Preparation and Energy Release Properties of nB@F2603@CL-20 Microspheres by Electrospray
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Compound Microspheres
2.3. Characterization
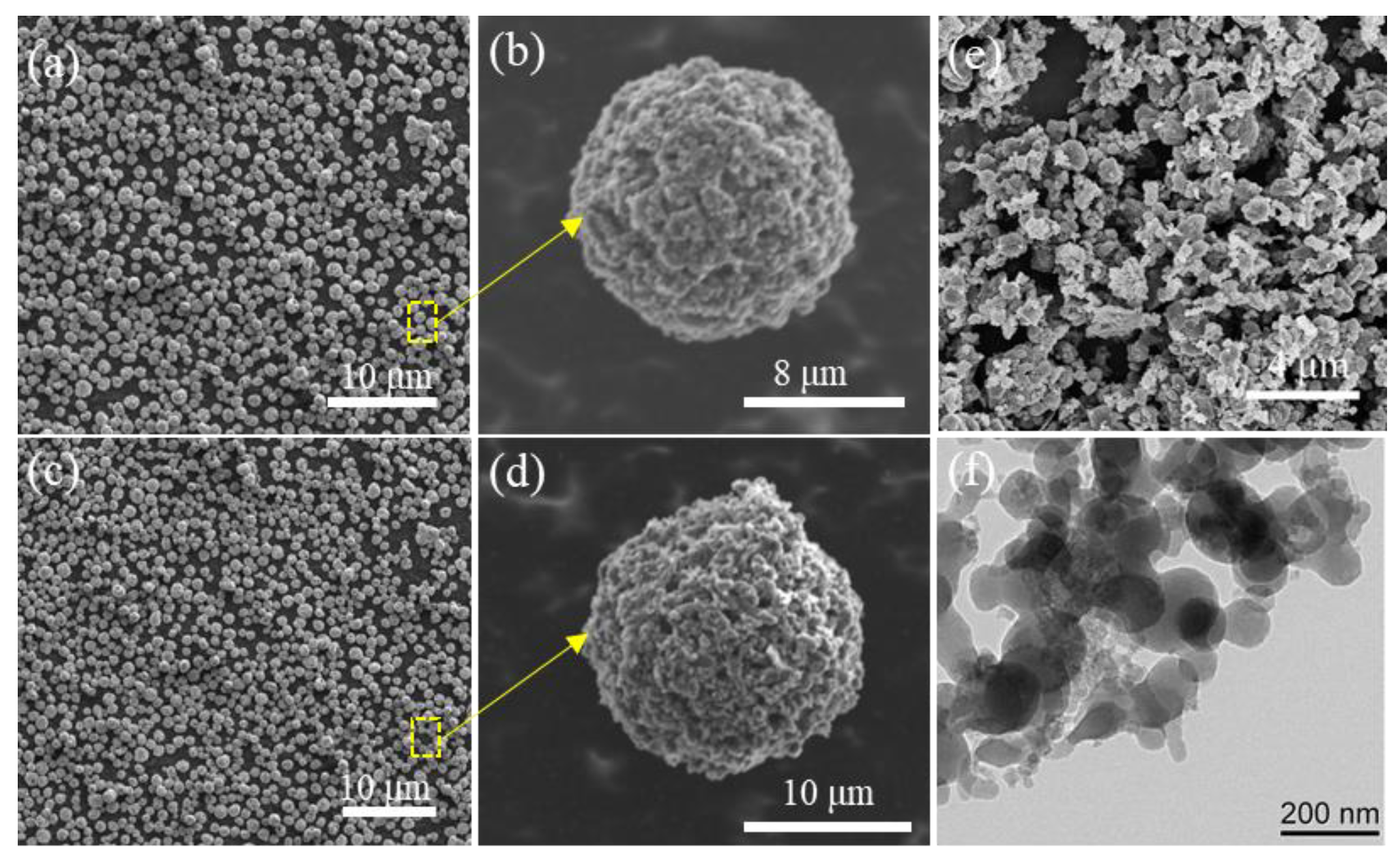
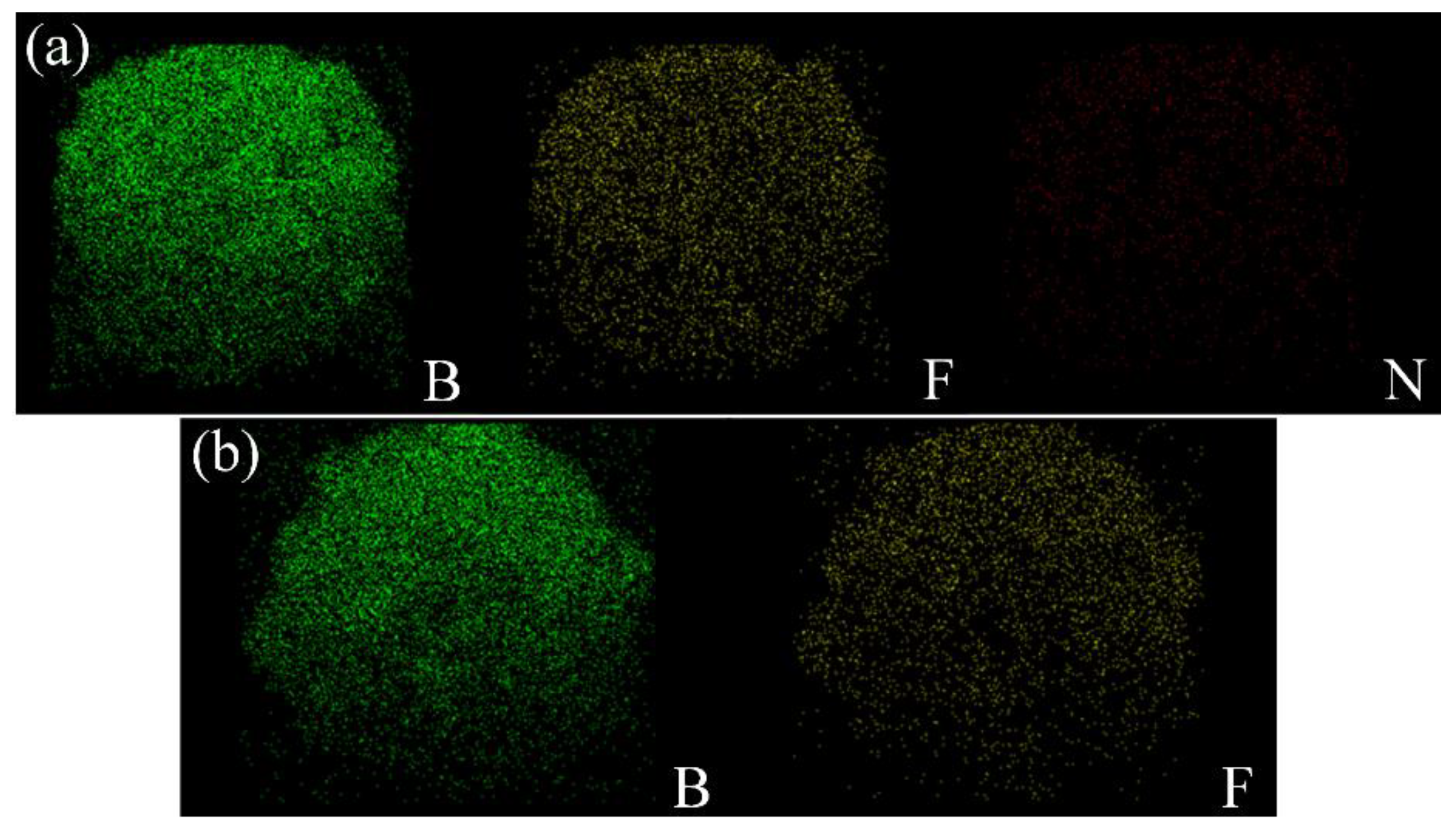
2.3.1. Morphological Characteristics
2.3.2. Hydrophobic and Acid Corrosion Resistance
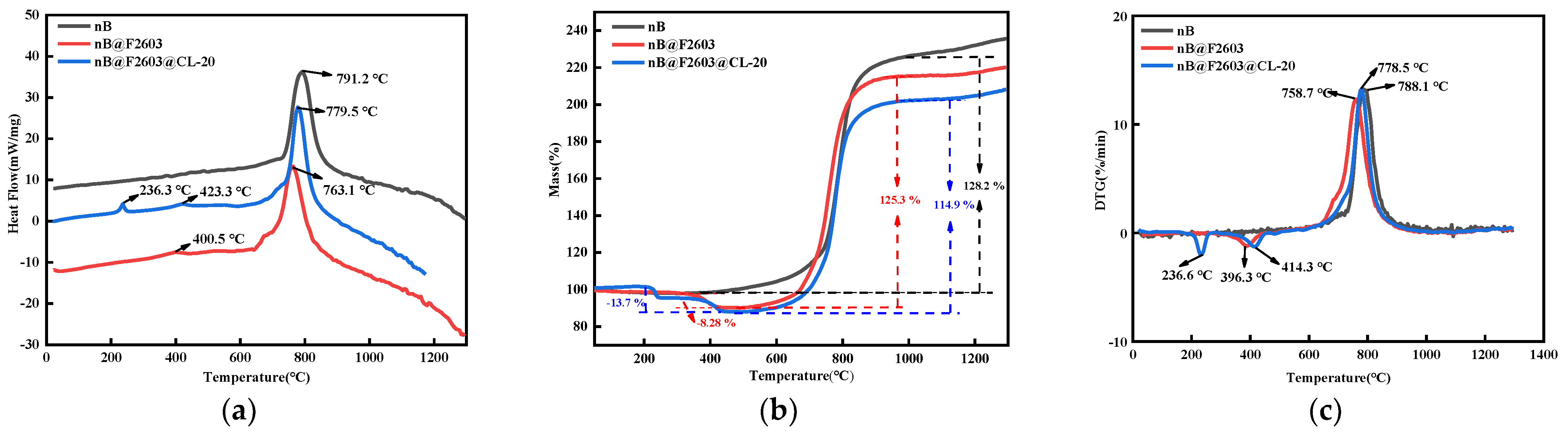
2.3.3. Thermal Analysis
2.3.4. Ignition and Combustion Performance
3. Results and Discussion
3.1. Morphology and Component Analysis
3.2. Hydrophobicity and Corrosion Resistance
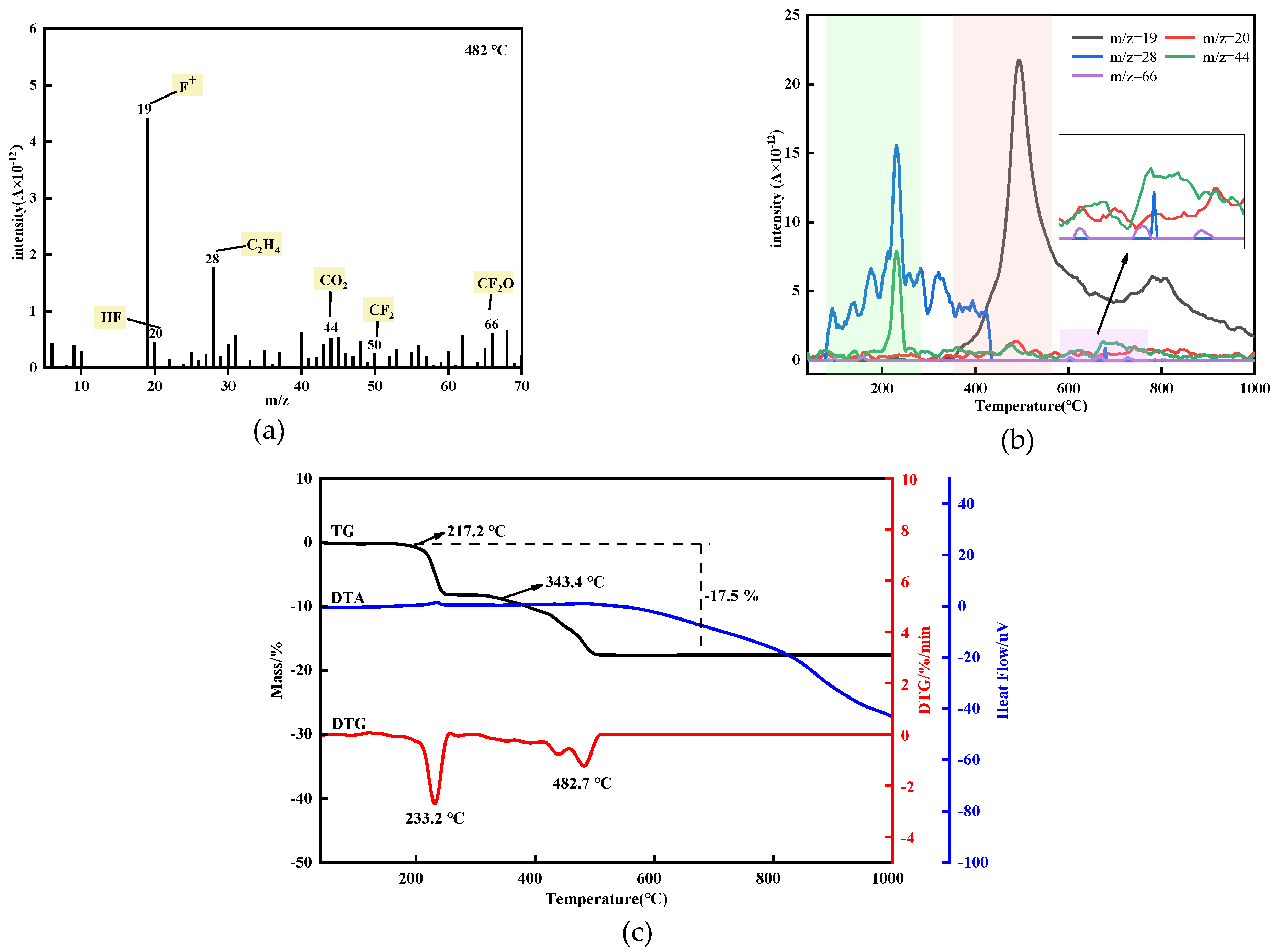
3.3. Thermal Analysis
3.4. Ignition and Combustion Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Wang, H.; Ren, H.; Yin, L.; Wu, X. High energy release boron-based material with oxygen vacancies promoting combustion. Chem. Eng. J. 2022, 430, 133027–133042. [Google Scholar] [CrossRef]
- Ma, M.; Liu, G.; Qin, Z.; Zhang, R.; Ying, Y.; Xu, L.; Liu, D. Effects of aluminum addition on flash ignition and combustion of boron nanoparticles. Combust. Flame 2022, 236, 111762–111775. [Google Scholar] [CrossRef]
- Chen, B.; Xia, Z.; Huang, L.; Hu, J. Ignition and combustion model of a single boron particle. Fuel Process. Technol. 2017, 165, 34–43. [Google Scholar] [CrossRef]
- Liang, D.; Liu, J.; Zhou, Y.; Zhou, J. Ignition and combustion characteristics of amorphous boron and coated boron particles in oxygen jet. Combust. Flame 2017, 185, 292–300. [Google Scholar] [CrossRef]
- Connell, T.L., Jr.; Yetter, R.A.; Risha, G.A.; Huba, Z.J.; Epshteyn, A.; TFisher, B. Enhancement of Solid Fuel Combustion in a Hybrid Rocket Motor Using Amorphous Ti-Al-B Nanopowder Additives. J. Propuls. Power 2019, 35, 662–665. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, H.; Du, F.; Yan, S.; Jiao, Q. Preparation and characterization of sintered B/MgB2 as heat release material. J. Alloy. Compd. 2018, 759, 100–107. [Google Scholar] [CrossRef]
- Xu, S.; Chen, Y.; Chen, X.; Wu, D.; Liu, D. Combustion heat of the Al/B powder and its application in metallized explosives in underwater explosions, Combustion. Explos. Shock. Waves 2016, 52, 342–349. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Wang, Y.; Huang, L.; Xiao, J. Effect of LiF coating on the thermal oxidation characteristics for boron powder. Chin. J. Energetic Mater. 2013, 21, 57–60. [Google Scholar]
- Gao, D.; Zhang, W.; Zhu, H.; Wang, C. Effect of coated and agglomerated boron on combustion characteristics of boron-based fuel-rich propellants. J. Propuls. Technol. 2009, 30, 119–123. [Google Scholar]
- Keerthi, V.; Nie, H.; Pisharath, S.; Hng, H.H. Combustion characteristics of fluoropolymer coated boron powders. Combust. Sci. Technol. 2020, 194, 1183–1198. [Google Scholar] [CrossRef]
- Bocanegra, P.E.; Chauveau, C.; Gökalp, I. Experimental studies on the burning of coated and uncoated micro and nano-sized aluminium particles. Aerosp. Sci. Technol. 2007, 11, 33–38. [Google Scholar] [CrossRef]
- Yetter, R.A.; Risha, G.A.; Son, S.F. Metal Particle Combustion and Nanotechnology. Proc. Combust. Inst. 2009, 32, 1819–1838. [Google Scholar] [CrossRef]
- Sumpter, B.G.; Beach, D.B.; Labinov, S.D.; Rondinone, A.J.; Richards, R.K. Solid-State Combustion of Metallic Nanoparticles: New Possibilities for an Alternative Energy Carrier. J. Energy Resour. Technol. 2007, 129, 29–32. [Google Scholar]
- Bergthorson, J.M. Recyclable metal fuels for clean and compact zero-carbon power. Prog. Energy Combust. Sci. 2018, 68, 169–196. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, C.; Yang, Y.; Li, Y.; Artiaga, R. Preparation and combustion performance of B/PVDF/Al composite microspheres. Propellants Explos. Pyrotech. 2020, 45, 657–664. [Google Scholar] [CrossRef]
- Wang, H.; Jian, G.; Yan, S.; Delisio, J.; Huang, C.; Zachariah, M. Electrospray formation of gelled nano-aluminum microspheres with superior reactivity. ACS Appl. Mater. Interfaces 2013, 5, 6797–6801. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Wang, H.; Zachariah, M.R. Application of nano-aluminum/nitrocellulose mesoparticles in composite solid rocket propellants. Propellants Explos. Pyrotech. 2015, 40, 413–418. [Google Scholar] [CrossRef]
- Wang, H.; Jian, G.; Egan, G.; Zachariah, M. Assembly and reactive properties of Al/CuO based nanothermite microparticles. Combust. Flame 2014, 161, 2203–2208. [Google Scholar] [CrossRef]
- Wang, H.; Jacob, J.; DeLisio, B.; Michael, R. Assembly and encapsulation of aluminum NP’s within AP/NC matrix and their reactive properties. Combust. Flame 2017, 180, 175–183. [Google Scholar] [CrossRef]
- Wang, H.; Zachariah, R.; Xie, L.; Rao, G. Ignition and combustion characterization of nano-Al-AP and nano-Al-CuO-AP micro-sized composites produced by electrospray technique. Energy Procedia 2015, 66, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.R.; Dubé, P.; Krawiec, M.; Salan, J.S.; Ende, D.J. Promising CL-20-Based Energetic Material by Cocrystallization, Propellants. Explos. Pyrotech. 2016, 41, 783–788. [Google Scholar] [CrossRef]
- Shi, Y.; Li, M.; Lu, S.; Jiao, Q.; Huang, R. Fabrication of Nano- and Micron- Sized Spheres of CL-20 by Electrospray. Cent. Eur. J. Energetic Mater. 2018, 15, 572–589. [Google Scholar]
- Cheng, L.A.; Yang, H.B.; Yang, Y.A.; Li, Y.A.; Meng, Y.A.; Li, Y.A. Preparation of B/Nitrocellulose/Fe particles and their effect on the performance of an ammonium perchlorate propellant. Combust. Flame 2020, 211, 456–464. [Google Scholar] [CrossRef]




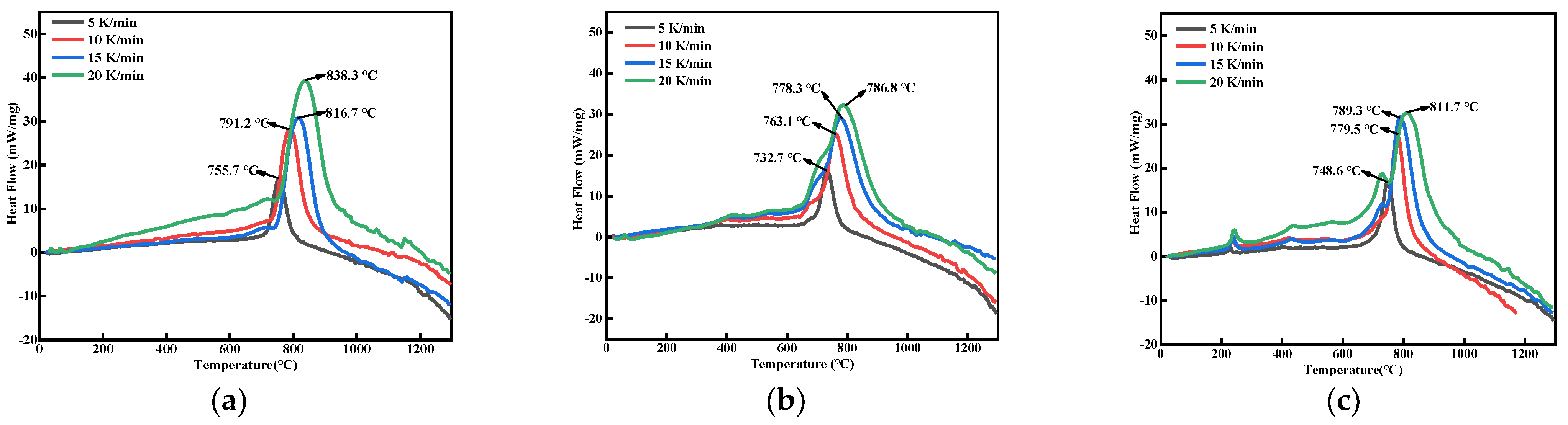


| Samples | Tp (°C) | Ea (kJ/mol) | r | |||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | |||
| nB | 755.7 | 791.2 | 816.7 | 838.3 | 152.9 | 0.998 |
| nB@F2603 | 732.7 | 763.1 | 778.3 | 786.8 | 211.7 | 0.996 |
| nB@F2603@CL-20 | 748.6 | 779.5 | 789.3 | 811.7 | 198.5 | 0.986 |
| Sample | n-B | nB@F2603 Microspheres | nB@F2603@CL-20 Microspheres |
|---|---|---|---|
| Experimental (kJ/g) | 16.76 ± 0.02 | 20.63 ± 0.03 | 32.00 ± 0.02 |
| Sample | Pmax (KPa) | Pressurization Rate (MPa s−1) |
|---|---|---|
| n-B | 64.0 ± 0.2 | 2.56 ± 0.03 |
| nB@F2603 microspheres | 189.6 ± 0.1 | 4.99 ± 0.02 |
| nB@F2603@CL-20 microspheres | 501.2 ± 0.1 | 15.19 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Huang, Y.; Chang, K.; Nie, J.; Guo, X.; Shen, C.; Yan, S. Preparation and Energy Release Properties of nB@F2603@CL-20 Microspheres by Electrospray. Metals 2022, 12, 1727. https://doi.org/10.3390/met12101727
Yao J, Huang Y, Chang K, Nie J, Guo X, Shen C, Yan S. Preparation and Energy Release Properties of nB@F2603@CL-20 Microspheres by Electrospray. Metals. 2022; 12(10):1727. https://doi.org/10.3390/met12101727
Chicago/Turabian StyleYao, Jie, Yanjie Huang, Kanghua Chang, Jianxin Nie, Xueyong Guo, Chen Shen, and Shi Yan. 2022. "Preparation and Energy Release Properties of nB@F2603@CL-20 Microspheres by Electrospray" Metals 12, no. 10: 1727. https://doi.org/10.3390/met12101727
APA StyleYao, J., Huang, Y., Chang, K., Nie, J., Guo, X., Shen, C., & Yan, S. (2022). Preparation and Energy Release Properties of nB@F2603@CL-20 Microspheres by Electrospray. Metals, 12(10), 1727. https://doi.org/10.3390/met12101727






