Corrosion Resistance of Mg(OH)2/Mn(OH)2 Hydroxide Film on ZK60 Mg Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Mg(OH)2/Mn(OH)2 Hydroxide Film
2.3. Characterization
3. Results and Discussions
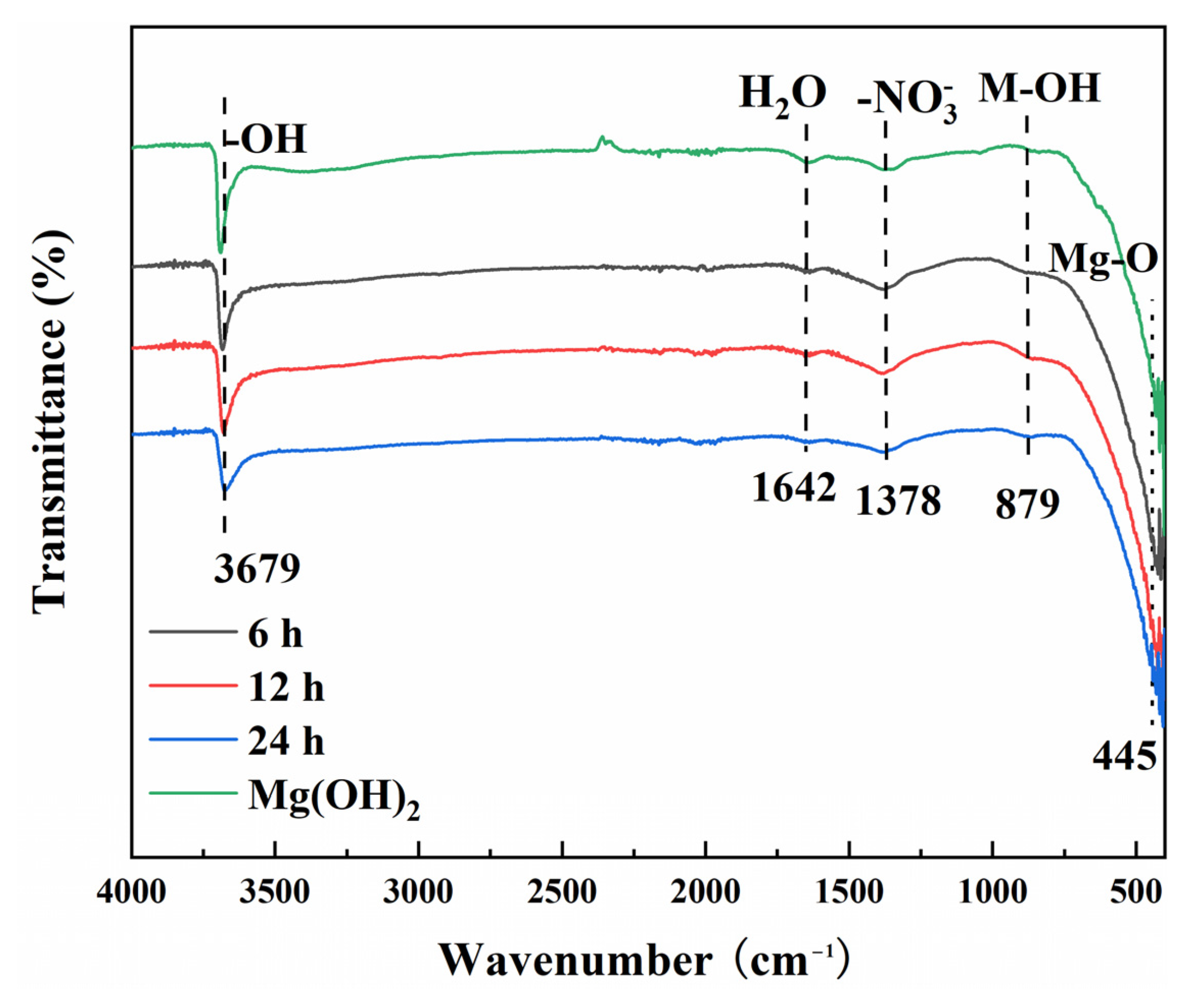
3.1. Composition of Films
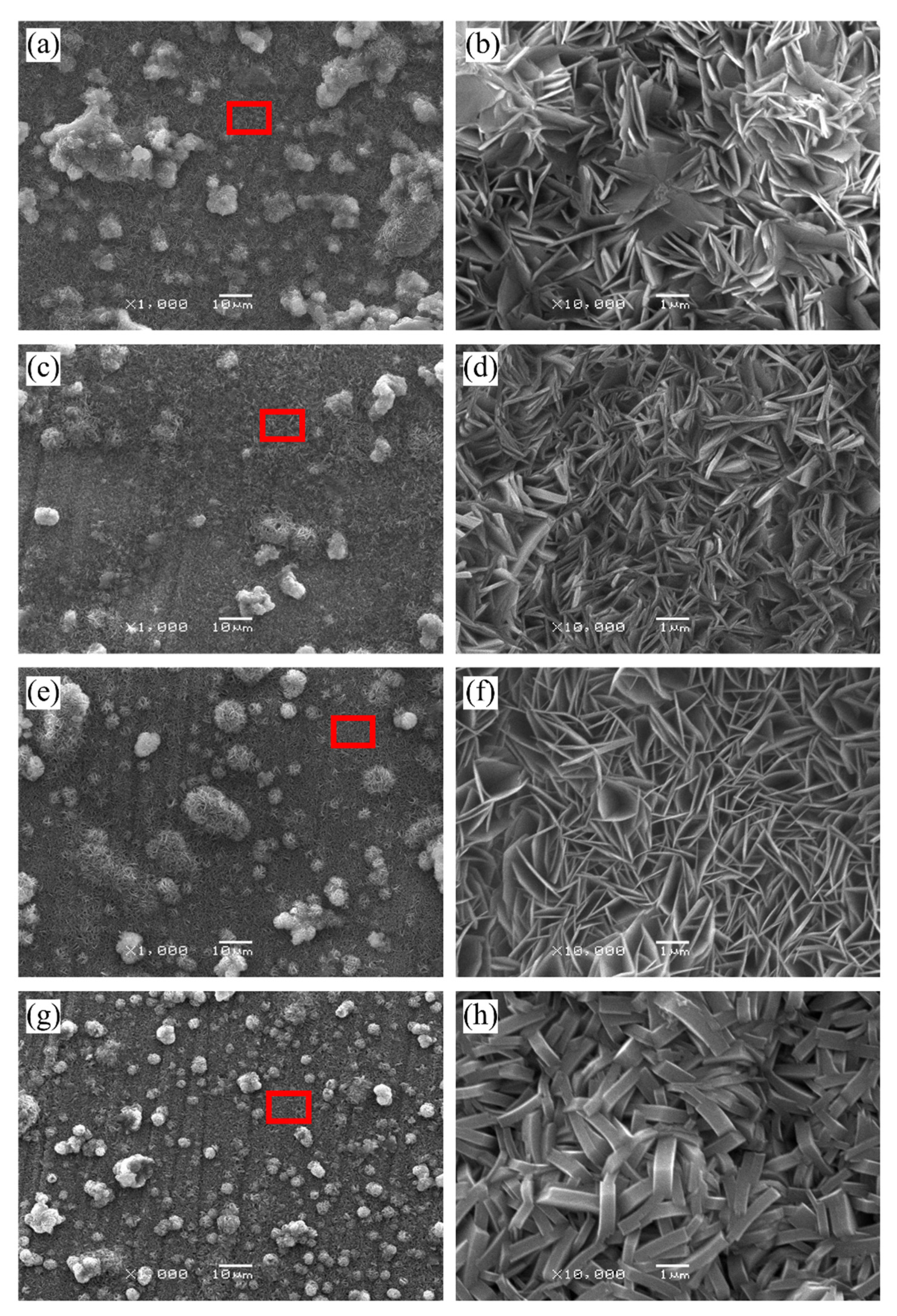
3.2. Morphology
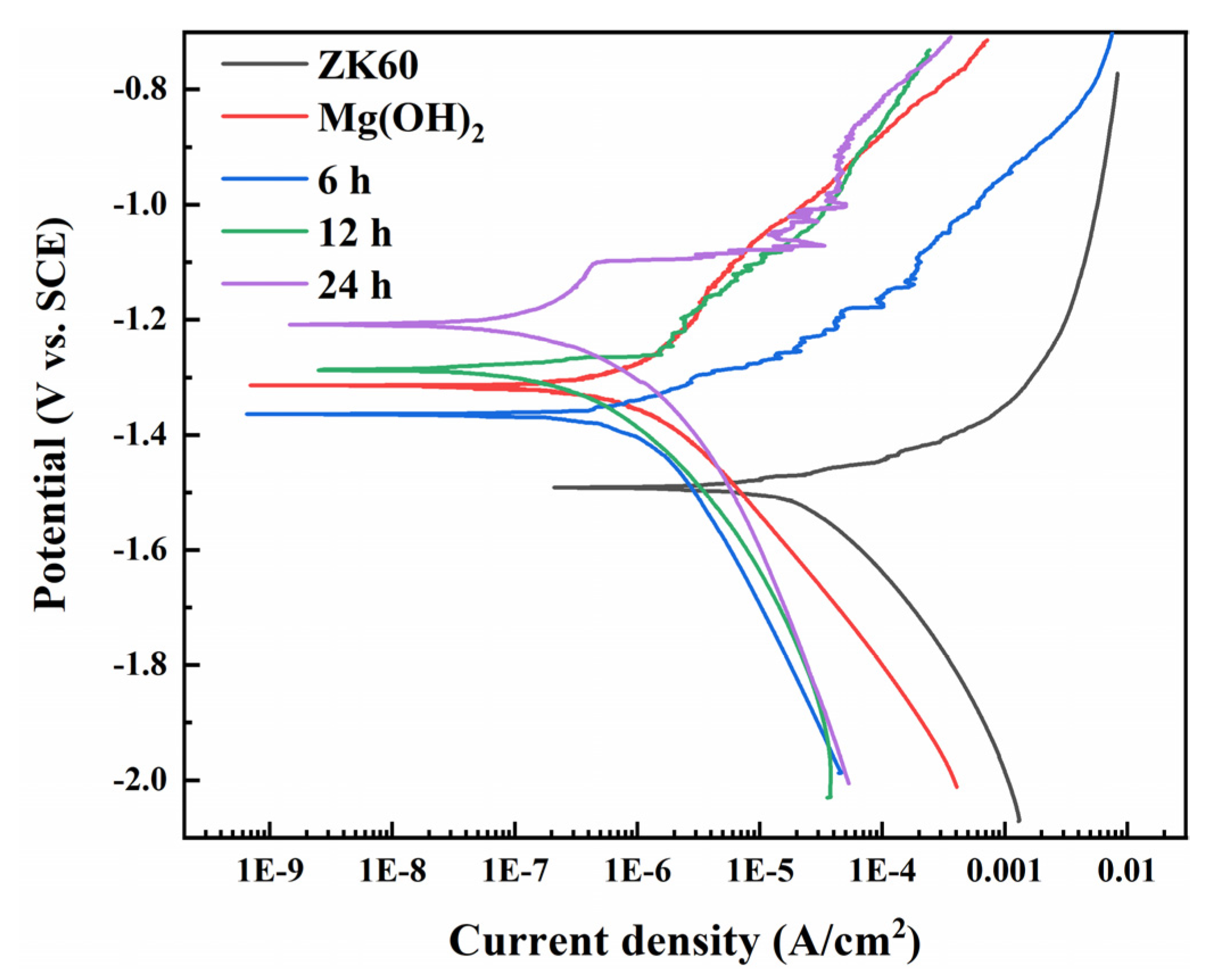
3.3. Corrosion Resistance
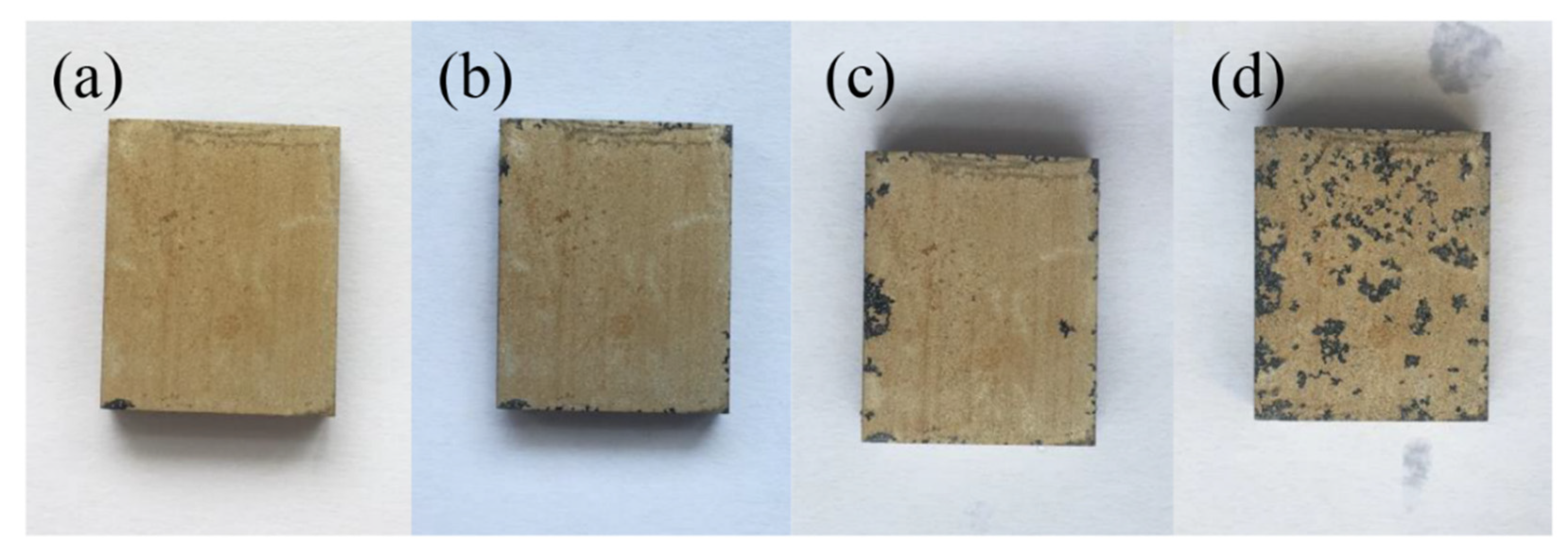
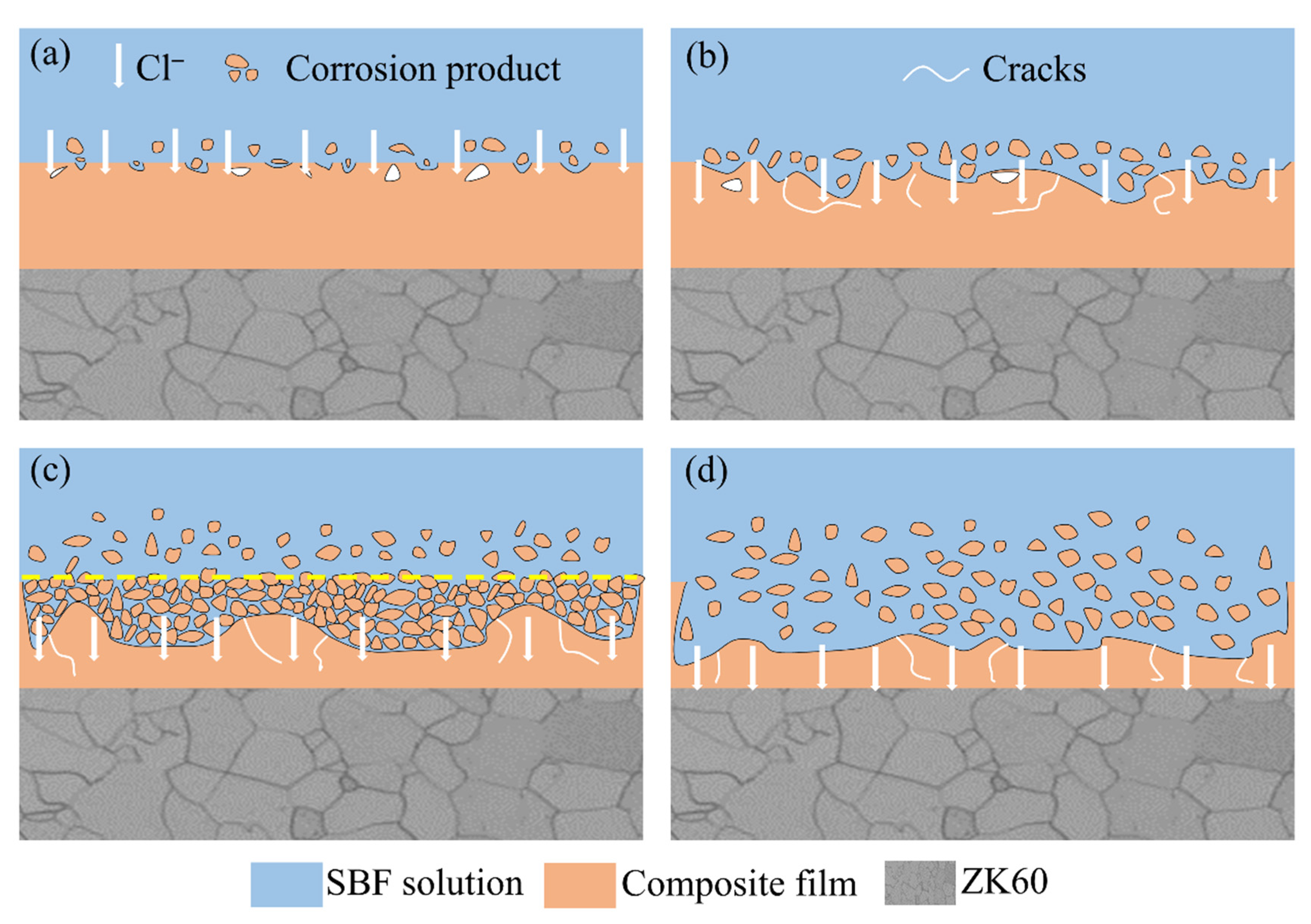
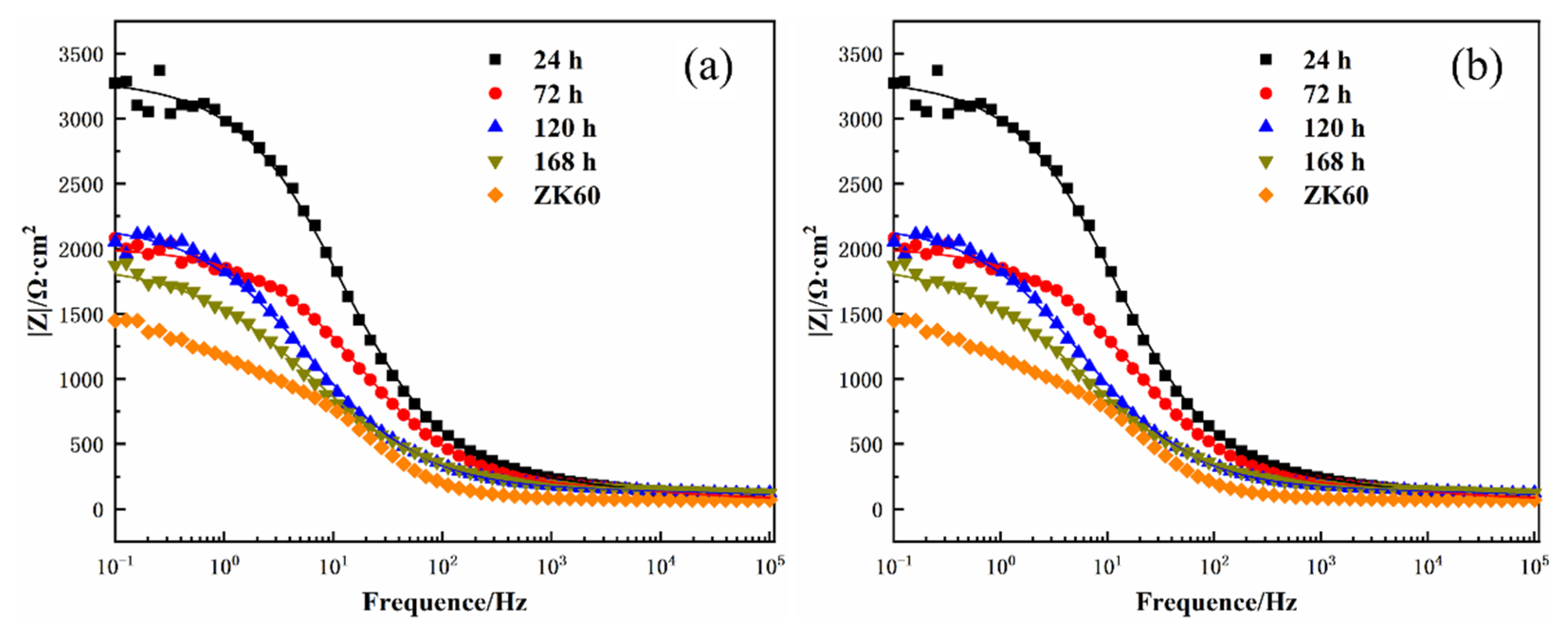
3.4. Corrosion Resistance of the 12 h Sample at Different Immersion Times
4. Conclusions
- (1)
- A simple hydrothermal process was demonstrated for the growth of Mg(OH)2/Mn(OH)2 hydroxide films on the ZK60 magnesium alloy substrates. The films were mainly composed of Mg(OH)2 and Mn(OH)2 phases;
- (2)
- By increasing the hydrothermal crystallization time from 6 h to 24 h, the structure of the hydroxide films changed from incomplete lamellae to complete lamellae and then to thick lamellae while decreasing the relative concentration of Mg and increasing the content of Mn elements;
- (3)
- After a 12 h hydrothermal crystallization, the Mg(OH)2/Mn(OH)2 hydroxide film exhibited the best corrosion resistance with a corrosion current density of 3.07 × 10−7 A·cm−2, which was approximately two orders and one order of magnitude lower than those of ZK60 magnesium alloy (3.04 × 10−5 A·cm−2) and Mg(OH)2 (1.31 × 10−6 A·cm−2), respectively. During the hydrogen evolution experiments, the 12 h sample had the least amount of hydrogen evolution, followed by 24 h, 6 h, Mg(OH)2, and ZK60 substrate, respectively, indicating that the hydroxide film could effectively improve the corrosion resistance of the substrate;
- (4)
- The experiments on the 12 h sample at various immersion times revealed that the corrosion rate of the hydroxide film was closely related to the surface phase composition and morphology. Even after immersion for 168 h, the hydroxide film could still provide protection for the substrate.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, W.H.; Chen, Y.H.; Wu, L.; Zhang, J.K.; Pan, F.S. Effective corrosion and wear protection of slippery liquid-infused porous surface on AZ31 Mg alloy. Surf. Coat. Technol. 2022, 429, 127953. [Google Scholar] [CrossRef]
- Lu, K. The future of metals. Science 2010, 328, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Xu, J.Q.; Choi, H.; Pozuelo, M.; Ma, X.; Bhowmick, S.; Yang, J.M.; Mathaudhu, S.; Li, X.C. Processing and properties of magnesium containing a dense uniform dispersion of nanoparticles. Nature 2015, 528, 539–543. [Google Scholar] [CrossRef]
- Zhao, D.W.; Witte, F.; Lu, F.Q.; Wang, J.L.; Li, J.L.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, H.G.; Hang, R.Q.; Shi, H.X.; Wang, L.F.; Ma, J.C.; Liu, X.P.; Yao, X.H. Enhanced anticorrosive and antibacterial performances of silver nanoparticles/polyethyleneimine/MAO composite coating on magnesium alloys. J. Mater. Res. Technol. 2021, 11, 2354–2364. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Nordlien, J.H.; Ono, S.; Masuko, N. A TEM investigation of naturally formed oxide films on pure magnesium. Corros. Sci. 1997, 39, 1397–1414. [Google Scholar] [CrossRef]
- Arrabal, R.; Pardo, A.; Merino, M.C.; Mohedano, M.; Casajús, P.; Matykina, E. Corrosion of Magnesium-Aluminum Alloys with Al-11Si/SiC Thermal Spray Composite Coatings in Chloride Solution. J. Therm. Spray Technol. 2011, 20, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.G.; Zhang, S.; Bu, J.F.; Lin, C.J.; Song, G.L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Ba, Z.X.; Dong, Q.S.; Yin, J.H.; Wang, J.X.; Ma, B.; Zhang, X.B.; Wang, Z.Z. Surface properties of Mg-Gd-Zn-Zr alloy modified by Sn ion implantation. Mater. Lett. 2016, 190, 90–94. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Zhang, Z.Y.; Wang, D.; Wen, Y.Q.; Peng, N.; Shang, W. ZIF-8-based micro-arc oxidation composite coatings enhanced the corrosion resistance and superhydrophobicity of a Mg alloy. J. Magnes. Alloys 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Y.; Liu, X.H.; Liu, T.D.; Zou, L.Y.; Wang, Y.T.; Ren, Y.M.; Fan, Z.J.; Lv, Y.Z.; Zhang, M.L. In-situ hydrothermal crystallization Mg(OH)2 films on magnesium alloy AZ91 and their corrosion resistance properties. Mater. Chem. Phys. 2013, 143, 322–329. [Google Scholar] [CrossRef]
- Tan, J.K.E.; Balan, P.; Birbilis, N.; Manivasagam, G. Corrosion-resistant Mg(OH)2/Mg-Fe layered double hydroxide (LDH) composite films on magnesium alloy WE43. J. Taiwan Inst. Chem. Eng. 2022, 131, 104169. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.L.; Zeng, R.C.; Song, L.; Guo, L.; Huang, X.W. Corrosion Resistance of the Superhydrophobic Mg(OH)2/Mg-Al Layered Double Hydroxide Coatings on Magnesium Alloys. Metals 2016, 6, 85. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Qian, S.; Qiao, Y.Q.; Liu, X.Y. Multifunctional Mn-containing titania coatings with enhanced corrosion resistance, osteogenesis and antibacterial activity. J. Mater. Chem. B. 2014, 2, 5397–5408. [Google Scholar] [CrossRef]
- Lüthen, F.; Bulnheim, U.; Müller, P.D.; Rychly, J.; Jesswein, H.; Nebe, J.G.B. Influence of manganese ions on cellular behavior of human osteoblasts in vitro. Biomol. Eng. 2007, 24, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ba, Z.; Wang, T.; Kuang, J.; Jia, Y. Fabrication and characterization of Mg–Mn hydrotalcite films on pure Mg substrates. Mater. Res. Express. 2019, 6, 116440. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, L.C.; Ling, H.; Diao, Y.P.; Pang, X.L.; Wang, Y.L.; Gao, K.W. Design and fabrication of enhanced corrosion resistance Zn-Al layered double hydroxides films based anion-exchange mechanism on magnesium alloys. Appl. Surf. Sci. 2017, 404, 246–253. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W. Mechanosynthesis and characterization of Hydrotalcite like Mg-Al-SO4-LDH. Mater. Lett. 2016, 165, 192–195. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhang, G.; Tang, A.T.; Chen, X.B.; Ma, Y.L.; Ying, L.; Peng, P.; Ding, X.X.; Pan, H.L.; Pan, F.S. Growth behavior of MgAl-layered double hydroxide films by conversion of anodic films on magnesium alloy AZ31 and their corrosion protection. Appl. Surf. Sci. 2018, 456, 419–429. [Google Scholar]
- Zhang, F.; Zhang, C.L.; Song, L.; Zeng, R.C.; Liu, Z.G.; Cui, H.Z. Corrosion of in-situ grown MgAl-LDH coating on aluminum alloy Trans. Nonferrous Met. Soc. China. 2015, 25, 3498–3504. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.G.; Zeng, R.C.; Li, S.Q.; Cui, H.Z.; Song, L.; Han, E.H.J.S.; Technology, C. Corrosion resistance of Mg-Al-LDH coating on magnesium alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Qiu, L.; Xie, R.; Ding, P.; Qu, B. Preparation and characterization of Mg(OH)2 nanoparticles and flame-retardant property of its nanocomposites with EVA. Compos. Struct. 2003, 62, 391–395. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Z.; Qi, K.; Li, H.; Qiu, Y.; Guo, X. Microstructure and Corrosion of Cast Magnesium Alloy ZK60 in NaCl Solution. Materials 2020, 13, 3833. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shen, Y.; Zheng, J.; Wen, Q.; Li, Z.; Yang, X.; Chu, P.K. Effects of one-step hydrothermal treatment on the surface morphology and corrosion resistance of ZK60 magnesium alloy. Surf. Coat. Technol. 2017, 309, 490–496. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Y.; Wen, Y.; Zhan, X.; Kong, D. Study on the Properties of Micro-arc Oxidation Self-assembled Composite coatings on Magnesium Alloy. Int. J. Electrochem. Sci. 2017, 12, 11875–11891. [Google Scholar] [CrossRef]
- Yin, X.; Mu, P.; Wang, Q.; Li, J. Superhydrophobic ZIF-8-Based Dual-Layer Coating for Enhanced Corrosion Protection of Mg Alloy. ACS Appl. Mater. Interfaces. 2020, 12, 35453–35463. [Google Scholar] [CrossRef]
- Huang, M.; Lu, G.; Pu, J.; Qiang, Y. Superhydrophobic and smart MgAl-LDH anti-corrosion coating on AZ31 Mg surface. J. Ind. Eng. Chem. 2021, 103, 154–164. [Google Scholar] [CrossRef]


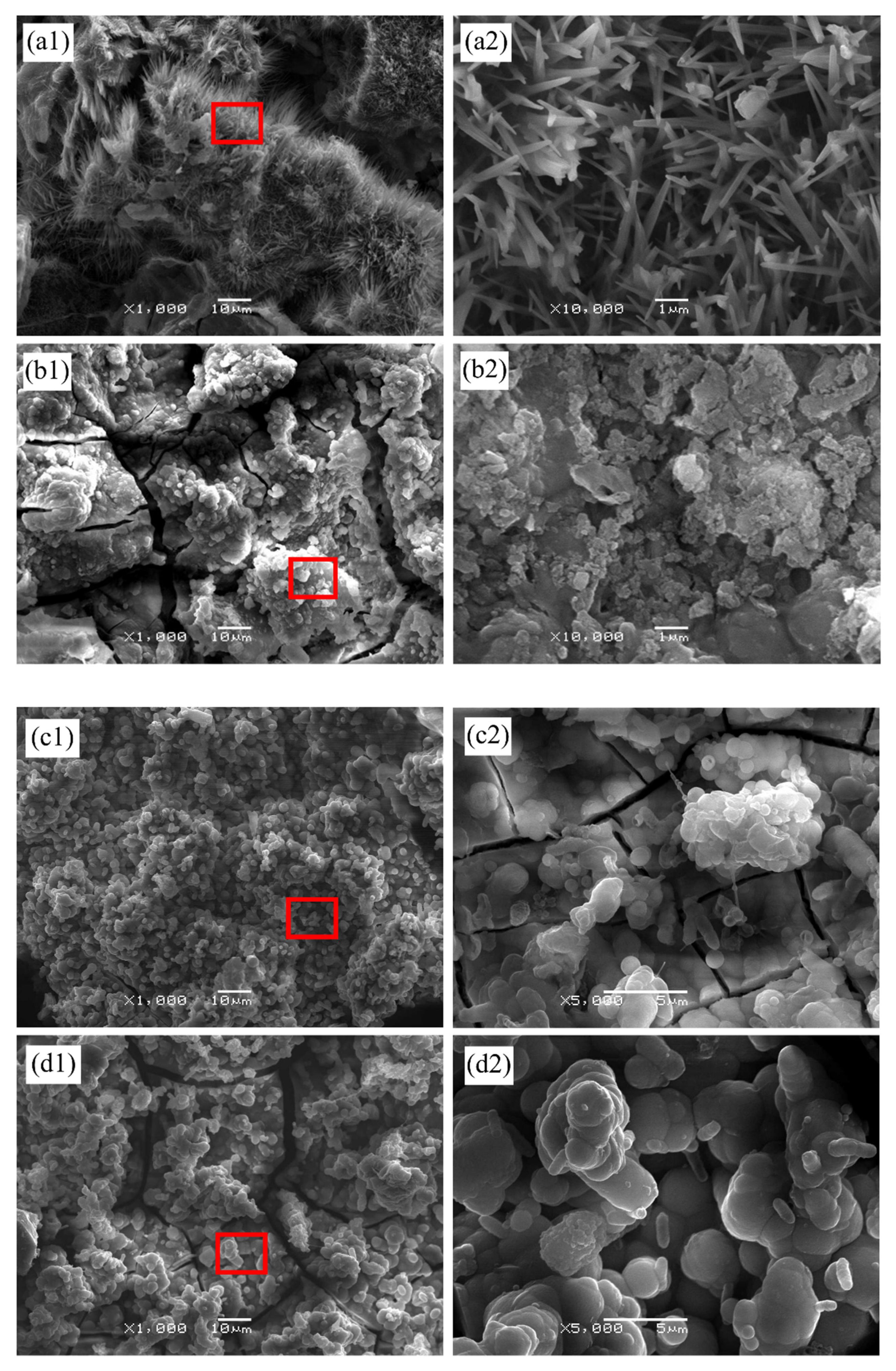
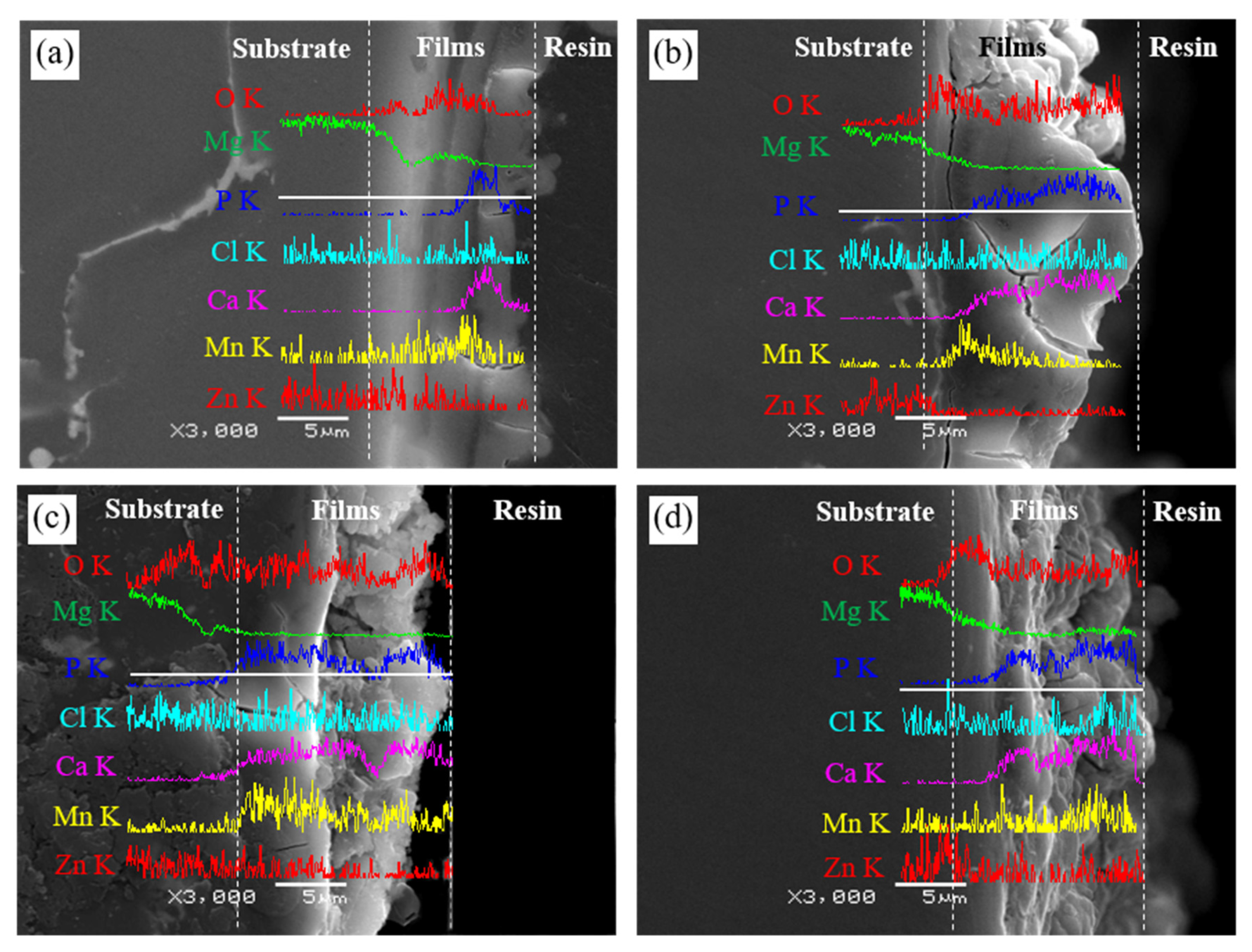


| Sample | Elementary Composition (at.%) | |||
|---|---|---|---|---|
| O | Mg | Mn | Zn | |
| Mg(OH)2 | 34.24 | 64.48 | - | 01.28 |
| 6 h | 29.46 | 63.71 | 04.67 | 02.17 |
| 12 h | 29.44 | 57.88 | 11.57 | 01.12 |
| 24 h | 29.27 | 56.07 | 13.92 | 00.73 |
| Sample | OCPs/V | Ecorr/V(vs. SCE) | icorr/A·cm−2 | Pi/mm/y | PH/mm/y |
|---|---|---|---|---|---|
| ZK60 | −1.62 | −1.49 | 3.04 × 10−5 | 0.70 | 0.34 |
| Mg(OH)2 | −1.42 | −1.32 | 1.31 × 10−6 | 2.99 × 10−2 | 0.19 |
| 6 h | −1.40 | −1.36 | 1.51 × 10−6 | 3.45 × 10−2 | 0.14 |
| 12 h | −1.35 | −1.29 | 3.07 × 10−7 | 7.02 × 10−3 | 9.6 × 10−2 |
| 24 h | −1.30 | −1.21 | 3.83 × 10−7 | 8.75 × 10−3 | 0.12 |
| Sample | Elementary Composition (at.%) | ||||||
|---|---|---|---|---|---|---|---|
| O | Na | Mg | P | Cl | Ca | Mn | |
| 24 h | 41.20 | 01.05 | 44.33 | - | 12.88 | - | 00.54 |
| 72 h | 28.84 | 01.74 | 24.93 | 21.79 | 00.41 | 21.58 | 00.71 |
| 120 h | 34.99 | 02.00 | 04.49 | 22.16 | 00.24 | 35.85 | 00.28 |
| 168 h | 28.85 | 01.40 | 04.74 | 23.94 | 00.40 | 40.42 | 00.53 |
| Sample | Rs/Ω·cm2 | Y0-out/μΩ–1⋅cm–2⋅s–1 | nout | Rout/Ω·cm2 | Y0-in/μΩ–1⋅cm–2⋅s–1 | nin | Rin/Ω·cm2 |
|---|---|---|---|---|---|---|---|
| 24 h | 81.51 | 7.40 | 0.67 | 208.3 | 12.02 | 0.74 | 3018 |
| 72 h | 70.82 | 7.76 | 0.64 | 136.5 | 17.45 | 0.73 | 1807 |
| 120 h | 104.3 | 7.92 | 0.66 | 59.95 | 45.79 | 0.68 | 2038 |
| 168 h | 0.01 | 3.00 | 0.50 | 158.3 | 68.05 | 0.63 | 1735 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Z.; Wang, Y.; Sun, T.; Ba, Z. Corrosion Resistance of Mg(OH)2/Mn(OH)2 Hydroxide Film on ZK60 Mg Alloy. Metals 2022, 12, 1760. https://doi.org/10.3390/met12101760
Wang Y, Li Z, Wang Y, Sun T, Ba Z. Corrosion Resistance of Mg(OH)2/Mn(OH)2 Hydroxide Film on ZK60 Mg Alloy. Metals. 2022; 12(10):1760. https://doi.org/10.3390/met12101760
Chicago/Turabian StyleWang, Yongmin, Zhuangzhuang Li, Yan Wang, Tianyi Sun, and Zhixin Ba. 2022. "Corrosion Resistance of Mg(OH)2/Mn(OH)2 Hydroxide Film on ZK60 Mg Alloy" Metals 12, no. 10: 1760. https://doi.org/10.3390/met12101760
APA StyleWang, Y., Li, Z., Wang, Y., Sun, T., & Ba, Z. (2022). Corrosion Resistance of Mg(OH)2/Mn(OH)2 Hydroxide Film on ZK60 Mg Alloy. Metals, 12(10), 1760. https://doi.org/10.3390/met12101760






