Static Recrystallization Behavior of Low-Carbon Nb-V-Microalloyed Forging Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
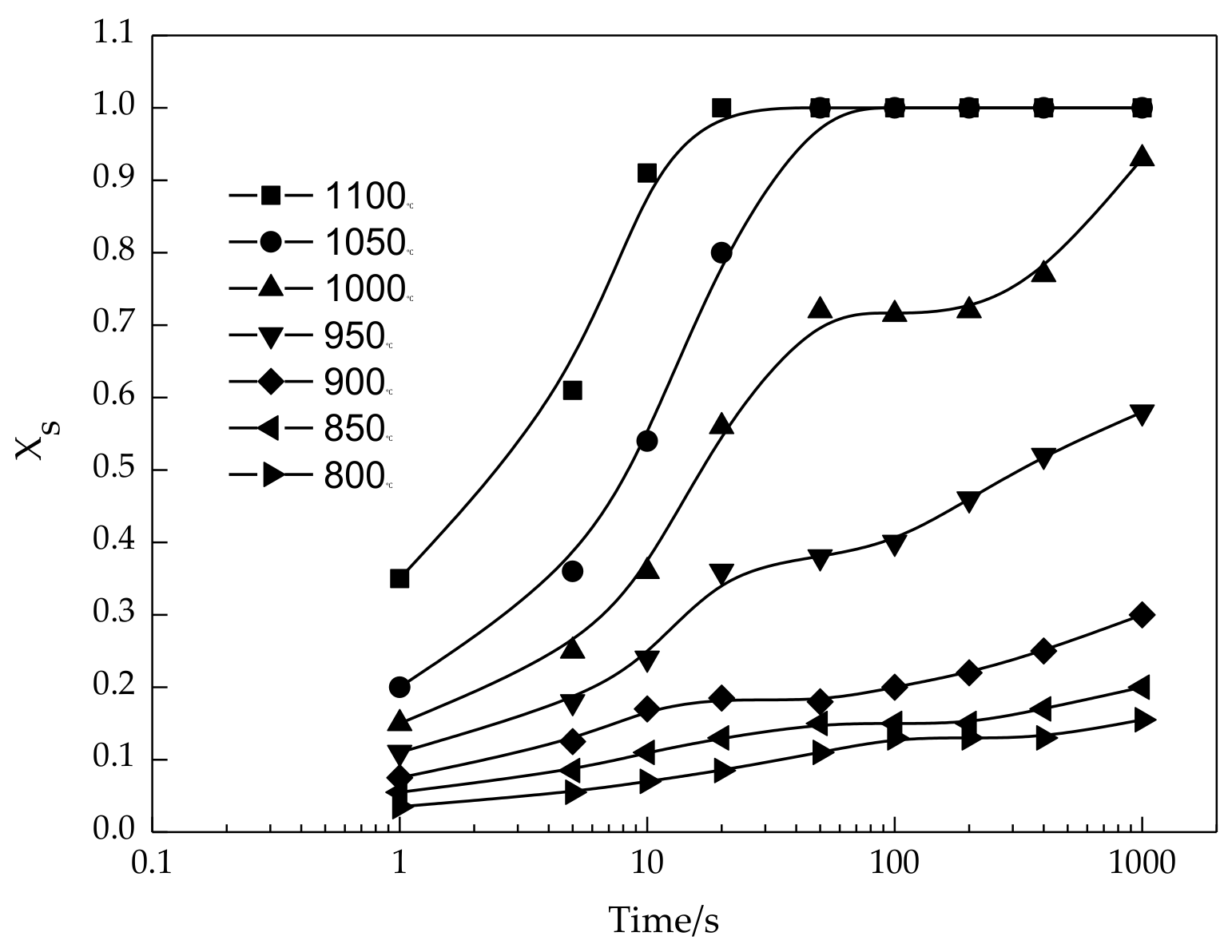
3.1. Static Recrystallization Fraction
3.2. Static Recrystallization Activation Energy
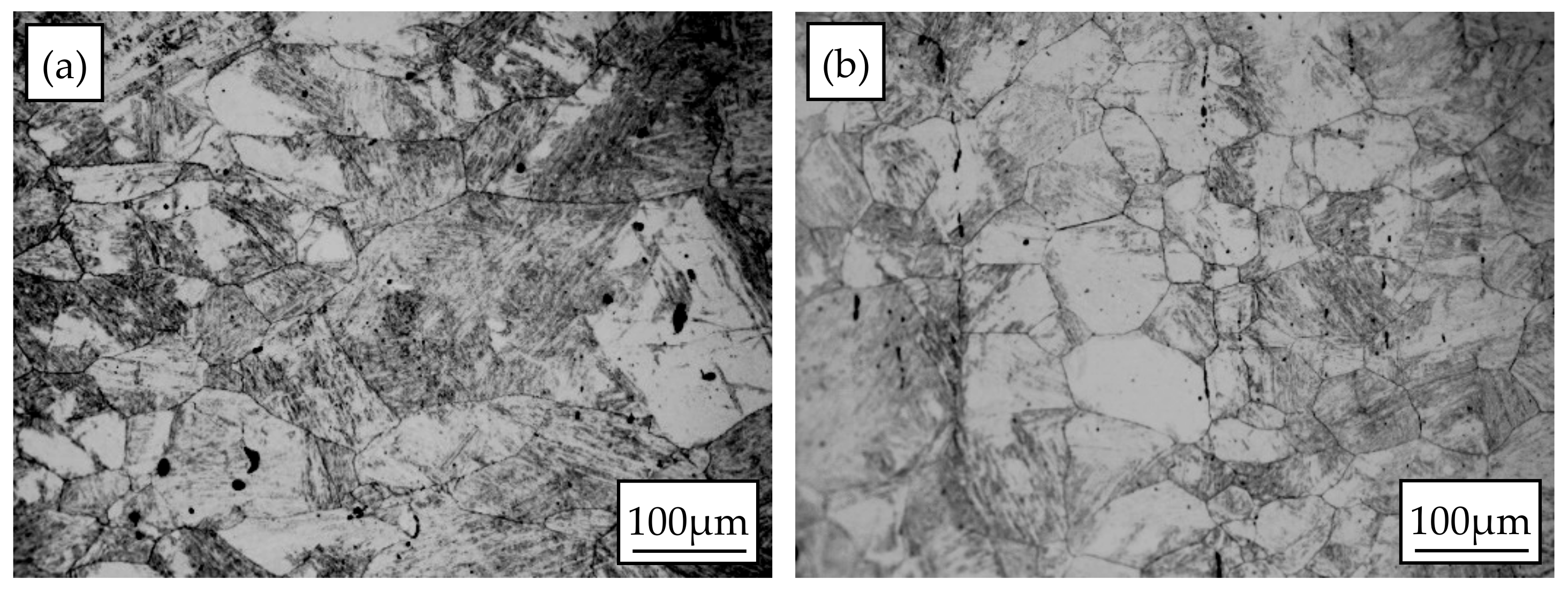
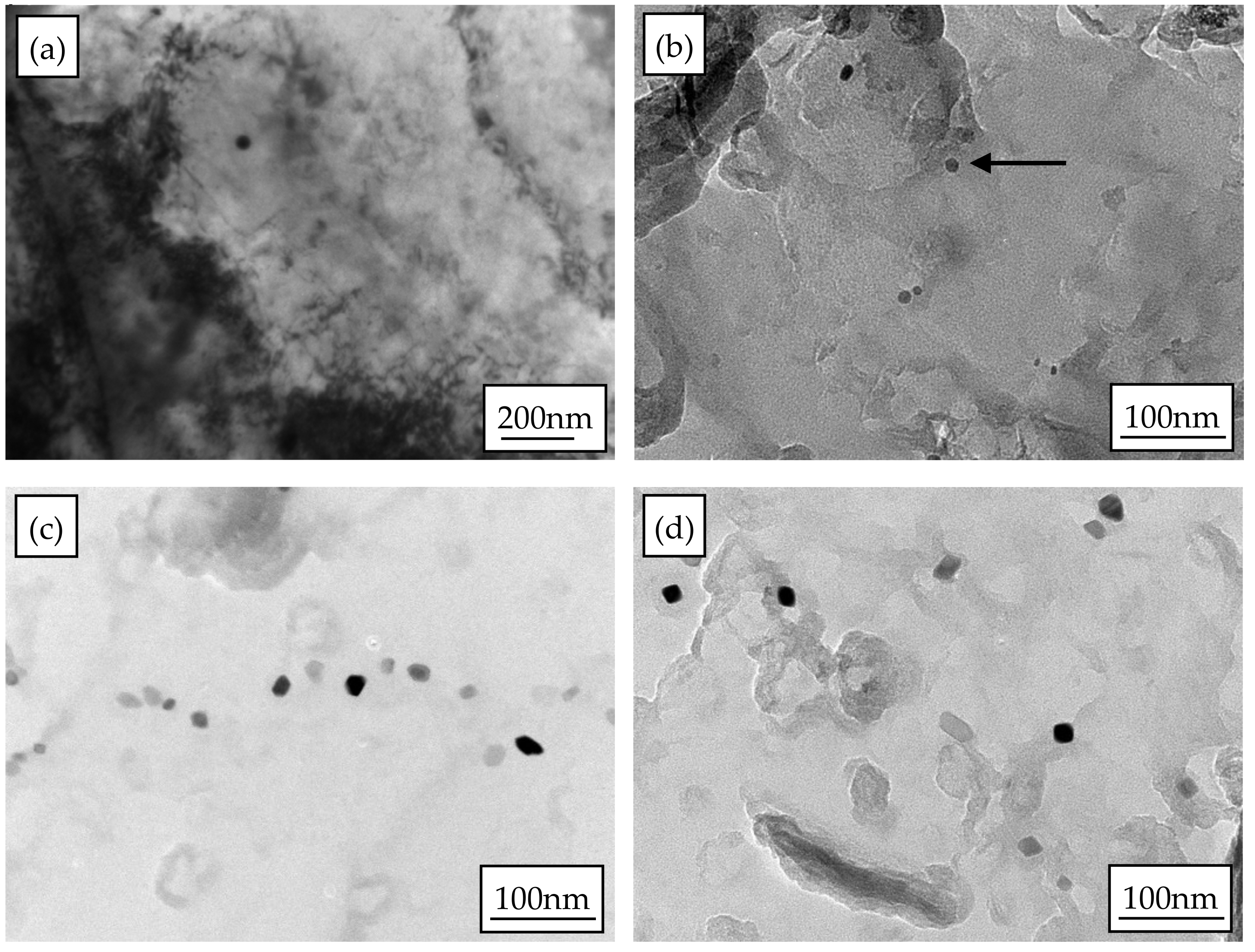
3.3. Static Recrystallization Microstructure Evolution
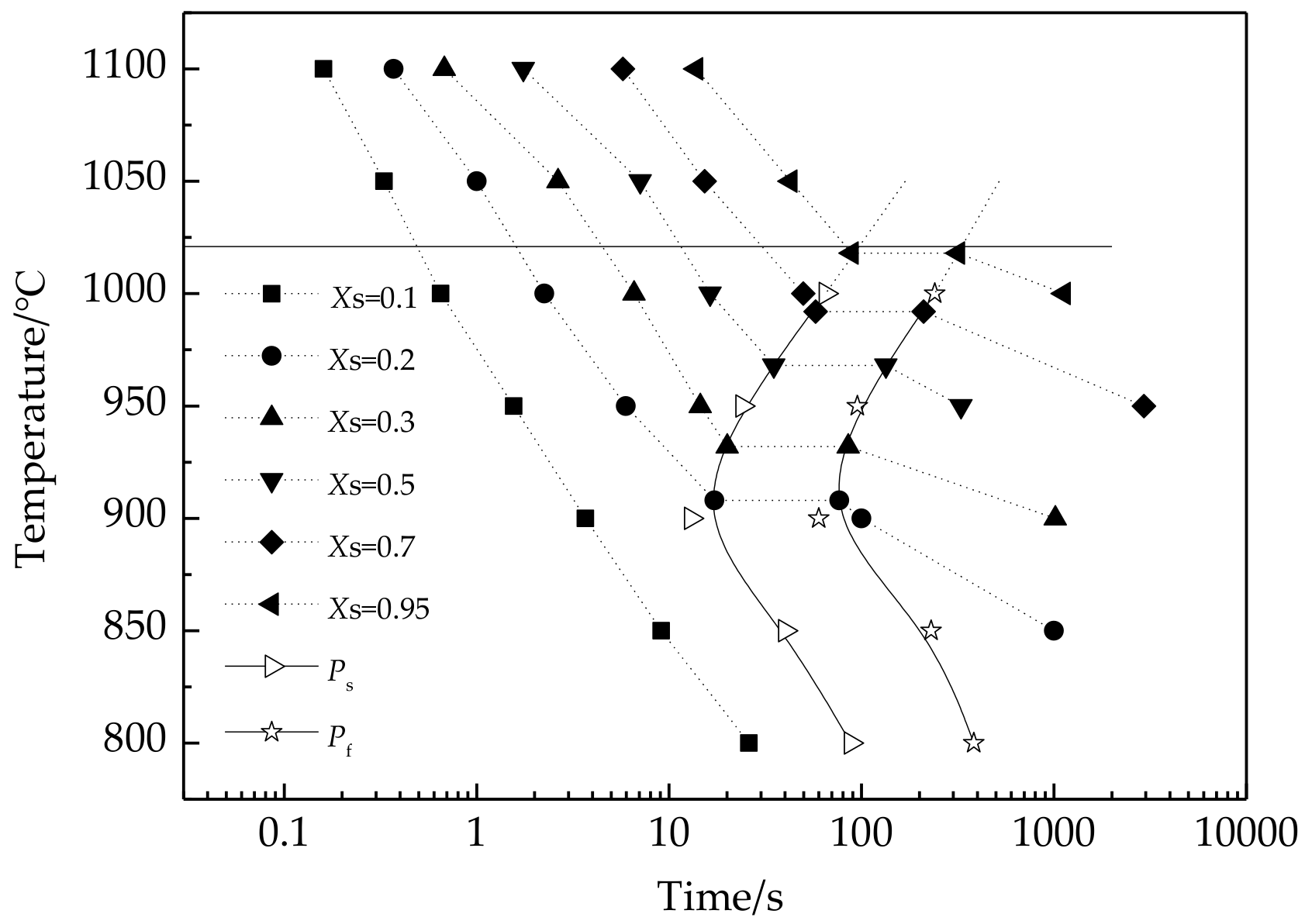
3.4. Recrystallization-Precipitation-Time-Temperature Diagram
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, F.; Jiang, B.; Xie, J.; Liu, Y. The optimized microstructure and properties of a V-Ti microalloyed forging steel by boron addition. Mater. Lett. 2019, 236, 440–443. [Google Scholar] [CrossRef]
- Jovanović, G.; Glišić, D.; Dikić, S.; Radović, N.; Patarić, A. Cleavage fracture of the air cooled medium carbon microalloyed forging steels with heterogeneous microstructures. Materials 2022, 15, 1760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, M.; Jiang, B.; Zhang, C.; Xie, J.; Liu, Y. Effect of nitrogen contents on the microstructure and mechanical properties of V-Ti microalloyed steels for the forging of crankshafts. Mater. Sci. Eng. A 2018, 731, 360–368. [Google Scholar] [CrossRef]
- Rasouli, D.; Asl, S.K.; Akbarzadeh, A.; Daneshi, G.H. Effect of cooling rate on the microstructure and mechanical properties of microalloyed forging steel. J. Mater. Process. Technol. 2008, 206, 92–98. [Google Scholar] [CrossRef]
- Matlock, D.K.; Speer, G.K. Microstructures and properties of direct-cooled microalloyed forging steels. J. Mater. Process. Technol. 2001, 117, 324–328. [Google Scholar] [CrossRef]
- Hui, W.J.; Xiao, N.; Zhao, X.L.; Zhang, Y.J.; Wu, Y.F. Effect of vanadium on dynamic continuous cooling transformation behavior of medium-carbon forging steels. J. Iron Steel Res. Int. 2017, 24, 641–648. [Google Scholar] [CrossRef]
- Miyamoto, G.; Shinyoshi, T.; Yamaguchi, J.; Furuhara, T.; Uemori, T.K. Crystallography of intragranular ferrite formed on (MnS+V(C,N)) complex precipitate in austenite. Scr. Mater. 2003, 48, 371–377. [Google Scholar] [CrossRef]
- Gómez, M.; Medina, S.F.; Quispe, A.; Valles, P. Static recrystallization and induced precipitation in a low Nb microalloyed steel. ISIJ Int. 2002, 42, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Lan, L.Y.; Qiu, C.L.; Zhao, D.W.; Gao, X.H.; Du, L.X. Dynamic and static recrystallization behavior of low carbon high niobium microalloyed Steel. J. Iron Steel Res. Int. 2011, 18, 55–60. [Google Scholar] [CrossRef]
- Zurob, H.S.; Brechet, Y.; Purdy, G. A model for the competition of precipitation and recrystallization in deformed austenite. Acta Mater. 2001, 49, 4183–4190. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, C.; Zhang, L.; Xu, Q.; Cui, Y.; Xu, Y. A modified Avrami equation for kinetics of static recrystallization of Nb-V microalloyed steel: Experiments and numerical simulation. Vacuum 2018, 150, 116–123. [Google Scholar] [CrossRef]
- García-Mateo, C.; López, B.; Rodriguez-Ibabe, J.M. Static recrystallization kinetics in warm worked vanadium microalloyed steels. Mater. Sci. Eng. A 2001, 303, 216–225. [Google Scholar] [CrossRef]
- Cho, S.H.; Kang, K.B.; Jonas, J.J. The dynamic, static and metadynamic recrystallization of a Nb-microalloyed steel. ISIJ Int. 2001, 41, 63–69. [Google Scholar] [CrossRef]
- Quispe, A.; Medina, S.F.; Valles, P. Recrystallization-induced precipitation interaction in a medium carbon vanadium microalloyed steel. ISIJ Int. 1997, 37, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.F.; Zhang, L.W.; Zhang, C.; Xu, Y.F.; Shi, X.H. Constitutive analysis of dynamic recrystallization and flow behavior of a medium carbon Nb-V microalloyed steel. J. Mater. Eng. Perform. 2016, 25, 2065–2073. [Google Scholar] [CrossRef]
- Khodabandeh, A.R.; Jahazi, M.; Yue, S.; Bocher, P. Impact toughness and tensile properties improvement through microstructure control in hot forged Nb-V microalloyed steel. ISIJ Int. 2005, 45, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Huang, L.; Ma, H.; Zhen, W. Strain energy method to determine static recrystallization volume fraction of microalloyed steel. Appl. Phys. A 2020, 126, 947. [Google Scholar] [CrossRef]
- Jin, M.; Lu, B.; Liu, X.G.; Guo, H.; Ji, H.P.; Guo, B.F. Static recrystallization behavior of 316LN austenitic stainless steel. J. Iron Steel Res. Int. 2013, 20, 67–72. [Google Scholar] [CrossRef]
- Li, G.; Maccagno, T.M.; Bai, D.B.; Jonas, J.J. Effect of initial grain size on the static recrystallization kinetics of Nb microalloyed steels. ISIJ Int. 1996, 36, 1479–1485. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, M.S.; Zhong, J. Study of static recrystallization kinetics in a low alloy steel. Comp. Mater. Sci. 2008, 44, 316–321. [Google Scholar] [CrossRef]
- Mandal, G.K.; Das, S.S.; Kumar, T.; Kamaraj, A.; Mondal, K.; Srivastava, V.C. Role of precipitates in recrystallization mechanisms of Nb-Mo microalloyed steel. J. Mater. Eng. Perform. 2018, 27, 6748–6757. [Google Scholar] [CrossRef]
- Hodgson, P.D.; Zahiri, S.H.; Whale, J.J. The static and metadynamic recrystallization behaviour of an X60 Nb microalloyed steel. ISIJ Int. 2004, 44, 1224–1229. [Google Scholar] [CrossRef]
- Wu, H.; Du, L.; Ai, Z.; Liu, X. Static recrystallization and precipitation behavior of a weathering steel microalloyed with vanadium. J. Mater. Sci. Technol. 2013, 29, 1197–1203. [Google Scholar] [CrossRef]
- Medina, S.F.; Mancilla, J.E. Influence of alloying elements in solution on static recrystallization kinetics of hot deformed steels. ISIJ Int. 1996, 36, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Medina, S.F.; Quispe, A. Improved model for static recrystallization kinetics of hot deformed austenite in low alloy and Nb/V microalloyed steels. ISIJ Int. 2001, 41, 774–781. [Google Scholar] [CrossRef]
- Graux, A.; Cazottes, S.; Castro, D.D.; San-Martín, D.; Capdevila, C.; Cabrera, J.M.; Molas, S.; Schreiber, S.; Mirković, D.; Danoix, F.; et al. Design and development of complex phase steels with improved combination of strength and stretch-flangeability. Metals 2020, 10, 824. [Google Scholar] [CrossRef]
- Quispe, A.; Medina, S.F.; Gómez, M.; Chaves, J.J. Influence of austenite grain size on recrystallisation–precipitation interaction in a V-microalloyed steel. Mater. Sci. Eng. A 2007, 447, 11–18. [Google Scholar] [CrossRef]
- Medina, S.F.; Gómez, M.; Gómez, P.P. Effects of V and Nb on static recrystallisation of austenite and precipitate size in microalloyed steels. J. Mater. Sci. 2010, 45, 5553–5557. [Google Scholar] [CrossRef]
- Medina, S.F.; Mancilla, J.E. Determination of static recrystallization critical temperature of austenite in microalloyed steels. ISIJ Int. 1993, 33, 1257–1264. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.; DeArdo, A.J. Interactions between recrystallization and precipitation in hot-deformed microalloyed steels. Acta Metall. Mater. 1991, 39, 529–538. [Google Scholar] [CrossRef]
- Dutta, B.; Sellars, C.M. Effect of composition and process variables on Nb(C,N) precipitation in niobium microalloyed austenite. Mater. Sci. Technol. 1987, 3, 197–206. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, L.; Zeng, R.; Wen, D.; Su, H.; Li, J. In-situ observations and modeling of static recrystallization in 300 M steel. Mater. Sci. Eng. A 2019, 765, 138300. [Google Scholar] [CrossRef]
- Quispe, A.B.; Medina, S.F.; Cabrera, J.M.; Prado, J.M. Effect of strain on recrystallisation-precipitation interaction in low vanadium microalloyed steel. Mater. Sci. Technol. 1999, 15, 635–642. [Google Scholar] [CrossRef]
- Medina, S.F.; Quispe, A.; Gomez, M. Model for strain-induced precipitation kinetics in microalloyed steels. Metall. Mater. Trans. A 2014, 45, 1524–1539. [Google Scholar] [CrossRef]





| C | Si | Mn | S | P | Cr | V | Nb |
|---|---|---|---|---|---|---|---|
| 0.24 | 0.30 | 1.99 | 0.050 | 0.008 | 0.51 | 0.08 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zheng, J.; Chen, L.; Liu, X. Static Recrystallization Behavior of Low-Carbon Nb-V-Microalloyed Forging Steel. Metals 2022, 12, 1745. https://doi.org/10.3390/met12101745
Zhao Y, Zheng J, Chen L, Liu X. Static Recrystallization Behavior of Low-Carbon Nb-V-Microalloyed Forging Steel. Metals. 2022; 12(10):1745. https://doi.org/10.3390/met12101745
Chicago/Turabian StyleZhao, Yang, Jiahao Zheng, Liqing Chen, and Xianghua Liu. 2022. "Static Recrystallization Behavior of Low-Carbon Nb-V-Microalloyed Forging Steel" Metals 12, no. 10: 1745. https://doi.org/10.3390/met12101745





