3D Printing Using Ti-Al Nanopowders: Mechanisms of Structure Formation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williams, J.C.; Starke, E.A., Jr. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Dimiduk, D.M. Gamma titanium aluminide alloys—An assessment within the competition of aerospace structural materials. Mater. Sci. Eng. A 1999, 263, 281–288. [Google Scholar] [CrossRef]
- Kaynak, Y.; Tascioglu, E. Finish machining-induced surface roughness, microhardness and XRD analysis of selective laser melted Inconel 718 alloy. Procedia CIRP 2018, 71, 500–504. [Google Scholar] [CrossRef]
- Brotzu, A.; Felli, F.; Marra, F.; Pilone, D.; Pulci, G. Mechanical properties of a TiAl-based alloy at room and high temperatures. Mater. Sci. Technol. 2018, 34, 1847–1853. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Intermetallic titanium aluminides in aerospace applications–processing, microstructure and properties. Mater. High Temp. 2016, 33, 560–570. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Castellanos, S.D.; Cavalerio, A.J.; de Jesus, A.M.P.; Neto, R.; Lino Alves, J. Machinability of titanium aluminides: A review. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 233, 426–451. [Google Scholar] [CrossRef]
- Loria, E.A. Quo vadis gamma titanium aluminide. Intermetallics 2001, 9, 997–1001. [Google Scholar] [CrossRef]
- Bünck, M.; Stoyanov, T.; Schievenbusch, J.; Michels, H.; Gußfeld, A. Titanium Aluminide Casting Technology Development. JOM 2017, 69, 2565–2570. [Google Scholar] [CrossRef]
- Wimler, D.; Lindemann, J.; Reith, M.; Kirchner, A.; Allen, M.; Vargas, W.G.; Franke, M.; Klöden, B.; Weißgärber, T.; Güther, V.; et al. Designing advanced intermetallic titanium aluminide alloys for additive manufacturing. Intermetallics 2021, 131, 107109. [Google Scholar] [CrossRef]
- Güther, V.; Allen, M.; Klose, J.; Clemens, H. Metallurgical processing of titanium aluminides on industrial scale. Intermetallics 2018, 103, 12–22. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications, 1st ed.; WILEY-VCH Verlag Gmbh & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Zhao, L.; Wang, S.; Jin, Y.; Chen, Y. Microstructural characterization and mechanical performance of Al–Cu–Li alloy electron beam welded joint. Aerosp. Sci. Technol. 2018, 82–83, 61–69. [Google Scholar] [CrossRef]
- Şuta, S.; Thalmaier, G.; Sechel, N.; Vida-Simiti, I.; Petrescu, V. Ti-Al Membranes for Microfiltration. Solid State Phenom. 2016, 254, 8–13. [Google Scholar] [CrossRef]
- Böhm, A.; Kieback, B. Investigation of swelling behaviour of Ti–Al elemental powder mixtures during reaction sintering. Int. J. Mater. Res. 1998, 89, 90–95. [Google Scholar]
- Yang, J.B.; Hwang, W.S. The preparation of TiAl-based intermetallics from elemental powders through a two-step pressureless sintering process. J. Mater. Eng. Perform. 1998, 7, 385–392. [Google Scholar] [CrossRef]
- Wang, G.X.; Dahms, M. An effective method for reducing porosity in the titanium aluminide alloy Ti52Al48 prepared by elemental powder metallurgy. Scr. Metall. Mater. 1992, 26, 1469–1474. [Google Scholar] [CrossRef]
- Dahms, M.; Schmelzer, F.; Seeger, J.; Wildhagen, B. Microstructure and mechanical properties of γ base titanium aluminide produced from extruded elemental powders. Mater. Sci. Technol. 1992, 8, 359–362. [Google Scholar] [CrossRef]
- Kennedy, S.; Kumaran, S.; Rao, T.S. Densification of γ-TiAl Powders by Spark Plasma Sintering. Mater. Sci. Forum 2012, 710, 303–307. [Google Scholar] [CrossRef]
- Mogale, N.F.; Matizamhuka, W.R. Spark Plasma Sintering of Titanium Aluminides: A Progress Review on Processing, Structure-Property Relations, Alloy Development and Challenges. Metals 2020, 10, 1080. [Google Scholar] [CrossRef]
- Park, K.; Kim, D.; Kim, K.; Cho, S.; Kwon, H. Behavior of Intermetallic Compounds of Al-Ti Composite Manufactured by Spark Plasma Sintering. Materials 2019, 12, 331. [Google Scholar] [CrossRef]
- Soliman, H.A.; Elbestawi, M. Titanium aluminides processing by additive manufacturing—A review. Int. J. Adv. Manuf. Technol. 2022, 119, 5583–5614. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies, 3rd ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Wong, K.V.; Hernandez, A. A review of additive manufacturing. Int. Sch. Res. Not. 2012, 2012, 208760. [Google Scholar] [CrossRef]
- Khorasani, M.; Ghasemi, A.; Rolfe, B.; Gibson, I. Additive manufacturing a powerful tool for the aerospace industry. Rapid Prototyp. J. 2022, 28, 87–100. [Google Scholar] [CrossRef]
- Qu, H.P.; Li, P.; Zhang, S.Q.; Li, A.; Wang, H.M. The effects of heat treatment on the microstructure and mechanical property of laser melting deposition γ-TiAl intermetallic alloys. Mater. Des. 2010, 31, 2201–2210. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z. 11—Additive manufacturing of titanium aluminides. In Additive Manufacturing for the Aerospace Industry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–263. [Google Scholar]
- Kim, Y.-W.; Dimiduk, D.M. Progress in the understanding of gamma titanium aluminides. JOM 1991, 43, 40–47. [Google Scholar] [CrossRef]
- Li, X.B.; Zhang, J.B.; Ji, X.C.; Wu, X.Q.; Luo, J.S.; Tang, Y.J. A Small Patch Production of γ-TiAl Alloys Nanoparticles via Physical Vapor-phase Method. Integr. Ferroelectr. 2013, 147, 146–153. [Google Scholar] [CrossRef]
- Lerner, M.; Pervikov, A.; Glazkova, E.; Rodkevich, N.; Toropkov, N. Electrical Explosion Synthesis, Oxidation and Sintering Behavior of Ti-Al Intermetallide Powders. Metals 2021, 11, 760. [Google Scholar] [CrossRef]
- Jones, S.A.; Kaufman, M.J. Phase equlibria and transformations in intermediate titanium-aluminum alloys. Acta Metall. Mater. 1993, 41, 387–398. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive Manufacturing of Metallic and Ceramic Components by the Material Extrusion of Highly-Filled Polymers: A Review and Future Perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef]
- Seyedzavvar, M.; Boğa, C. A study on the effects of internal architecture on the mechanical properties and mixed-mode fracture behavior of 3D printed CaCO3/ABS nanocomposite samples. Rapid Prototyp. J. 2022. [Google Scholar] [CrossRef]
- Batalu, D.; Cosmeleata, G.; Aloman, A. Critical analysis of the Ti-Al phase diagrams. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2006, 68, 77–90. [Google Scholar]
- Villars, P.; Calvert, L.D. Pearson’s Handbook of Crystallographic Data for Intermediate Phases; American Society of Metals: Cleveland, OH, USA, 1985. [Google Scholar]
- Manoun, B.; Zhang, F.X.; Saxena, S.K.; El-Raghy, T.; Barsoum, M.W. X-ray high-pressure study of Ti2AlN and Ti2AlC. J. Phys. Chem. Solids 2006, 67, 2091–2094. [Google Scholar] [CrossRef]
- Hug, G.; Jaouen, M.; Barsoum, M.W. X-ray absorption spectroscopy, EELS, and full-potential augmented plane wave study of the electronic structure of Ti2AlC, Ti2AlN, Nb2AlC, and (Ti0.5Nb0.5)2AlC. Phys. Rev. B. 2005, 71, 024105. [Google Scholar] [CrossRef]
- Barsoum, M.W. The MN+ 1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Barsoum, M.W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides, 1st ed.; WILEY-VCH Verlag Gmbh & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Hanaor, D.; Hu, L.; Kan, W.H.; Proust, G.; Foley, M.; Karaman, I.; Radovic, M. Compressive performance and crack propagation in Al alloy/Ti2AlC composites. Mater. Sci. Eng. A 2016, 672, 247–256. [Google Scholar] [CrossRef]
- Kattner, U.R.; Lin, J.-C.; Chang, Y.A. Thermodynamic Assessment and Calculation of the Ti-Al System. Met. Mater. Trans. A 1992, 23, 2081–2090. [Google Scholar] [CrossRef]
- Chlubny, L.; Lis, J.; Buko, M.M. Influence of nitrogen pressure on shs synthesis of Ti2AlN Powders. In Proceedings of the Developments in Strategic Ceramic Materials: A Collection of Papers Presented at the 39th International Conference on Advanced Ceramics and Composites, 1st ed.; Kriven, W.M., Wang, J., Zhu, D., Fisher, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 253. [Google Scholar]
- Tian, J.J.; Zhang, L.L.; Bi, X.W.; Liu, G.Y.; Ding, Z.M. Ti2AlN Prepared by Self-Propagating High-Temperature Combustion Method Using TiN as Additive. Adv. Mater. Res. 2013, 710, 37–40. [Google Scholar] [CrossRef]
- Bailey, E.; Ray, N.; Hector, A.L.; Crozier, P.; Petuskey, W.T.; McMillan, P.F. Mechanical properties of titanium nitride nanocomposites produced by chemical precursor synthesis followed by high-P, T treatment. Materials 2011, 4, 1747–1762. [Google Scholar] [CrossRef]
- Ding, Z.H.; Yao, B.; Qiu, L.X.; Bai, S.Z.; Guo, X.Y.; Xue, Y.F.; Wang, W.R.; Zhou, X.D.; Su, W.H. Formation of titanium nitride by me-chanical milling and isothermal annealing of titanium and boron nitride. J. Alloys Compd. 2005, 391, 77–81. [Google Scholar] [CrossRef]
- Deopura, B.L.; Alagirusamy, R.; Joshi, M.; Gupta, B. Polyesters and Polyamides, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2008. [Google Scholar]
- Procopio, A.; El-Raghy, T.; Barsoum, M. Synthesis of Ti 4 AlN 3 and phase equilibria in the Ti-Al-N system. Metall. Mater. Trans. A 2000, 31, 373–378. [Google Scholar]
- Chen, Q.; Sundman, B. Thermodynamic assessment of the Ti-Al-N system. J. Phase Equilibria Diffus. 1998, 19, 146–160. [Google Scholar] [CrossRef]
- Magnan, J.; Weatherly, G.C.; Cheynet, M.-C. The nitriding behavior of Ti-Al alloys at 1000 °C. Met. Mater. Trans. A 1999, 30, 19–29. [Google Scholar] [CrossRef]
- Huang, K.; Marthinsen, K.; Zhao, Q.; Logé, R.E. The double-edge effect of second-phase particles on the recrystallization behaviour and associated mechanical properties of metallic materials. Prog. Mater. Sci. 2018, 92, 284–359. [Google Scholar] [CrossRef]
- Sadovnikov, S.I.; Gusev, A.I. The Effect of Temperature on the Particle Sizes and the Recrystallization of Silver Sulfide Nanopowders. Phys. Solid State 2018, 60, 1308–1315. [Google Scholar] [CrossRef]
- Osinkina, T.V.; Krasikov, S.A.; Zhilina, E.M.; Agafonov, S.N.; Vedmid’, L.B.; Zhidovina, S.V. Influence of niobium and tantalum on the phase formation during the metallothermic interaction of aluminum with titanium dioxide. Russ. Metall. (Met.) 2019, 2019, 85–89. [Google Scholar] [CrossRef]
- Lin, Z.; Zhuo, M.; Li, M.; Wang, J.; Zhou, Y. Synthesis and microstructure of layered-ternary Ti2AlN ceramic. Scr. Mater. 2007, 56, 1115–1118. [Google Scholar] [CrossRef]
- Seipenbusch, M.; Rothenbacher, S.; Kirchhoff, M.; Schmid, H.-J.; Kasper, G.; Weber, A.P. Interparticle forces in silica nanoparticle agglomerates. J. Nanopart. Res. 2010, 12, 2037–2044. [Google Scholar] [CrossRef]
- Xuan, C.; Karasev, A.V.; Jönsson, P.G.; Nakajima, K. Attraction force estimations of Al2O3 particle agglomerations in the melt. Steel Res. Int. 2017, 88, 1600090. [Google Scholar] [CrossRef]


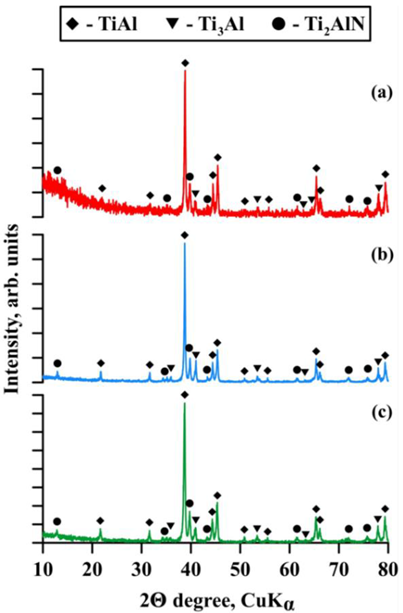

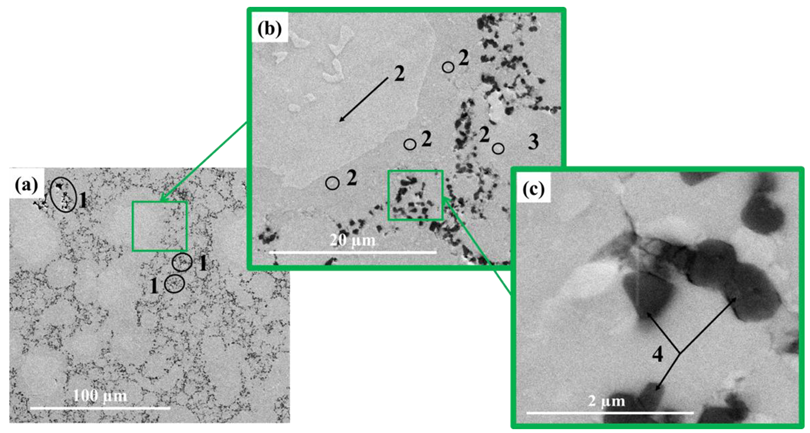

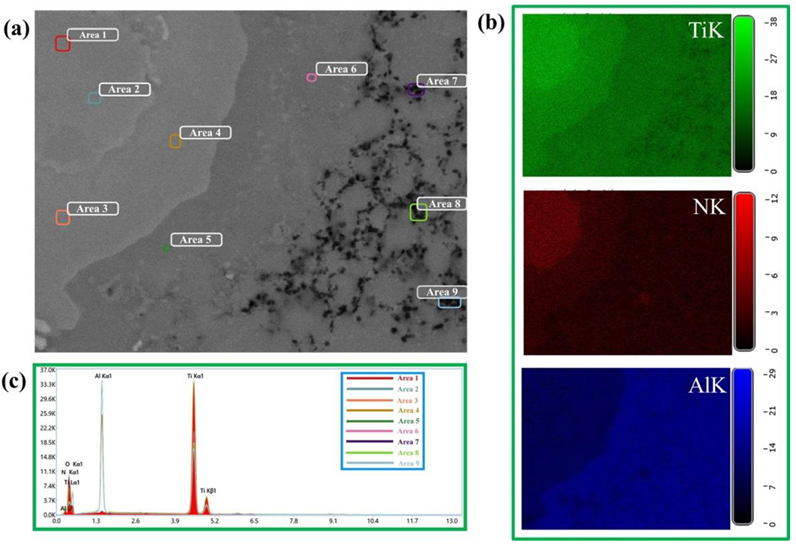
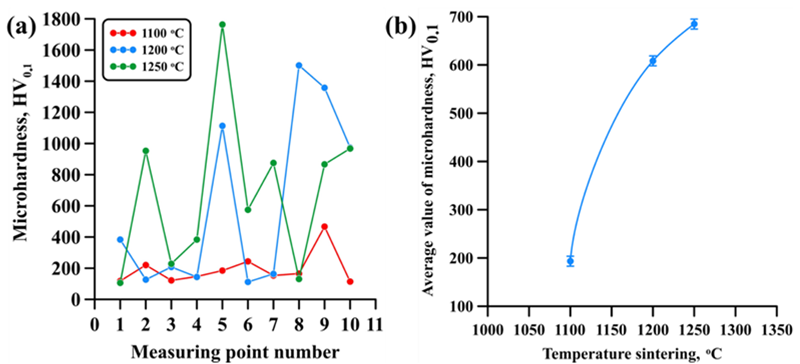
| Sintering Temperature, °C | Discovered Phases | Phase Content, wt% | Lattice Parameters, Ǻ |
|---|---|---|---|
| 1100 | TiAl | 82 | a = 3.9900 c = 4.0715 |
| Ti3Al | 8 | a = 5.7691 c = 4.6726 | |
| Ti2AlN | 10 | a = 3.0087 c = 13.6947 | |
| 1200 | TiAl | 75 | a = 4.0394 c = 4.0107 |
| Ti3Al | 12 | a = 5.7699 c = 4.6814 | |
| Ti2AlN | 13 | a = 3.0184 c = 13.6517 | |
| 1250 | TiAl | 74 | a = 3.9911 c = 4.0728 |
| Ti3Al | 9 | a = 5.8631 c = 4.6334 | |
| Ti2AlN | 17 | a = 3.0095 c = 13.6022 |
| Area | Element, wt% | |||
|---|---|---|---|---|
| NK | OK | AlK | TiK | |
| 1 | 8.4 | <1 | 20.1 | 70.5 |
| 2 | 9.5 | <1 | 19.9 | 69.6 |
| 3 | 4.3 | <1 | 19.5 | 75.2 |
| 4 | 3.8 | <1 | 18.8 | 76.4 |
| 5 | 1.5 | <1 | 37.2 | 60.3 |
| 6 | 2.7 | <1 | 36.4 | 59.9 |
| 7 | <1 | 46.7 | 51.3 | <1 |
| 8 | <1 | 47.3 | 50.7 | <1 |
| 9 | <1 | 46.5 | 51.5 | <1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promakhov, V.; Matveev, A.; Babaev, A.; Schulz, N.; Toropkov, N.; Vorozhtsov, A.; Lerner, M. 3D Printing Using Ti-Al Nanopowders: Mechanisms of Structure Formation. Metals 2022, 12, 1737. https://doi.org/10.3390/met12101737
Promakhov V, Matveev A, Babaev A, Schulz N, Toropkov N, Vorozhtsov A, Lerner M. 3D Printing Using Ti-Al Nanopowders: Mechanisms of Structure Formation. Metals. 2022; 12(10):1737. https://doi.org/10.3390/met12101737
Chicago/Turabian StylePromakhov, Vladimir, Alexey Matveev, Artem Babaev, Nikita Schulz, Nikita Toropkov, Alexander Vorozhtsov, and Marat Lerner. 2022. "3D Printing Using Ti-Al Nanopowders: Mechanisms of Structure Formation" Metals 12, no. 10: 1737. https://doi.org/10.3390/met12101737
APA StylePromakhov, V., Matveev, A., Babaev, A., Schulz, N., Toropkov, N., Vorozhtsov, A., & Lerner, M. (2022). 3D Printing Using Ti-Al Nanopowders: Mechanisms of Structure Formation. Metals, 12(10), 1737. https://doi.org/10.3390/met12101737







