Parameters
3.1.1. Pseudo-Vapour Pressure
3.1.2. Molar Volume of the Adsorbed Phase
3.2. Pure-Gas Experimental Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Equation | Parameters | R-Square | ||
|---|---|---|---|---|
| At temperature | ||||
| Langmuir | 4.130 | 9.917 | N/A | 0.9997 |
| Sips | 3.900 | 2.110 | 0.200 | 0.9997 |
| Toth | 3.956 | 10.480 | 0.9685 | 0.9996 |
| Freundlich (K and n) | 7.104 × 10−7 | 1.048 | 0.9994 | |
| Jovanovic | 3.993 | 9.494 | 0.984 | 0.9996 |
| UNILAN | 4.006 | 6.435 | 1.140 | 0.9997 |
| OBMR | 4.020 | 1.709 | 3.095 | 0.9995 |
| Potential Theory | 4.139 | 6.229 × 1011 | 1.630 | 0.9997 |
| At temperature | ||||
| Langmuir | 3.687 | 9.243 | N/A | 0.9985 |
| Sips | 3.731 | 46.370 | 5.086 | 0.9991 |
| Toth | 3.547 | 9.081 | 1.504 | 0.9989 |
| Freundlich | 3.283 × 10−7 | 1.002 | 0.9983 | |
| Jovanovic | 3.508 | 10.600 | 1.047 | 0.9993 |
| UNILAN | 3.969 | 2.808 | 1.463 | 0.9997 |
| OBMR | 3.606 | 3.414 | 1.889 | 0.9996 |
| Potential Theory | 3.811 | 6.708 × 1011 | 1.69 | 0.9993 |
| At temperature | ||||
| Langmuir | 3.026 | 9.42 | N/A | 0.9966 |
| Sips | 3.1 | 14.35 | 1.563 | 0.9967 |
| Toth | 2.892 | 9.536 | 1.169 | 0.9979 |
| Freundlich | 1.832 × 10−7 | 0.97331 | 0.9991 | |
| Jovanovic | 3.088 | 10.27 | 1.056 | 0.9998 |
| UNILAN | 3.081 | 1.878 | 1.737 | 0.998 |
| OBMR | 3.248 | 3.229 | 1.812 | 0.9995 |
| Potential Theory | 3.418 | 7.211 × 1011 | 1.741 | 0.9996 |
| Equation | Parameters | R-Square | ||
|---|---|---|---|---|
| At temperature | ||||
| Langmuir | 5.116 | 7.402 | N/A | 0.9991 |
| Sips (LF) | 5.039 | 7.687 | 1.016 | 0.9993 |
| Toth | 5.338 | 7.774 | 0.8918 | 0.9999 |
| Freundlich (K and n) | 1.145 × 10−4 | 1.4 | 0.9974 | |
| Jovanovic | 4.921 | 5.224 | 0.8336 | 0.9998 |
| UNILAN | 5.134 | 6.282 | 1.03 | 0.9999 |
| OBMR | 5.449 | 6.248 | 1.03 | 0.9997 |
| Potential Theory | 5.501 | 6.926 × 109 | 1.769 | 0.9999 |
| At temperature | ||||
| Langmuir | 4.638 | 6.099 | N/A | 0.9999 |
| Sips | 4.638 | 6.214 | 1.019 | 0.9999 |
| Toth | 4.512 | 6.239 | 1.02 | 0.9995 |
| Freundlich | 3.581 × 10−5 | 1.275 | 0.9996 | |
| Jovanovic | 4.5 | 4.654 | 0.8937 | 0.9994 |
| UNILAN | 5.25 | 4.334 | 1.03 | 0.9999 |
| OBMR | 4.964 | 1.58 | 2.244 | 0.9999 |
| Potential Theory | 4.876 | 7.208 × 109 | 1.823 | 0.9998 |
| At temperature | ||||
| Langmuir | 4.306 | 5.221 | N/A | 0.9996 |
| Sips | 4.308 | 6.554 | 1.256 | 0.9997 |
| Toth | 4.115 | 5.454 | 1.025 | 0.9997 |
| Freundlich | 2.714 × 10−5 | 1.265 | 0.9989 | |
| Jovanovic | 4.324 | 3.791 | 0.8892 | 0.9998 |
| UNILAN | 4.525 | 3.589 | 1.079 | 0.9998 |
| OBMR | 4.159 | 5.477 | 0.004049 | 0.9997 |
| Potential Theory | 4.52 | 7.413 × 109 | 1.781 | 0.9998 |
| Equation | Parameters | R-Square | ||
|---|---|---|---|---|
| At temperature | ||||
| Langmuir | 6.367 | 2.073 | N/A | 0.9978 |
| Sips | 6.368 | 2.115 | 1.021 | 0.9979 |
| Toth | 6.4 | 2.149 | 0.9673 | 0.998 |
| Freundlich (K and n) | 2.147 × 10-3 | 1.805 | 0.9936 | |
| Jovanovic | 6.214 | 1.293 | 0.7586 | 0.9997 |
| UNILAN | 6.206 | 1.68 | 1.062 | 0.9988 |
| OBMR | 6.5 | 1.98 | 0.3916 | 0.9982 |
| Potential Theory | 6.489 | 8.382 × 109 | 2.081 | 0.9992 |
| At temperature | ||||
| Langmuir | 3.041 | 1.541 | N/A | 0.9993 |
| Sips | 6.042 | 1.57 | 1.019 | 0.9993 |
| Toth | 7.025 | 1.463 | 0.8436 | 0.9997 |
| Freundlich | 7.772 × 10-4 | 1.624 | 0.9934 | |
| Jovanovic | 6.021 | 0.984 | 0.776 | 0.9988 |
| UNILAN | 6.01 | 1.21 | 1.086 | 0.9997 |
| OBMR | 6.4 | 1.363 | 0.4485 | 0.9995 |
| Potential Theory | 6.2 | 8.288 × 109 | 2.013 | 0.9998 |
| At temperature | ||||
| Langmuir | 5.724 | 1.215 | N/A | 0.9996 |
| Sips | 5.724 | 1.368 | 1.126 | 0.9995 |
| Toth | 5.9 | 1.207 | 0.9611 | 0.9997 |
| Freundlich | 3.51 × 10-4 | 1.511 | 0.9996 | |
| Jovanovic | 4.553 | 1.207 | 0.8881 | 0.9998 |
| UNILAN | 5.901 | 0.921 | 1.054 | 0.9998 |
| OBMR | 5.805 | 0.243 | 2.66 | 0.9983 |
| Potential Theory | 5.93 | 8.302 × 109 | 1.94 | 1.0000 |
References
- Yue, B.; Liu, S.; Chai, Y.; Wu, G.; Guan, N.; Li, L. Zeolites for separation: Fundamental and application. J. Energy Chem. 2022, 71, 288–303. [Google Scholar] [CrossRef]
- Sayılgan, Ş.Ç.; Mobedi, M.; Ülkü, S. Effect of regeneration temperature on adsorption equilibria and mass diffusivity of zeolite 13x-water pair. Micropor. Mesopor. Mat. 2016, 224, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Xu, L.; Wang, Q.; Chen, C.; Fu, M. Adsorption of Indium(III) Ions from an Acidic Solution by Using UiO-66. Metals 2022, 12, 579. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Q.R.; He, H.Z. Multicomponent adsorptive separation of CO2, CH4, N2, and H2 over M-MOF-74 and AX-21@ M-MOF-74 composite adsorbents. Micropor. Mesopor. Mat. 2022, 336, 111899. [Google Scholar] [CrossRef]
- Chumakova, L.S.; Bakulin, A.V.; Hocker, S.; Schmauder, S.; Kulkova, S.E. Interaction of Oxygen with the Stable Ti5Si3 Surface. Metals 2022, 12, 492. [Google Scholar] [CrossRef]
- Weinberger, B.; Darkrim Lamari, F.; Levesque, D. Capillary condensation and adsorption of binary mixtures. J. Chem. Phys. 2006, 124, 234712. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.V.; Gadipelli, S.; Howard, C.A.; Kwapinski, W.; Brett, D.J. Probing adsorbent heterogeneity using Toth isotherms. J. Mater. Chem. A 2021, 9, 944–962. [Google Scholar] [CrossRef]
- Goswami, A.; Ma, H.; Schneider, W.F. Consequences of adsorbate-adsorbate interactions for apparent kinetics of surface catalytic reactions. J. Catal. 2022, 405, 410–418. [Google Scholar] [CrossRef]
- Cho, Y.; Lo, R.; Krishnan, K.; Yin, X.; Kazemi, H. Measuring Absolute Adsorption in Porous Rocks Using Oscillatory Motions of a Spring-Mass System. Chin. J. Chem. Eng. 2022, 4, 131–139. [Google Scholar] [CrossRef]
- Chen, L.; Liu, K.; Jiang, S.; Huang, H.; Tan, J.; Zuo, L. Effect of adsorbed phase density on the correction of methane excess adsorption to absolute adsorption in shale. Chem. Eng. J. 2021, 420, 127678. [Google Scholar] [CrossRef]
- Ekundayo, J.M.; Rezaee, R.; Fan, C. Experimental investigation and mathematical modelling of shale gas adsorption and desorption hysteresis. J. Nat. Gas Sci. Eng. 2021, 88, 103761. [Google Scholar] [CrossRef]
- Keller, J.U.; Staudt, R. Gas Adsorption Equilibria: Experimental Methods and Adsorptive Isotherms; Springer Science and Business Media: Boston, MA, USA, 2005; pp. 359–413. [Google Scholar]
- Do, D.D.; Do, H.D. Adsorption of supercritical fluids in non-porous and porous carbons: Analysis of adsorbed phase volume and density. Carbon 2003, 41, 1777–1791. [Google Scholar] [CrossRef]
- Chilev, C.; Darkrim Lamari, F.; Kirilova, E.; Pentchev, I. Comparison of gas excess adsorption models and high pressure experimental validation. Chem. Eng. Res. Des. 2012, 90, 2002–2012. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Amer. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Zeldowitsch, J. Adsorption site energy distribution. Acta Phys. Chim. URSS 1934, 1, 961–973. [Google Scholar]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar] [CrossRef] [Green Version]
- Sips, R. The Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Tóth, J. Gas-(Steam-) Adsorption on solid surfaces of inhomogeneous activity III. Acta Chim. Acad. Sci. Hung. 1962, 32, 39–57. [Google Scholar]
- Jovanovic, D.S. Physical adsorption of gases—II: Practical application of derived isotherms for monolayer and multilayer adsorption. Kollozd Z. 1969, 235, 1214–1225. [Google Scholar] [CrossRef]
- Quinones, I.; Guiochon, G. Extension of a Jovanovic–Freundlich isotherm model to multicomponent adsorption on heterogeneous surfaces. Chromatogr. J. 1998, 796, 15–40. [Google Scholar] [CrossRef]
- Hazlett, J.D.; Hsu, C.C.; Wojciechowski, B.W. Is the Jovanovic isotherm theoretically valid? J. Chem. Soc. Faraday Trans 2 1979, 75, 602–605. [Google Scholar] [CrossRef]
- Grady, C.; McWhorter, S.; Sulic, M.; Sprik, S.J.; Thornton, M.J.; Brooks, K.P.; Tamburello, D.A. Design tool for estimating adsorbent hydrogen storage system characteristics for light-duty fuel cell vehicles. Int. J. Hydrog. Energ. 2022, 47, 29847–29857. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Myers, A.L. Physical adsorption of gases on heterogeneous surfaces. Series expansion of isotherms using central moments of the adsorption energy distribution. J. Chem. Soc. Faraday Trans. 1 1984, 80, 1467–1477. [Google Scholar] [CrossRef]
- Bering, B.P.; Dubinin, M.M.; Serpinsky, V.V. On thermodynamics of adsorption in micropores. J. Colloid. Int. Sci. 1972, 38, 185–194. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of the activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–337. [Google Scholar]
- Wu, Q.; Zhou, L.; Wu, J.; Zhou, Y. Adsorption equilibrium of the mixture CH4+N2+H2 on activated carbon. J. Chem. Eng. Data 2005, 50, 635–642. [Google Scholar] [CrossRef]
- Chilev, C.; Darkrim Lamari, F. Hydrogen storage at low temperature and high pressure for application in automobile manufacturing. Int. J. Hydrog. Energ. 2016, 41, 1744–1758. [Google Scholar] [CrossRef]
- van der Waals, J.D. Over de Continuited van den Gas-en Vloeistoftoestand. Ph.D. Thesis, University of Leiden, Holland, The Netherlands, 14 June 1873. [Google Scholar]
- Peng, D.Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Patel, N.C.; Teja, A.S. A new cubic equation of state for fluids and fluid mixtures. Chem. Eng. Sci. 1982, 37, 463–473. [Google Scholar] [CrossRef]
- McCarty, R.D.; Weber, L.A. Thermophysical properties of parahydrogen from the freezing liquid line to 5000 R for pressures to 10,000 psia. In National Bureau of Standards—Technical Note 617; NBS: Boulder, CO, USA, 1972; pp. 1–169. [Google Scholar]
- Lemmon, E.W.; McLinden, M.O.; Friend, D.G. Thermophysical properties of fluid systems. In the NIST Chemistry Webbook; Database Reference Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1998. [Google Scholar] [CrossRef]
- Dubinin, M.M. Modern state of the theory of gas and vapour adsorption by microporous adsorbents. Pure Appl. Chem. 1965, 10, 309–322. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Fomkin, A.F.; Seliverstova, I.I.; Serpinsky, V.V. Direct determination of the density of adsorbates in zeolite micropores. In Proceedings of the Fifth Int. Conference on Zeolite, Naples, Italy, 2–6 June 1980. [Google Scholar]
- Rogers, K.A. Adsorption on Activated Carbon of Hydrogen, Methane, and Carbon Dioxide gases and Their Mixtures at 212–301 Degrees K and up to Thirty-Fie Atmospheres. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 1973. [Google Scholar]
- Findenegg, G. High-pressure physical adsorption of gases on homogeneous surfaces. In Fundamentals of Adsorption; Myers, A.L., Belfort, G., Eds.; United Engineering Trustees: New York, NY, USA, 1984; Volume 1, pp. 207–218. [Google Scholar]
- Mehta, S.D.; Danner, R.P. An improved potential theory method for predicting gas-mixture adsorption equilibria. Ind. Eng. Chem. Fund. 1985, 24, 325–330. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Ozawa, S.; Kusumi, S.; Ogino, Y. Physical adsorption of gases at high pressure. IV. An improvement of the Dubinin—Astakhov adsorption equation. J. Coll. Int. Sci. 1976, 56, 83–91. [Google Scholar] [CrossRef]
- Dubinin, M.M. Physical adsorption of gases and vapors in micropores. In Progress in Surface and Membrane Science, 1st ed.; Cadenhead, D.A., Danielli, J.E., Rosenberg, M.D., Eds.; Academic Press: New York, NY, USA, 1975; Volume 9, pp. 1–70. [Google Scholar]
- Do, D.D. Adsorption Analysis: Equilibria and kinetics; Imperial College Press: London, UK, 1998. [Google Scholar]
- Maslan, F.D.; Altman, M.; Alberth, E.R. Prediction of Gas–Adsorbent Equilibria. J. Phys. Chem. 1953, 57, 106–109. [Google Scholar] [CrossRef]
- Lewis, W.K.; Gilliland, E.R.; Chertow, L.; Cadogen, W.P. Adsorption equilibria hydrocarbon gas mixtures. Ind. Eng. Chem. 1950, 42, 1319–1326. [Google Scholar] [CrossRef]
- Grant, R.J.; Manes, M. Adsorption of normal paraffins and sulfur compounds on activated carbon. AIChE J. 1962, 8, 403–406. [Google Scholar] [CrossRef]
- Cook, W.H.; Basmadjian, D. Correlation of adsorption equilibria of pure gases on activated carbon. Can. J. Chem. Eng. 1964, 42, 146–151. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–618. [Google Scholar] [CrossRef]

| Isotherm | Equation 1 | Parameters |
|---|---|---|
| Langmuir | ||
| Freundlich | ||
| Sips | ||
| Toth | ||
| Jovanovic | ||
| UNILAN | ||
| OBMR | ||
| Potential Theory (PT) |
| Pure Gas | ||
|---|---|---|
| First Model (Equation (5)) | Second Model (Equation (6)) | |
| Nitrogen | 18.939 | 3.1216 × 106 |
| Methane | 11.245 | 1.1478 × 104 |
| Hydrogen | 104.790 | 6.1633 × 108 |
| Pure Gas | |||
|---|---|---|---|
| Nitrogen | 17.080 | 18.939 | 20.893 |
| Methane | 10.141 | 11.245 | 12.406 |
| Hydrogen | 94.510 | 104.790 | 115.610 |
| Temperature Range | Authors | |
|---|---|---|
| First group | ||
| Rogers [36], Findenegg [37] | ||
| Metha and Danner [38] | ||
| Second group 1 | ||
| Dubinin [35] | ||
| Dubinin [39] | ||
| Ozawa [40], Dubinin [41] | ||
| Ozawa [40] | ||
| Dubinin [41] | ||
| Do [42] | ||
| Third group 2 | ||
| for compressed gas | Any T | Maslan et al. [43] |
| Any T | Lewis et al. [44] Grant and Manes [45] | |
| Cook and Basmadjian [46] | ||
| Dubinin | Do | ||
|---|---|---|---|
| Nitrogen | 0.139392551516816 | 0.145142438708709 | |
| 0.143982509390866 | 0.149710518000801 | ||
| 0.148723606712872 | 0.154187496548527 | ||
| critical molar volume for N2 | 0.0894 | ||
| Methane | 0.130748871040833 | 0.124989933925346 | |
| 0.134019508224059 | 0.128923751862843 | ||
| 0.137371959250105 | 0.154187496548527 | ||
| critical molar volume for CH4 | 0.09860 | ||
| Hydrogen | 0.158161733097024 | 2.335716203132310 | |
| 0.145960056635246 | 2.409228381339168 | ||
| 0.137371959250105 | 2.481274513594026 | ||
| critical molar volume for H2 | 0.645 | ||
| Nitrogen | 0.14514 | 0.14971 | 0.15419 |
| Methane | 0.12499 | 0.12892 | 0.15419 |
| Hydrogen | 2.33572 | 2.40923 | 2.48127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilev, C.; Dicko, M.; Langlois, P.; Lamari, F. Modelling of Single-Gas Adsorption Isotherms. Metals 2022, 12, 1698. https://doi.org/10.3390/met12101698
Chilev C, Dicko M, Langlois P, Lamari F. Modelling of Single-Gas Adsorption Isotherms. Metals. 2022; 12(10):1698. https://doi.org/10.3390/met12101698
Chicago/Turabian StyleChilev, Chavdar, Moussa Dicko, Patrick Langlois, and Farida Lamari. 2022. "Modelling of Single-Gas Adsorption Isotherms" Metals 12, no. 10: 1698. https://doi.org/10.3390/met12101698
APA StyleChilev, C., Dicko, M., Langlois, P., & Lamari, F. (2022). Modelling of Single-Gas Adsorption Isotherms. Metals, 12(10), 1698. https://doi.org/10.3390/met12101698






