Hydrogen Generation from Mg Wastes by Cold Rolling and Ball Milling by Hydrolysis Reaction: Elektron 21 (UNS-M12310) in Simulated Seawater
Abstract
:1. Introduction
2. Experimental Details
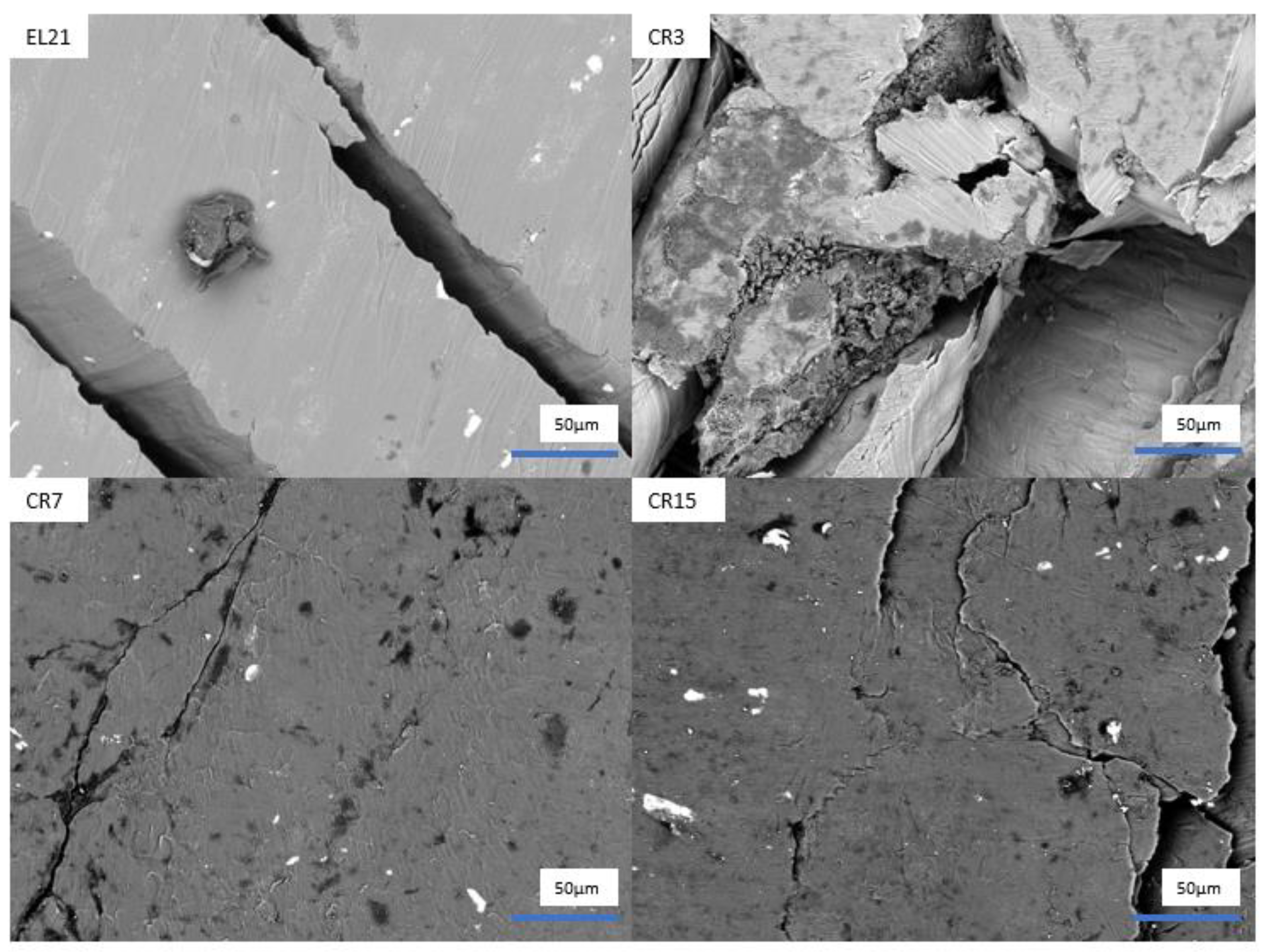
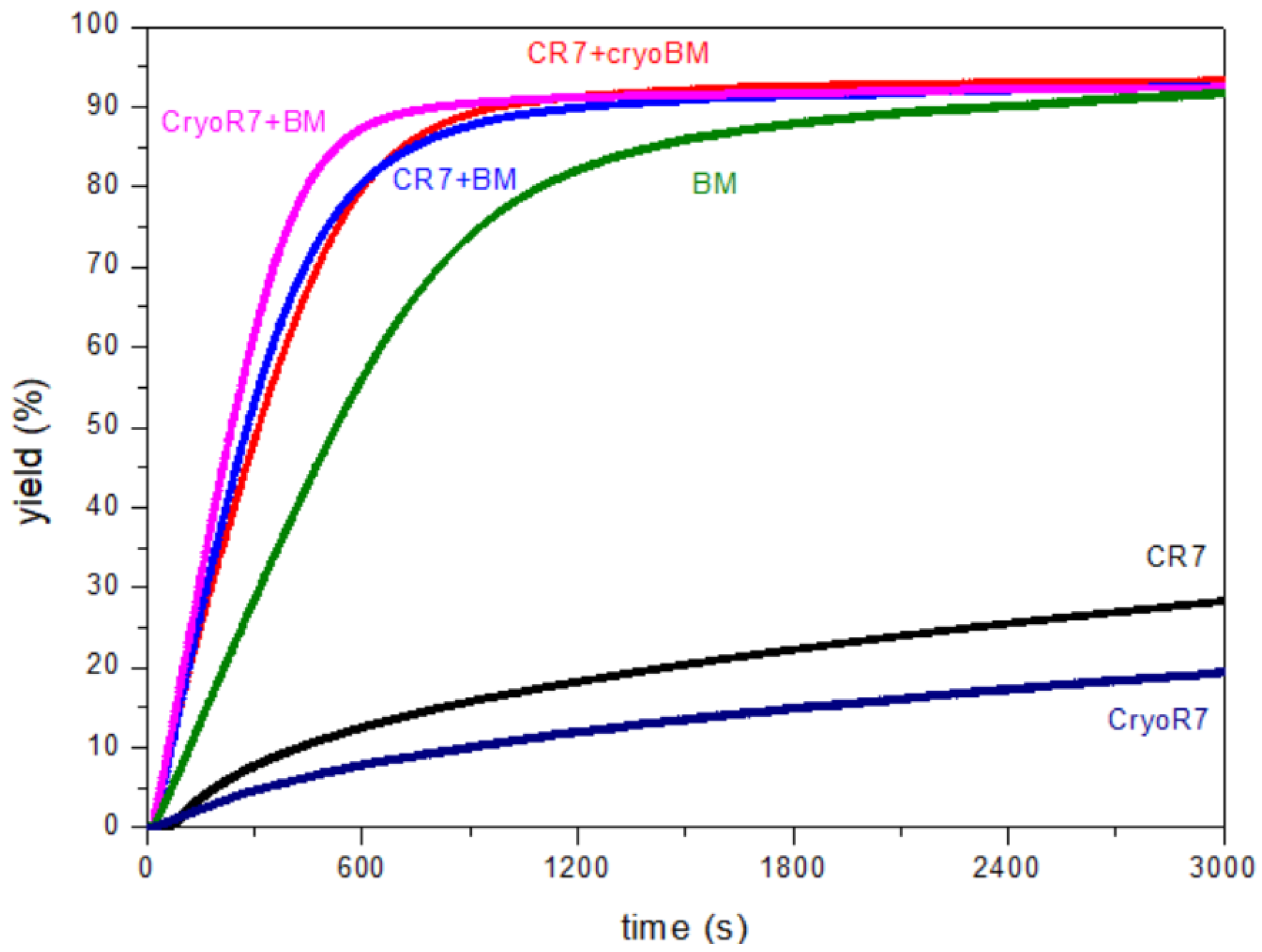
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oshiro, K.; Fujimori, S. Role of hydrogen-based energy carriers as an alternative option to reduce residual emissions associated with mid-century decarbonization goals. Appl. Energy 2022, 313, 118803. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Dicks, A.L. Hydrogen generation from natural gas for the fuel cell systems of tomorrow. J. Power Sources 1996, 61, 113–124. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Oh, S.K.; Cho, T.; Kim, M.; Lim, J.; Eom, K.; Kim, D.; Cho, E.; Kwon, H. Fabrication of Mg–Ni–Sn alloys for fast hydrogen generation in seawater. Int. J. Hydrogen Energy 2017, 42, 7761–7769. [Google Scholar] [CrossRef]
- Uan, J.-Y.; Cho, C.-Y.; Liu, K.-T. Generation of hydrogen from magnesium alloy scraps catalyzed by platinum-coated titanium net in NaCl aqueous solution. Int. J. Hydrogen Energy 2007, 32, 2337–2343. [Google Scholar] [CrossRef]
- Uan, J.-Y.; Yu, S.-H.; Lin, M.-C.; Chen, L.-F.; Lin, H.-I. Evolution of hydrogen from magnesium alloy scraps in citric acid-added seawater without catalyst. Int. J. Hydrogen Energy 2009, 34, 6137–6142. [Google Scholar] [CrossRef]
- AlBacha, S.; Thienpont, A.; Zakhour, M.; Nakhl, M.; Bobet, J.L. Clean hydrogen production by the hydrolysis of magnesium-based material: Effect of the hydrolysis solution. J. Clean. Prod. 2021, 282, 124498–124507. [Google Scholar] [CrossRef]
- Figen, A.K.; Coşkuner, B.; Pişkin, S. Hydrogen generation from waste Mg based material in various saline solutions (NiCl2, CoCl2, CuCl2, FeCl3, MnCl2). Int. J. Hydrogen Energy 2015, 40, 7483–7489. [Google Scholar] [CrossRef]
- Rodríguez, M.; Niro, F.; Urretavizcaya, G.; Bobet, J.-L.; Castro, F.J. Hydrogen production from hydrolysis of magnesium wastes reprocessed by mechanical milling under air. Int. J. Hydrogen Energy 2022, 47, 5074–5084. [Google Scholar] [CrossRef]
- Bai, J.; Li, D.; Cao, Q.; Hou, X.; Xu, Y. Hydrolysis H2 generation behavior of AM50 alloy waste coactivated by Mg-based master alloys. Int. J. Hydrogen Energy 2022, 47, 31191–31201. [Google Scholar] [CrossRef]
- Al Bacha, S.; Pighin, S.; Urretavizcaya, G.; Zakhour, M.; Nakhl, M.; Castro, F.; Bobet, J.-L. Effect of ball milling strategy (milling device for scaling-up) on the hydrolysis performance of Mg alloy waste. Int. J. Hydrogen Energy 2020, 45, 20883–20893. [Google Scholar] [CrossRef]
- Figen, A.K.; Filiz, B.C. Hydrogen production by the hydrolysis of milled waste magnesium scraps in nickel chloride solutions and nickel chloride added in Marmara Sea and Aegean Sea Water. Int. J. Hydrogen Energy 2015, 40, 16169–16177. [Google Scholar] [CrossRef]
- Mouls, B.; Arurault, L.; Taberna, P.-L.; Bonningue, C. Macroscopic, thermodynamic, kinetic and microscopic study of nitric acid pickling of Elektron 21 (EV31A) magnesium alloy. J. Magnes. Alloys 2014, 2, 363–376. [Google Scholar] [CrossRef]
- Awad, A.S.; El-Asmar, E.; Tayeh, T.; Mauvy, F.; Nakhl, M.; Zakhour, M.; Bobet, J.-L. Effect of carbons (G and CFs), TM (Ni, Fe and Al) and oxides (Nb2O5 and V2O5) on hydrogen generation from ball milled Mg-based hydrolysis reaction for fuel cell. Energy 2016, 95, 175–186. [Google Scholar] [CrossRef]
- Grosjean, M.-H.; Roué, L. Hydrolysis of Mg–salt and MgH2–salt mixtures prepared by ball milling for hydrogen production. J. Alloys Compd. 2006, 416, 296–302. [Google Scholar] [CrossRef]
- Wang, S.; Sun, L.-X.; Xu, F.; Jiao, C.-L.; Zhang, J.; Zhou, H.-Y.; Huang, F.-L. Hydrolysis reaction of ball-milled Mg-metal chlorides composite for hydrogen generation for fuel cells. Int. J. Hydrogen Energy 2012, 37, 6771–6775. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Chen, Z.; Peng, C.; Zhu, X.; Zhu, M. Hydrogen production via hydrolysis of Mg-oxide composites. Int. J. Hydrogen Energy 2017, 42, 22305–22311. [Google Scholar] [CrossRef]
- Tayeh, T.; Awad, A.S.; Nakhl, M.; Zakhour, M.; Silvain, J.-F.; Bobet, J.-L. Production of hydrogen from magnesium hydrides hydrolysis. Int. J. Hydrogen Energy 2014, 39, 3109–3117. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Liu, H.; Dong, Z.; Cao, G.; Yan, M. Hydrogen generation from Mg–LiBH4 hydrolysis improved by AlCl3 addition. Energy 2014, 68, 548–554. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Dong, Z.; Liu, H.; Li, S.; Ge, H.; Yan, M. Hydrogen generation from the hydrolysis of Mg powder ball-milled with AlCl3. Energy 2013, 53, 147–152. [Google Scholar] [CrossRef]
- Shetty, T.; Szpunar, J.A.; Faye, O.; Eduok, U. A comparative study of hydrogen generation by reaction of ball milled mixture of magnesium powder with two water-soluble salts (NaCl and KCl) in hot water. Int. J. Hydrogen Energy 2020, 45, 25890–25899. [Google Scholar] [CrossRef]
- Grosjean, M.-H.; Zidoune, M.; Roué, L.; Huot, J.; Schulz, R. Effect of ball milling on the corrosion resistance of magnesium in aqueous media. Electrochim. Acta 2004, 49, 2461–2470. [Google Scholar] [CrossRef]
- Yu, S.-H.; Uan, J.-Y.; Hsu, T.-L. Effects of concentrations of NaCl and organic acid on generation of hydrogen from magnesium metal scrap. Int. J. Hydrogen Energy 2012, 37, 3033–3040. [Google Scholar] [CrossRef]
- Pighin, S.A.; Urretavizcaya, G.; Bobet, J.-L.; Castro, F.J. Nanostructured Mg for hydrogen production by hydrolysis obtained by MgH2 milling and dehydriding. J. Alloys Compd. 2020, 827, 154000. [Google Scholar] [CrossRef]
- Teatum, E.T., Jr.; Gschneidner, K.A.; Waber, J.T. Compilation of Calculated Data Useful in Predicting Metallurgical Behavior of the Elements in Binary Alloy Systems; Los Alamos Scientific Laboratory: Los Alamos, NM, USA, 1968. [Google Scholar] [CrossRef] [Green Version]
- Hancock, J.D.; Sharp, J.H. Method of Comparing Solid-State Kinetic Data and Its Application to the Decomposition of Kaolinite, Brucite, and BaCO3. J. Am. Ceram. Soc. 1972, 55, 74–77. [Google Scholar] [CrossRef]
- Sharp, J.H.; Brindley, G.W.; Achar, B.N.N. Numerical Data for Some Commonly Used Solid State Reaction Equations. J. Am. Ceram. Soc. 1966, 49, 379–382. [Google Scholar] [CrossRef]
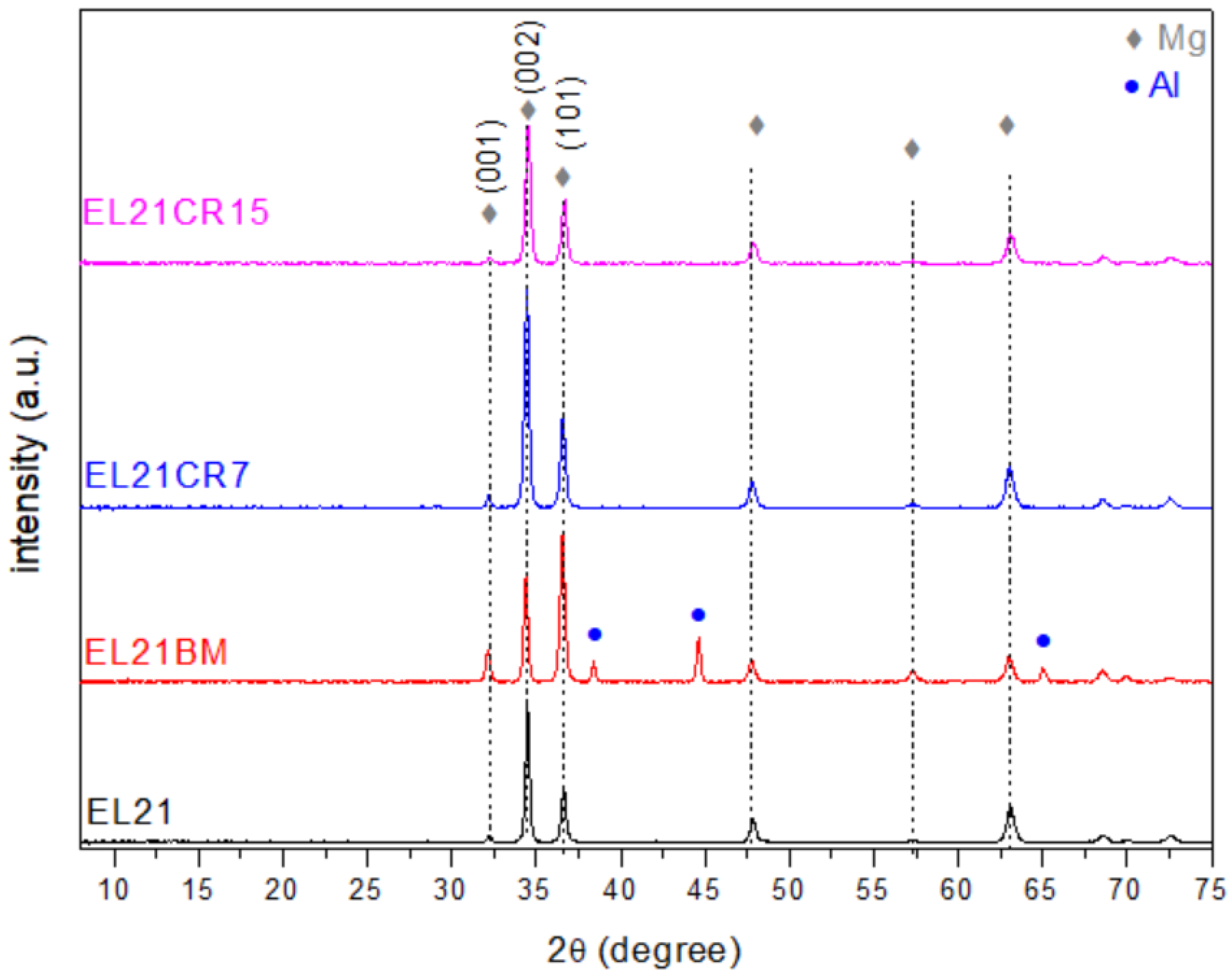


| EL21 | EL21-BM | EL21-CR7 | EL21-CR15 | Mg (According to ICSD n° 01-089-4894) | |
|---|---|---|---|---|---|
| a (Å) | 3.212 (3) | 3.218 (4) | 3.214 (4) | 3.213 (4) | 3.209 |
| c (Å) | 5.203 (5) | 5.216 (7) | 5.208 (7) | 5.197 (7) | 5.210 |
| (nm) | 36 (2) | 34 (3) | 30 (3) | 28 (3) | |
| (nm) | 32 (2) | 30 (3) | 34 (3) | 32 (3) |
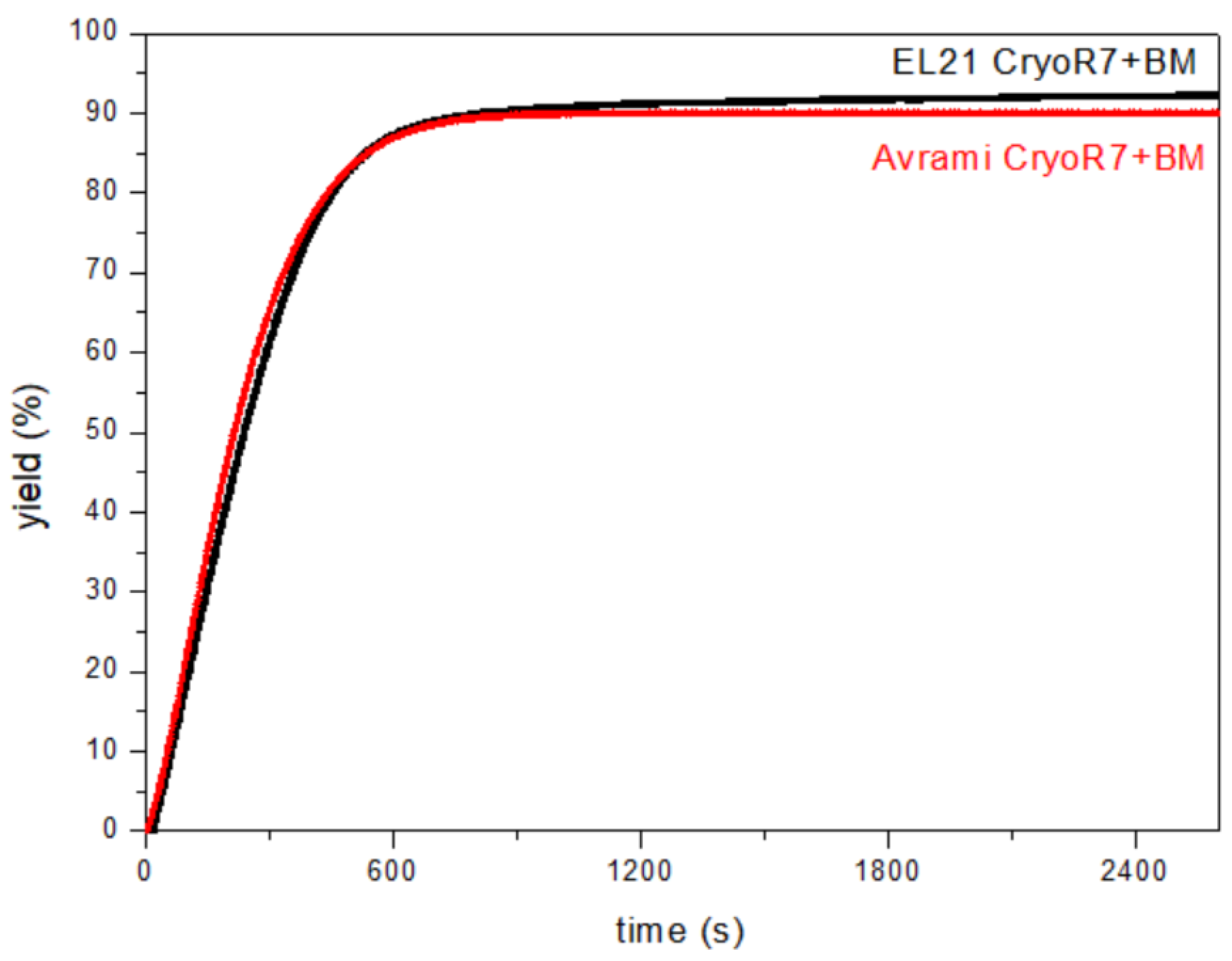
| CryoR7BM | CR7+CryoBM | CR7+bm | BM | CR7 | CryoR7 | |
|---|---|---|---|---|---|---|
| n | 1.381 | 1.276 | 1.278 | 1.328 | 0.958 | 0.882 |
| k ( | 4.9 × 10−4 | 6.0 × 10−4 | 6.5 × 10−4 | 2.0 × 10−4 | 1.2 × 10−3 | 1.2 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donadey, G.; Caillaud, S.; Coeuret, P.; Moussa, M.; Cuzacq, L.; Bobet, J.-L. Hydrogen Generation from Mg Wastes by Cold Rolling and Ball Milling by Hydrolysis Reaction: Elektron 21 (UNS-M12310) in Simulated Seawater. Metals 2022, 12, 1821. https://doi.org/10.3390/met12111821
Donadey G, Caillaud S, Coeuret P, Moussa M, Cuzacq L, Bobet J-L. Hydrogen Generation from Mg Wastes by Cold Rolling and Ball Milling by Hydrolysis Reaction: Elektron 21 (UNS-M12310) in Simulated Seawater. Metals. 2022; 12(11):1821. https://doi.org/10.3390/met12111821
Chicago/Turabian StyleDonadey, Guillaume, Simon Caillaud, Pierre Coeuret, Maria Moussa, Laurent Cuzacq, and Jean-Louis Bobet. 2022. "Hydrogen Generation from Mg Wastes by Cold Rolling and Ball Milling by Hydrolysis Reaction: Elektron 21 (UNS-M12310) in Simulated Seawater" Metals 12, no. 11: 1821. https://doi.org/10.3390/met12111821






