Influence of Pulse Current on Inclusion Properties of Alumina in Molten Steel
Abstract
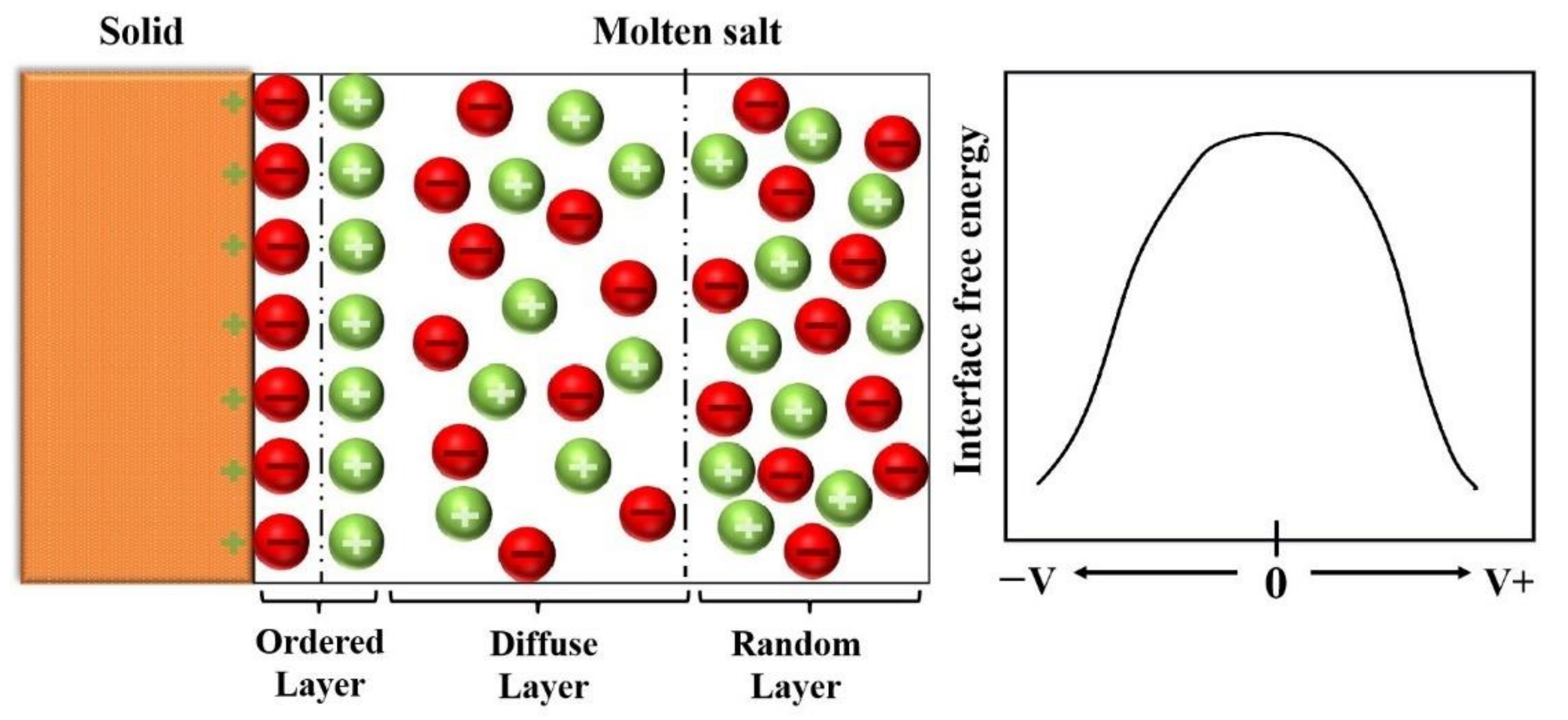
1. Introduction
2. Experiment Schemes
2.1. Experimental Materials
2.2. Experimental Device and Scheme
2.3. Experimental Results and Discussion
3. Conclusions
- (1)
- The results show that the number of small alumina inclusions in the steel after electric pulse treatment (the pulse current density was 0.1–3 A/cm2, the frequency was 100 Hz, and the pulse waveform was a square wave) was significantly increased compared with the sample without electric pulse treatment, and the particle size was concentrated at 2–5 μm, and the inclusions were concentrated in the upper and lower parts of the sample. However, in the samples without electrification treatment, the large particles of inclusions were the majority, and the particle size is concentrated at about 10 μm, and the inclusions are concentrated in the upper and middle parts of the samples.
- (2)
- When the pulse current was applied to the molten steel, the interfacial free energy of the internal system of liquid steel alumina inclusion was reduced, the nucleation of alumina inclusion was promoted, the aggregation growth between alumina particles was restrained, and then the migration and distribution of inclusions were also affected.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.N. Mechanism of alumina buildup in tundish nozzles during continuous casting ofaluminum-killed steels. Metall. Mater. Trans. B 1974, 5, 2165–2178. [Google Scholar] [CrossRef]
- Sambasivam, R. Clogging resistant submerged entry nozzle design through mathematical modeling. Ironmak. Steelmak. 2006, 33, 439–453. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, C.S.; Girase, N.U. Nozzle clogging behaviour of Ti-bearing Al-killed ultra lowcarbon steel. ISIJ Int. 2004, 44, 1653–1660. [Google Scholar] [CrossRef]
- Sun, M.K.; Jung, I.H.; Lee, H.G. Morphology and chemistry of oxide inclusions after Al and Ticomplex deoxidation. Met. Mater. Int. 2008, 14, 791–798. [Google Scholar] [CrossRef]
- Yu, J.K.; Yang, X.; Liu, Z.Y.; Hou, X.H.; Kai, Z.K. Anti-clogging of submerged entry nozzle through control of electrical characteristics. Ceram. Int. 2017, 43, 13025–13029. [Google Scholar] [CrossRef]
- Zhang, X.F.; Qin, R.S. Controlled motion of electrically neutral microparticles by pulsed direct current. Sci. Rep. 2015, 5, 10162. [Google Scholar] [CrossRef]
- Zhang, X.F.; Lu, W.J.; Qin, R.S. Removal of MnS inclusions in molten steel using electropulsing. Scr. Mater. 2013, 69, 453–456. [Google Scholar] [CrossRef]
- Zhang, X.F.; Qin, R.S. Separation of electrically neutral non-metallic inclusions from molten steel by pulsed electric current. Mater. Sci. Technol. 2017, 33, 1399–1403. [Google Scholar] [CrossRef]
- Paik, Y.H.; Shin, H.C.; Lee, J.M. Electrical charge of metal oxides in liquid metals. Met. Mater. 1998, 4, 995–1000. [Google Scholar] [CrossRef]
- Paik, Y.H.; Yoon, W.J.; Shin, H.C. Static electrification of solid oxide in liquid metal and electrical double layer at the interface. J. Colloid Interface Sci. 2004, 269, 353–354. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.M.; Shin, H.C. Separation of oxide inclusions from liquid metal in an applied electrostatic field. Met. Mater. Int. 2003, 9, 593. [Google Scholar] [CrossRef]
- Zhang, B.W.; Li, B.W. Growth kinetics of single inclusion particle in molten melts. Acta Metall. Sin. Engl. Lett. 2007, 20, 416–424. [Google Scholar] [CrossRef]
- Wu, J.X.; Ren, Z.M.; Zhang, B.W. Electromagnetic purification of aluminum alloy melt only by alternating current. Chin. J. Nonferrous Met. 2004, 14, 354–358. [Google Scholar]
- Wang, S.; Zhang, L.; Tian, Y.; Ling, H. Separation of Non-metallic Inclusions from Molten Steel Using High Frequency Electromagnetic Ficlds. Metall. Mater. Trans. B 2014, 45, 1915–1935. [Google Scholar] [CrossRef]
- Zhang, X.F.; Lu, W.J.; Qin, R.S. Morphology and distribution control of MnS inclusions in molten steel by electropulsing. Mater. Res. Innov. 2015, 18, 244–248. [Google Scholar] [CrossRef]
- Lu, W.J.; Zhang, X.F.; Qin, R.S. Stability of precipitates under electropulsing in 316L stainless steel. Mater. Sci. Technol. 2015, 13, 2–11. [Google Scholar] [CrossRef]
- Fu, J.W.; Yang, Y.S. Formation of the solidified microstructure of Mg-Al-Zn alloy under a low-voltage pulsed magnetic field. J. Mater. Res. 2011, 26, 1688–1695. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Luo, J.; Jing, J.X.; Xia, Z.Q.; Zhai, Q.J. Structure refinement of pure aluminum by pulse magneto-oscillation. Mater. Sci. Eng. A 2008, 497, 147–152. [Google Scholar] [CrossRef]
- Nightingale, S.A.; Monaghan, B.J.; Brooks, G.A. Degradation of MgO refractory in CaO-SiO2-MgO-FeOx and CaO-SiO2-Al2O3-MgO-FeOx slags under forced convection. Metall. Mater. Trans. B 2005, 36, 453–461. [Google Scholar] [CrossRef]
- Nightingale, S.A.; Monaghan, B.J. Kinetics of Spinel Formation and Growth during Dissolution of MgO in CaO-Al2O3-SiO2 Slag. Metall. Mater. Trans. B 2008, 39, 643–648. [Google Scholar] [CrossRef]
- Yang, X.; He, Z.J.; Yu, J.K.; Zhang, Y.Y.; Yuan, L.; Mao, F.X. Influence of interface electric field on interaction between molten iron and refractory interface. Ceram. Int. 2020, 46, 10180–10185. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.Y.; Yuan, L.; Mao, F.X.; Yu, J.K.; He, Z.J. Influence of Interface Electric Field on Wettability Between Molten Iron and Submerged Entry Nozzle Interface. JOM 2020, 72, 3521–3528. [Google Scholar] [CrossRef]
- Nogi, K. Wetting Phenomena in Materials Processing. Tetsu Hagane 2010, 84, 1–6. [Google Scholar]
- Zhang, L.; Thomas, B.G. State of the art in the control of inclusions during steel ingot casting. Metall. Mater. Trans. B 2006, 37, 733–761. [Google Scholar] [CrossRef]


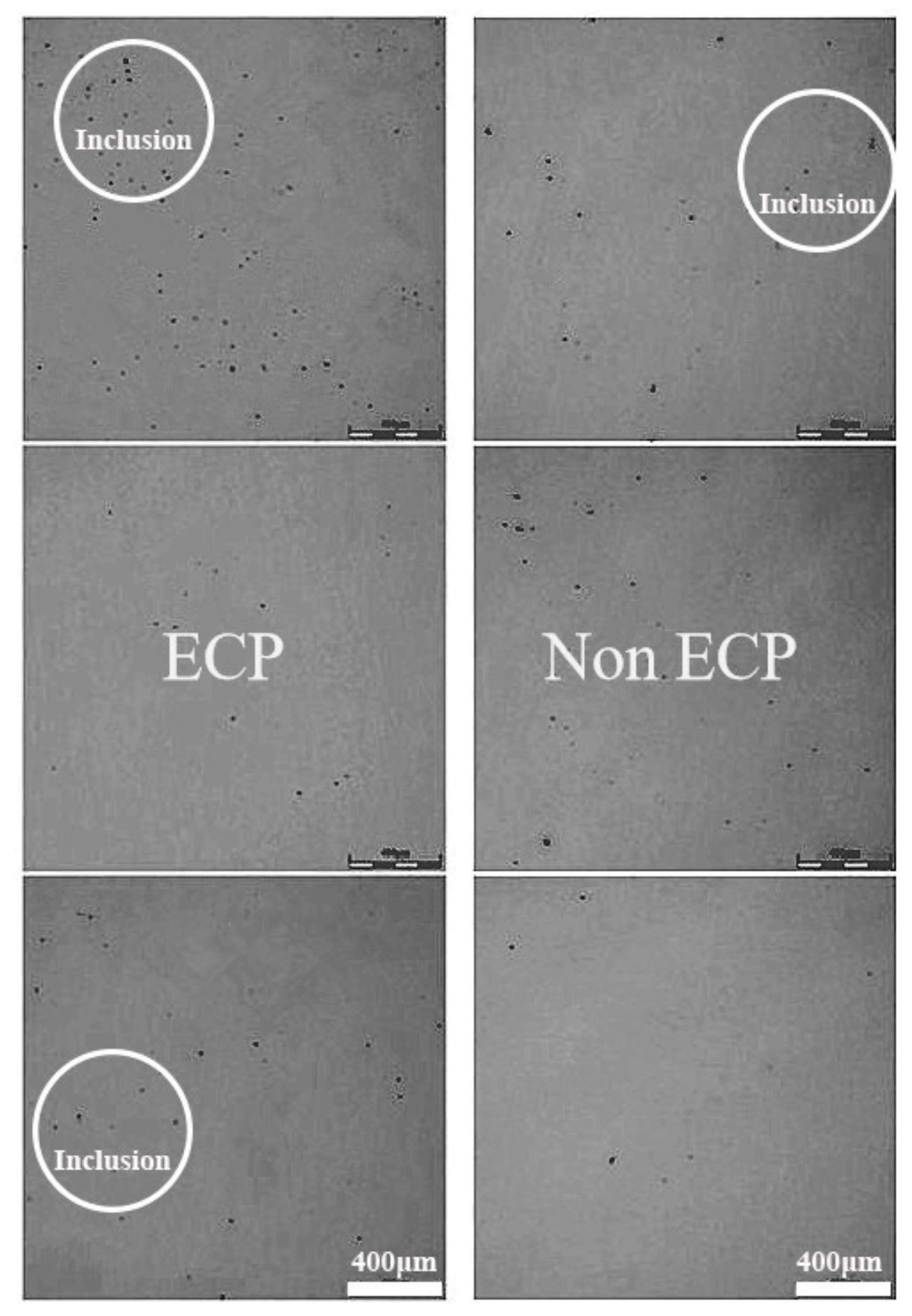
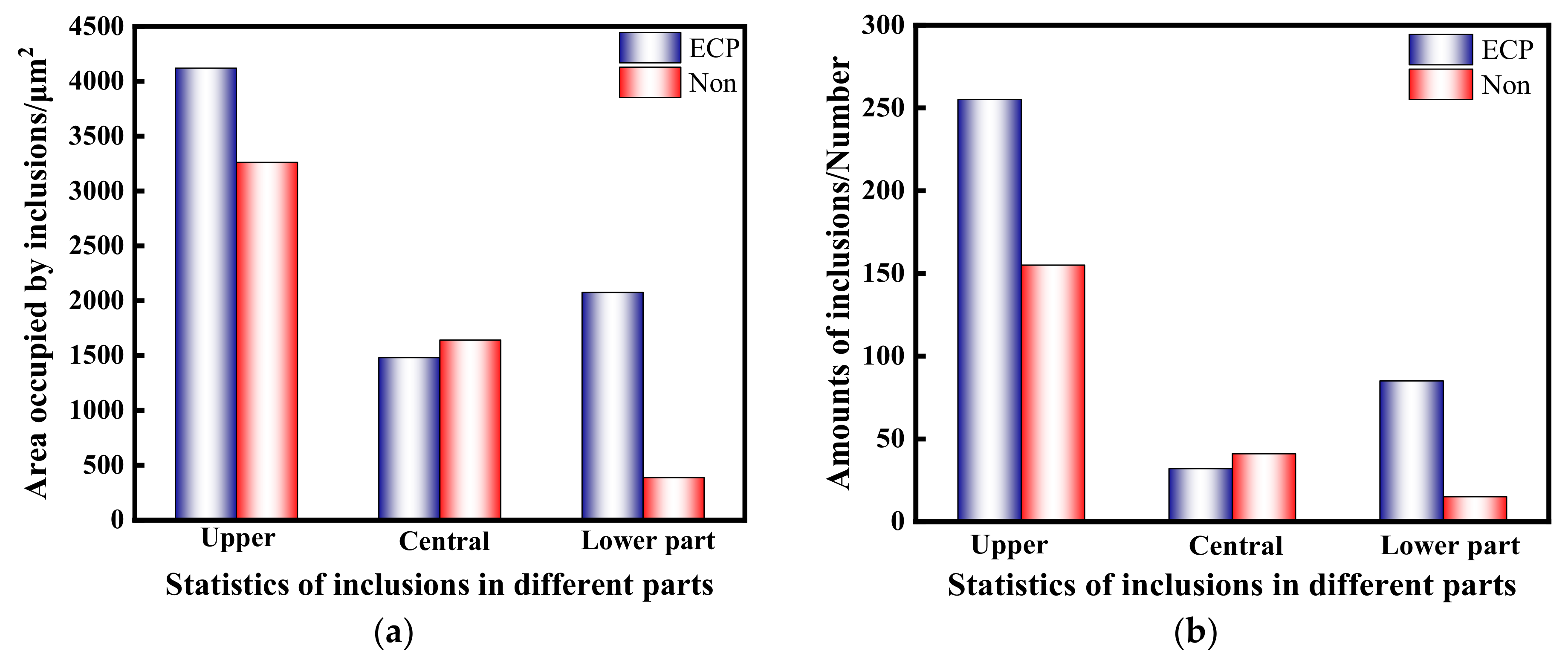
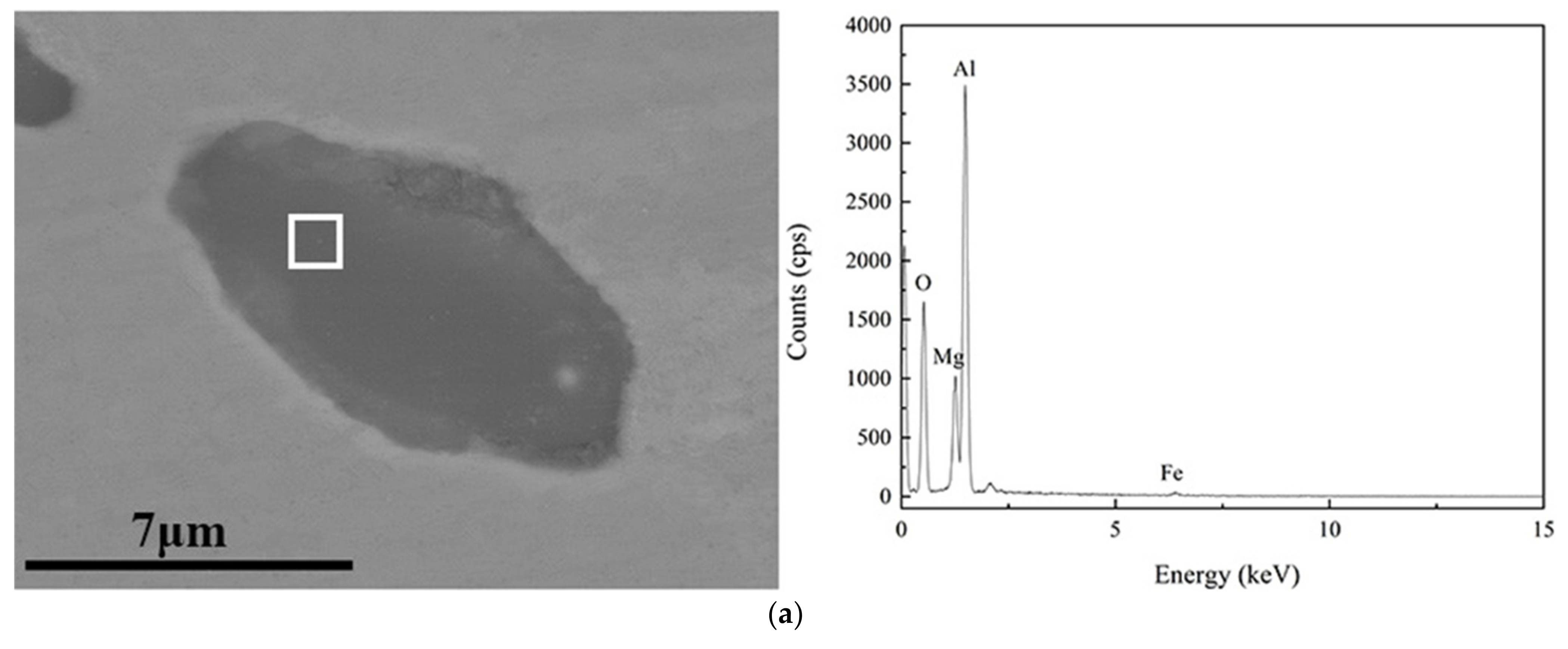
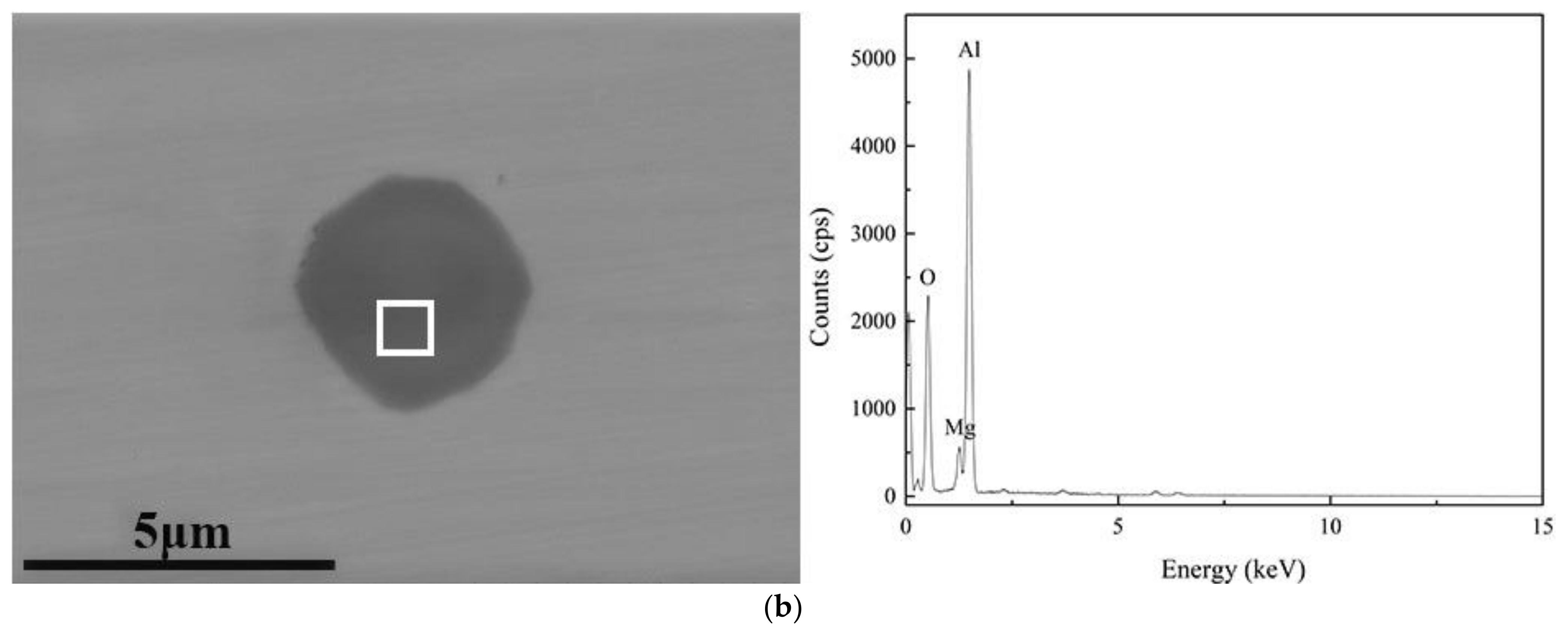
| C | Si | Mn | P | S | Als |
|---|---|---|---|---|---|
| 0.220 | 0.032 | 0.854 | 0.025 | 0.018 | 0.035 |
| Model Inclusion (μm) | ECP | No ECP |
|---|---|---|
| 2–5 | 260 | 38 |
| 5–10 | 98 | 152 |
| 10–20 | 4 | 19 |
| >20 | 0 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, L.; Cao, D.; Han, X.; Zhang, Y.; He, Z. Influence of Pulse Current on Inclusion Properties of Alumina in Molten Steel. Metals 2022, 12, 1742. https://doi.org/10.3390/met12101742
Yang X, Zhang L, Cao D, Han X, Zhang Y, He Z. Influence of Pulse Current on Inclusion Properties of Alumina in Molten Steel. Metals. 2022; 12(10):1742. https://doi.org/10.3390/met12101742
Chicago/Turabian StyleYang, Xin, Liguo Zhang, Dong Cao, Xiao Han, Yuanyuan Zhang, and Zhijun He. 2022. "Influence of Pulse Current on Inclusion Properties of Alumina in Molten Steel" Metals 12, no. 10: 1742. https://doi.org/10.3390/met12101742
APA StyleYang, X., Zhang, L., Cao, D., Han, X., Zhang, Y., & He, Z. (2022). Influence of Pulse Current on Inclusion Properties of Alumina in Molten Steel. Metals, 12(10), 1742. https://doi.org/10.3390/met12101742








