Effects of Alloying Elements on Solidification Structures and Macrosegregation in Slabs
Abstract
:1. Introduction
2. Model Descriptions
2.1. Solidification Heat Transfer Model
- (1)
- Heat transfer along the casting direction is ignored.
- (2)
- The effect of the fluid flow of molten steel on the internal heat transfer and structure is ignored during the calculations.
- (3)
- The heat transfer of each part of the secondary cooling zone is uniform, and the heat transfer coefficient can be used to describe the heat exchange of the slab in the secondary cooling zone.
- (4)
- The heat transfer in the mold is calculated by the average heat flux.
- (5)
- The influence of mold vibration and protective slag film on heat transfer was ignored.
2.2. Nucleation Model
2.3. Dendrite Tip Growth Kinetics Model
2.4. SDAS Model
3. Solution Conditions and Model Validation
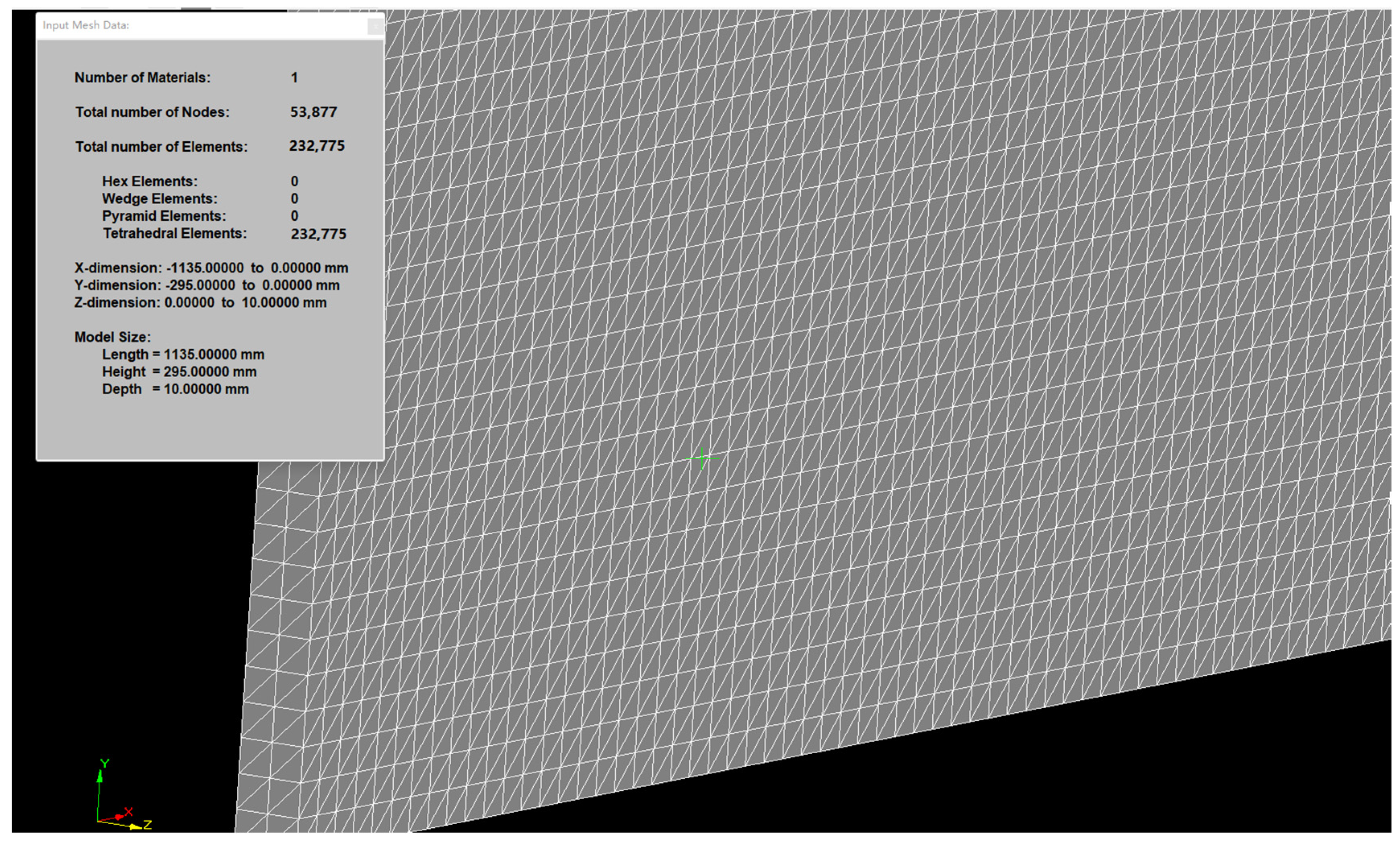
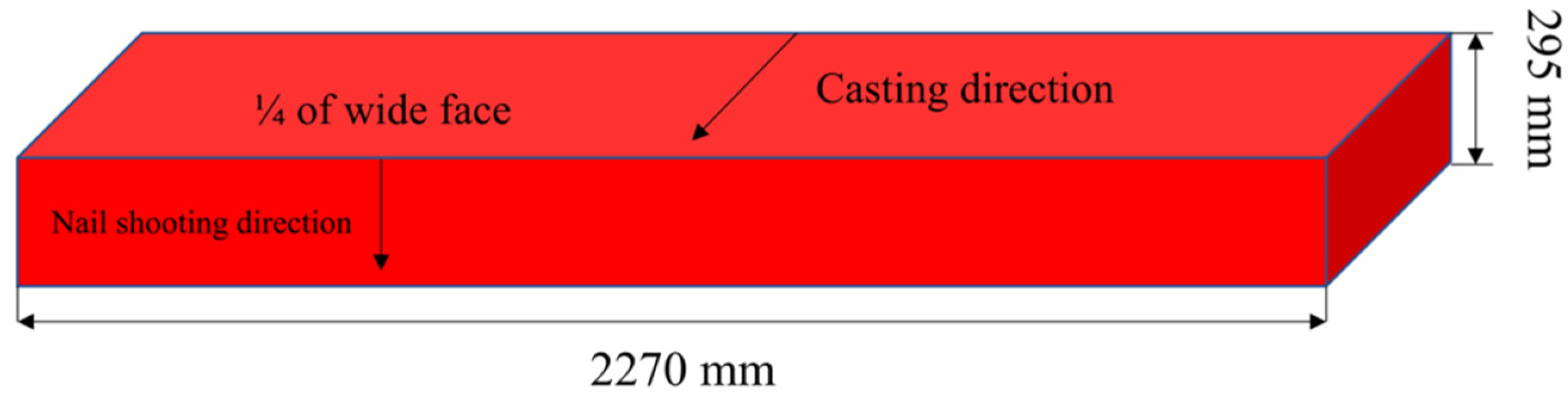
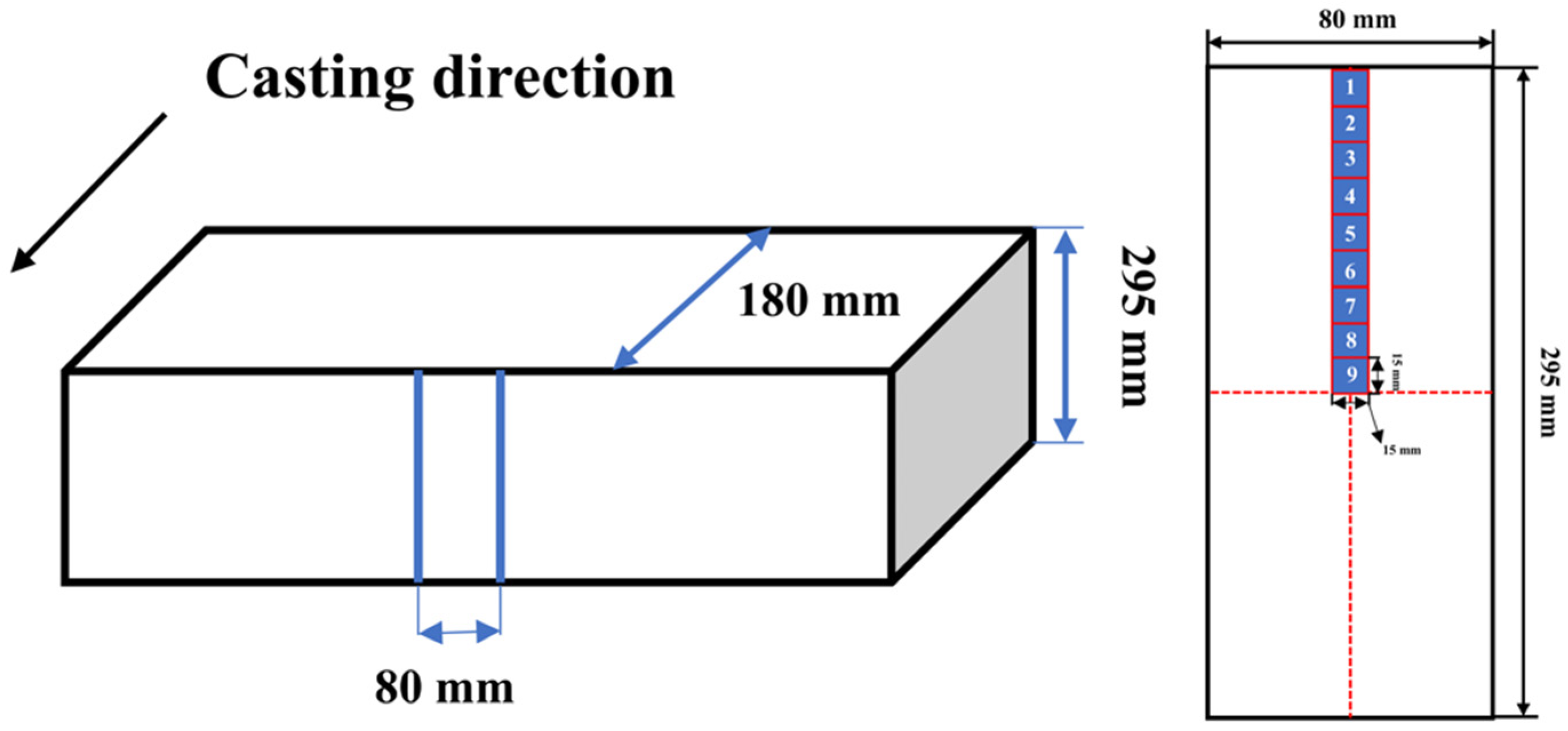
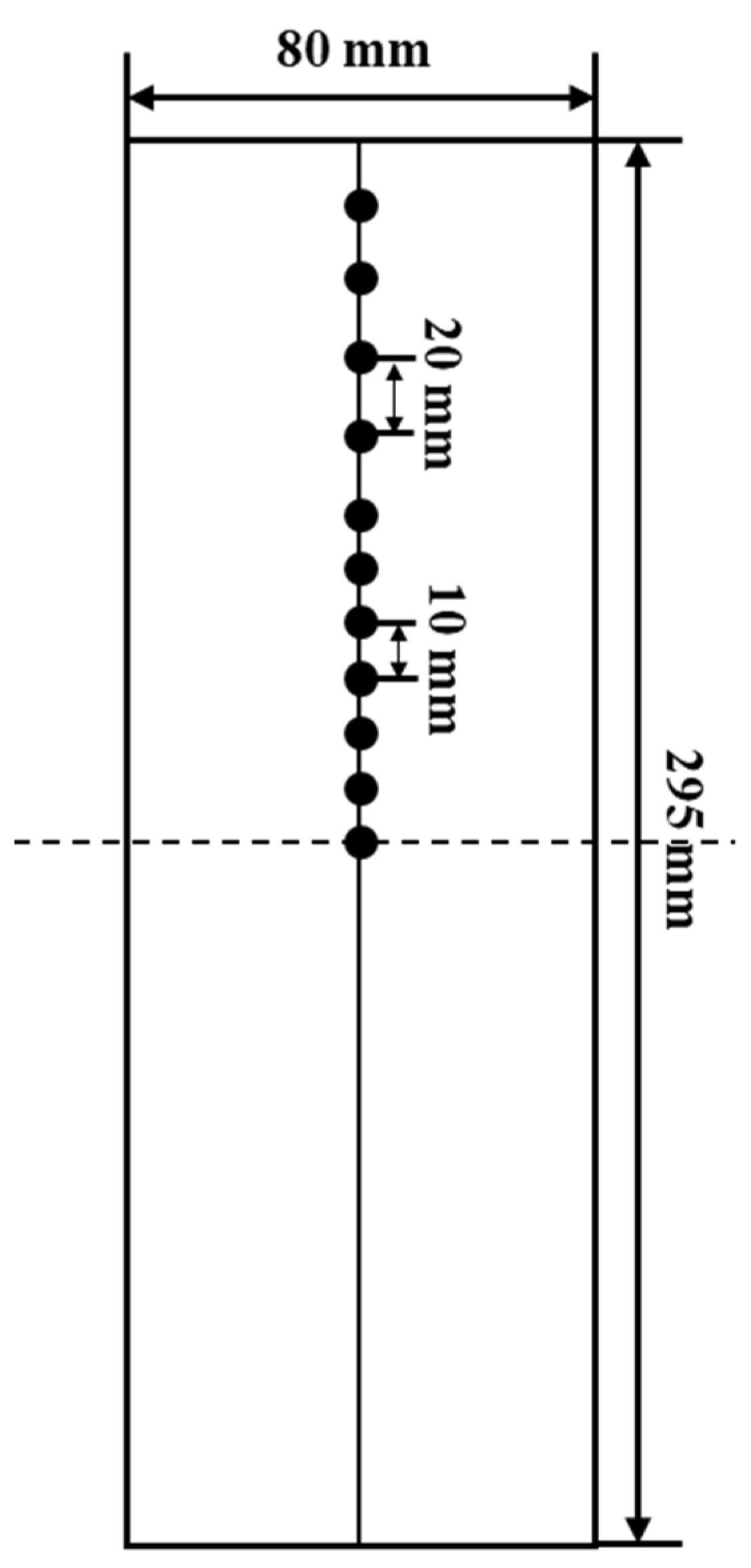
3.1. Geometric Model
3.2. Selection of the Calculation Parameters
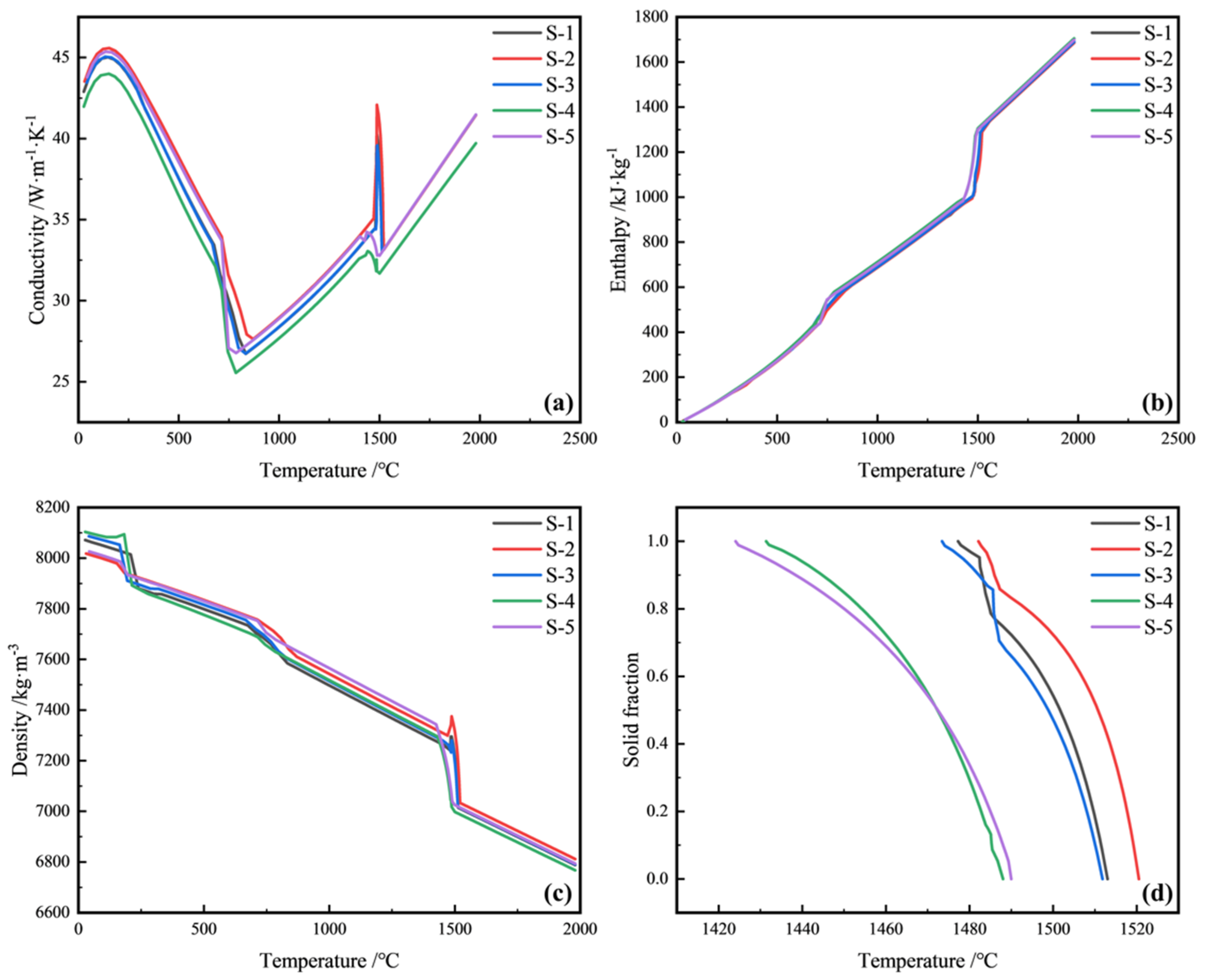
3.2.1. Thermal properties of the Material
3.2.2. Initial Condition and Calculation method for Boundary Conditions
3.3. Model Validation
3.3.1. Validation of Heat Transfer
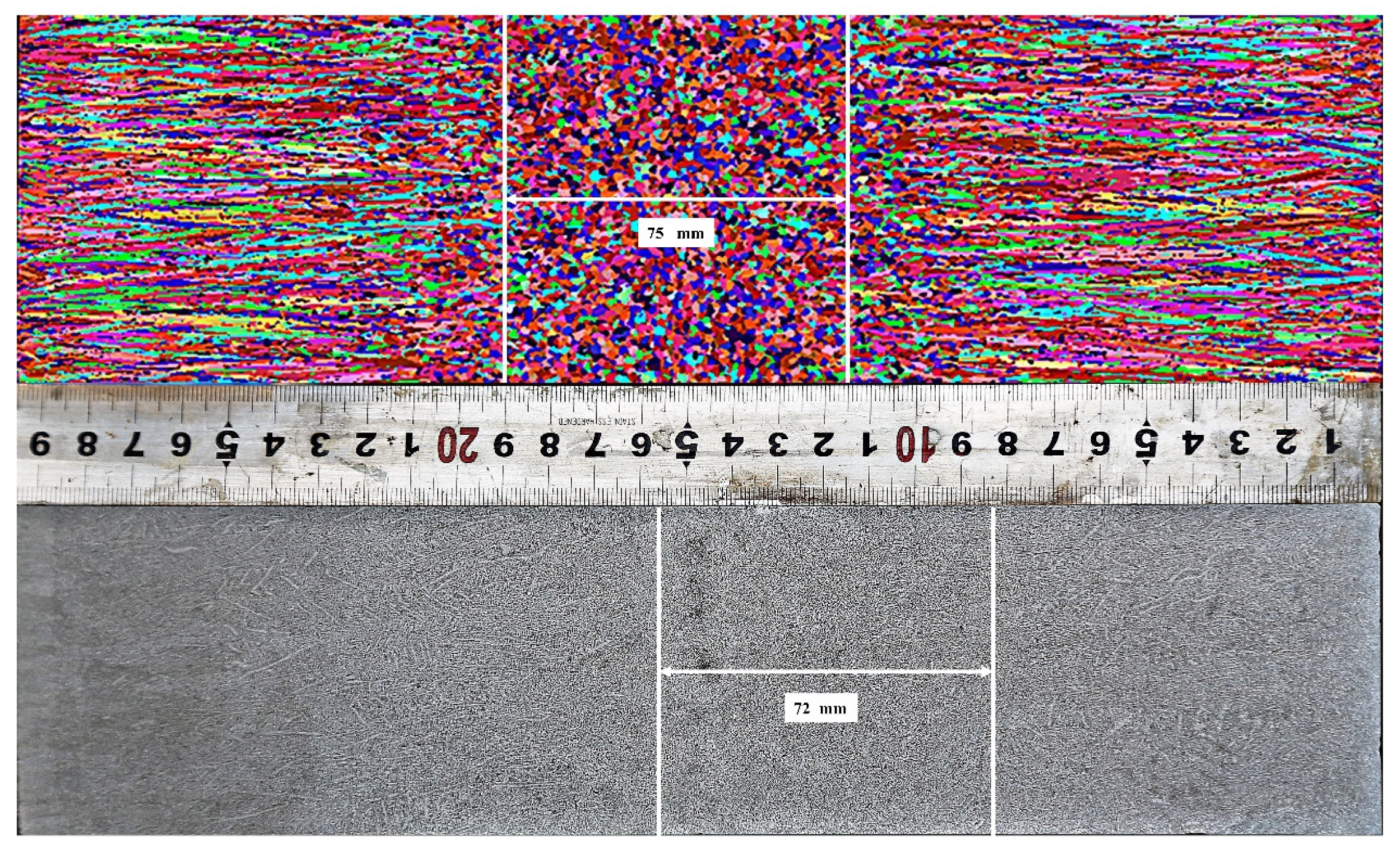
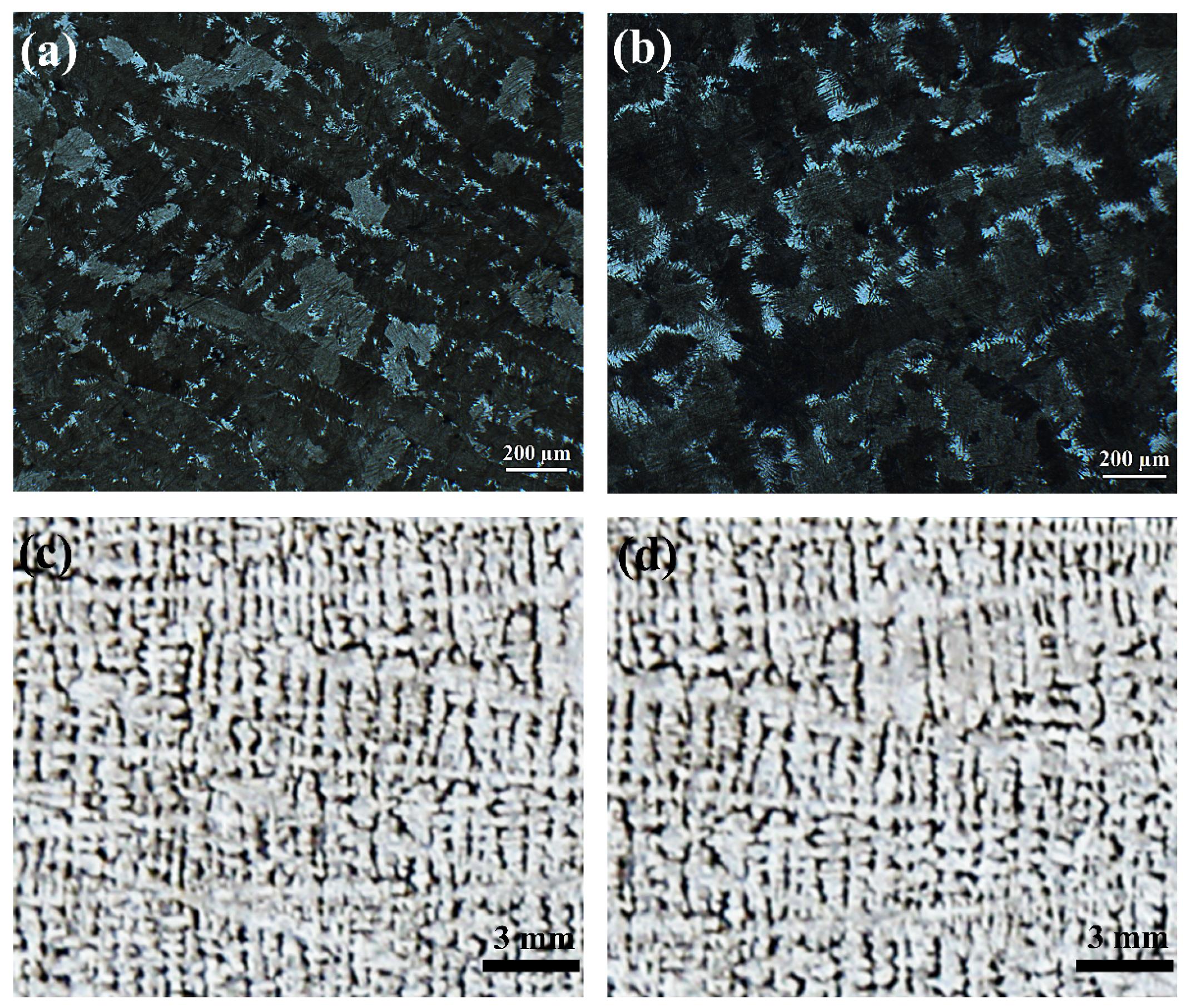
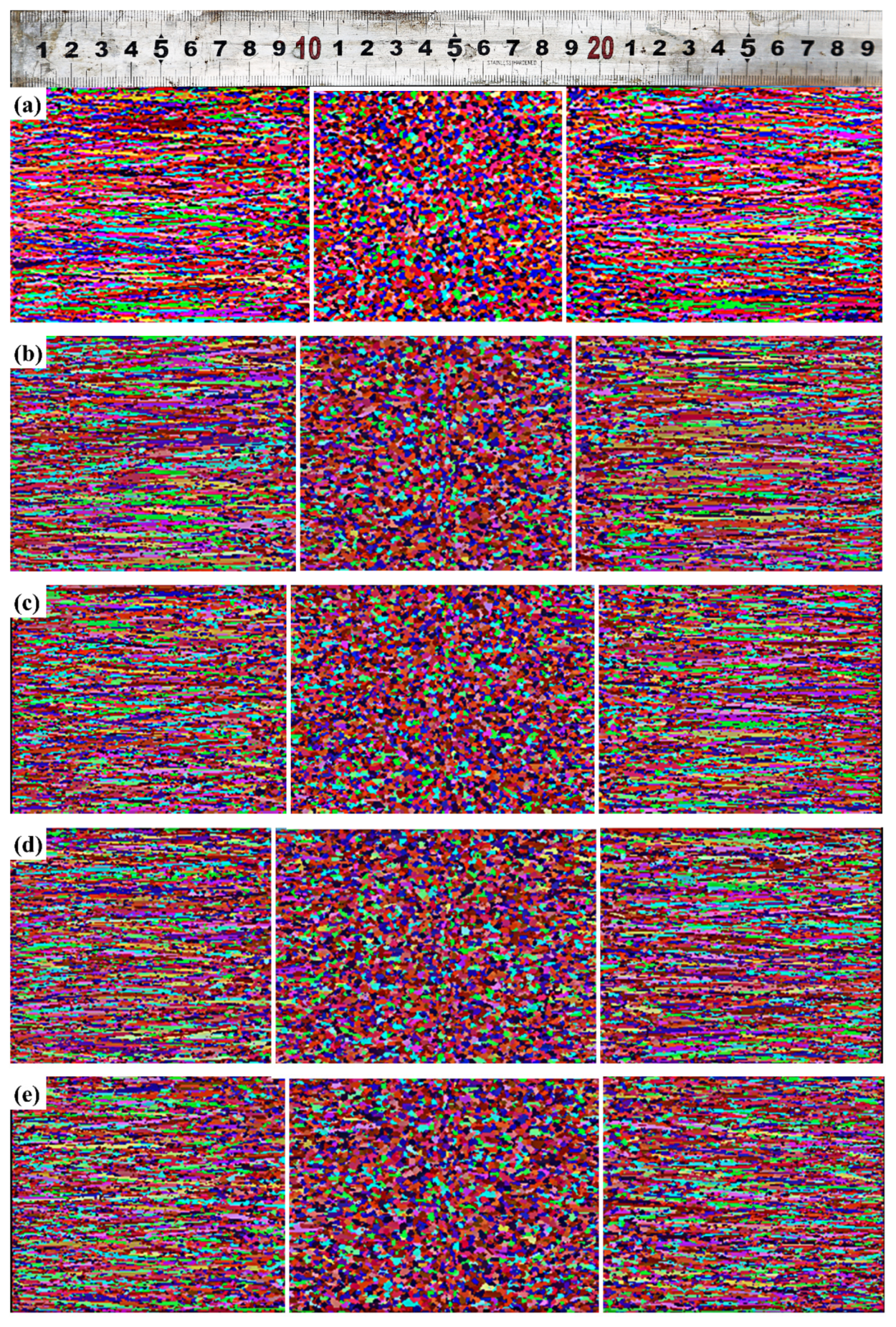
3.3.2. Validation of Solidification Structure
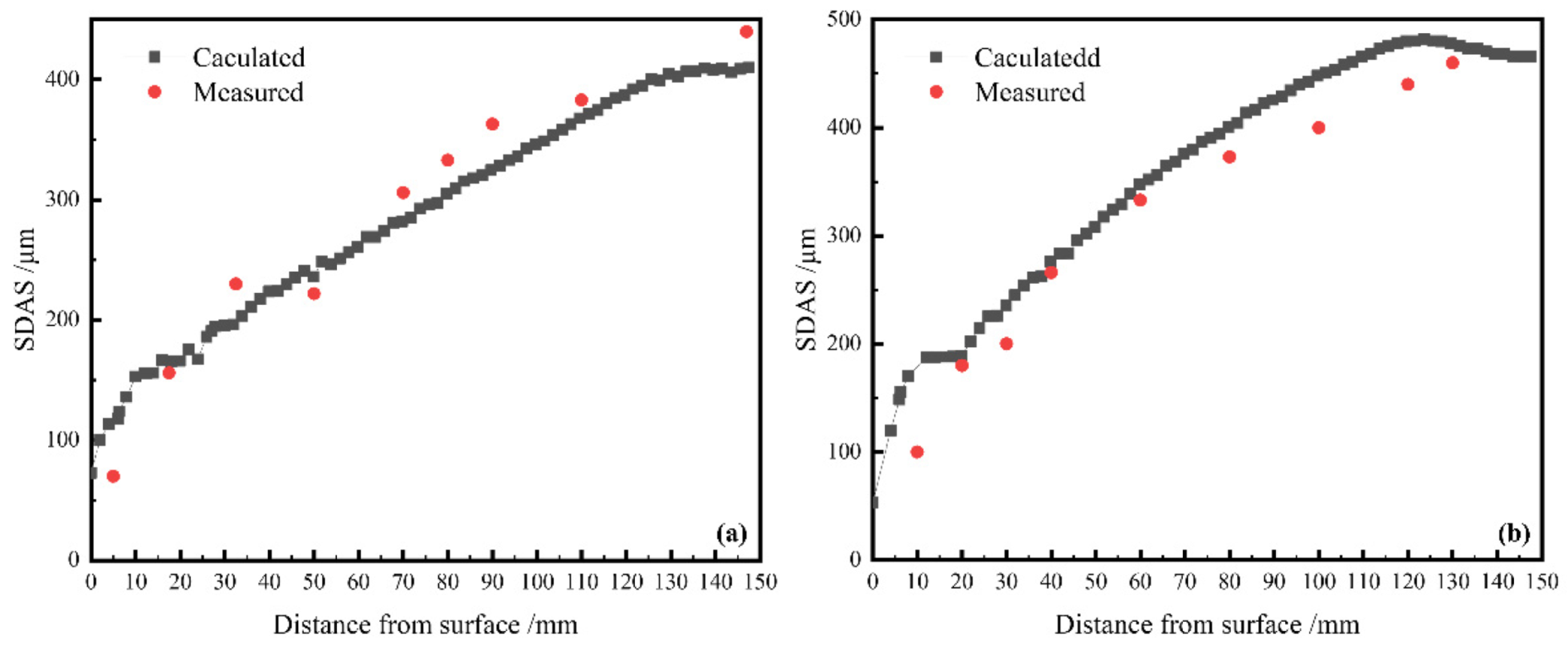
3.3.3. Validation of SDAS Model
4. Results and Discussion
4.1. Effect of Alloying Elements on the Solidification Structure of the Slab
4.2. Effect of Alloying Elements on Macrosegregation
5. Conclusions
- (1)
- The heat transfer model is established and verified by surface temperature measurements and the nail shooting test. Based on the temperature field, the solidification structure of different slabs is simulated, which is in good agreement with the results of macro-etch experiments. The SDAS model for experimental slabs was obtained, and the calculated value of the model is in good agreement with the value obtained through metallographic observation.
- (2)
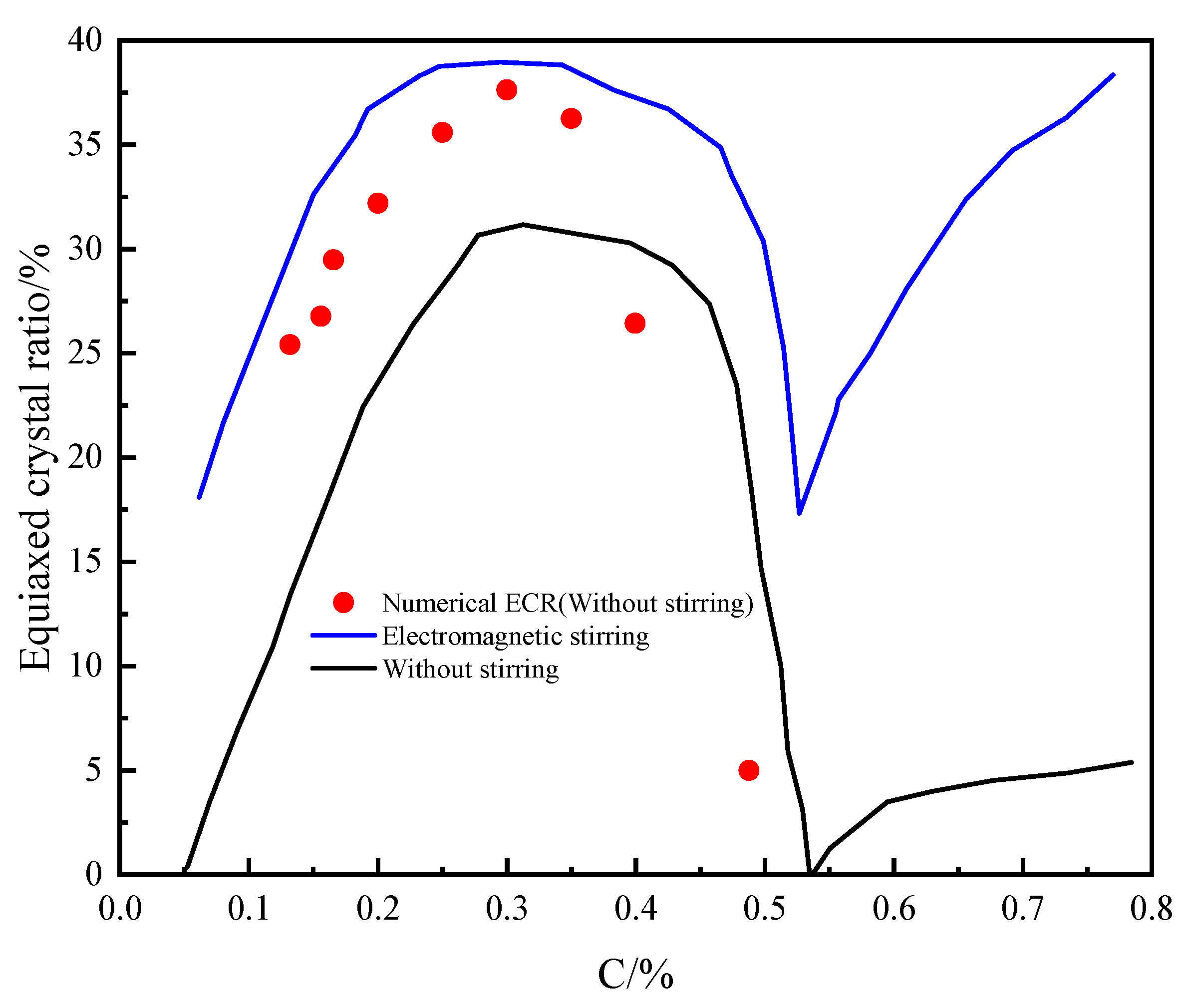
- With the increase in carbon content, the ECR increases at first, and then decreases. The ECR reaches the maximum value when the carbon content is 0.3%. When the carbon content is about 0.53 wt%, the equiaxed crystal rate reaches the lowest value. Therefore, in the actual production, within the acceptable range of carbon content, the content of C can be controlled by this rule to improve the ECR.
- (3)
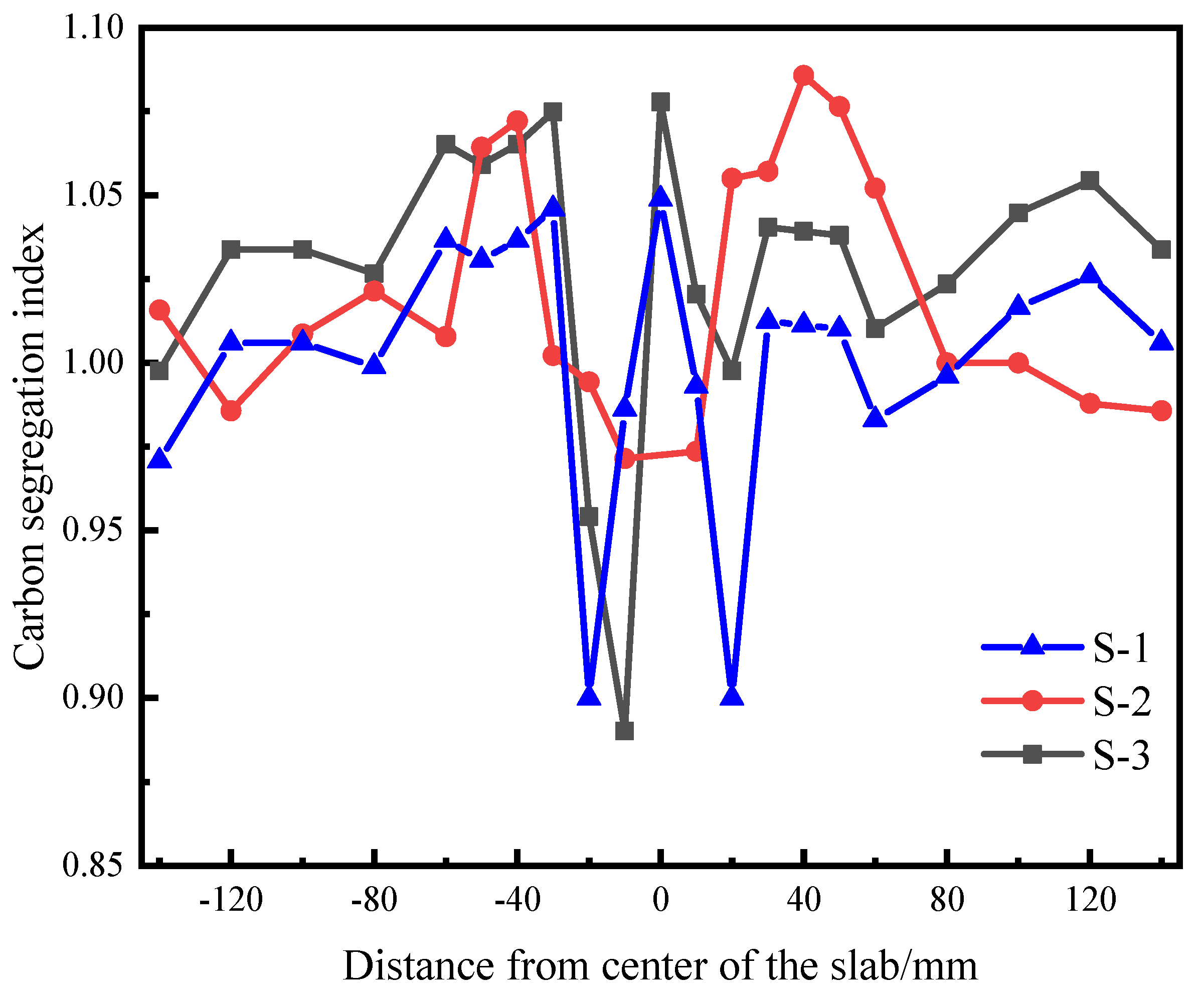
- The SDAS increases with the increase in C and Si content. Compared with S-2 steel, the degree of central segregation in S-1 steel is lower. The effect of Si content on SDAS is second only to that of C content. Properly reducing Si content can reduce SDAS and effectively reduce central segregation.
- (4)
- The average grain size of the equiaxed crystal zone can be expressed by the primary dendrite spacing, which is closely related to the element content. Additions of C, Si, and Ni increase the arm spacing, while additions of Mn, Al, and Cr diminish them. C promotes the largest increases in PDAS, and conversely Al produces the strongest diminution. The grain size of the equiaxed zone can be controlled by controlling the alloying content to reduce the macrosegregation.
- (5)
- The macrosegregation is closely related to the SDAS, ECR and average grain size. Smaller SDAS can make the solidification structure more compact, and a high ECR and small grain size can reduce macrosegregation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hisashi, M.; Nobuyoshi, T.; Norio, S.; Masazumi, H. Macrostructure of and Segregation in Continuously Cast Carbon Steel Billets. Trans. Iron Steel Inst. Jpn. 1972, 12, 102–111. [Google Scholar]
- Cicutti, C.; Boeri, R. On the relationship between primary and secondary dendrite arm spacing in continuous casting products. Scr. Mater. 2001, 45, 1455–1460. [Google Scholar] [CrossRef]
- Ludlow, V.; Normanton, A.; Anderson, A. Strategy to minimize central segregation in high carbon steel grades during billet casting. Ironmak. Steelmak. 2005, 32, 68–74. [Google Scholar] [CrossRef]
- Ayata, K.; Mori, T.; Fujimoto, T.; Ohnishi, T.; Wakasugi, I. Improvement of macrosegregation in continuously cast bloom and billet by electromagnetic stirring. Trans. Iron Steel Inst. Jpn. 1984, 24, 931–939. [Google Scholar] [CrossRef]
- Imagumbai, M. Relationship between Primary- and Secondary-dendrite Arm Spacing of C-Mn Steel Uni-directionally Solidified in Steady State. ISIJ Int. 1994, 34, 986. [Google Scholar] [CrossRef]
- Ayata, K.; Mor, T.; Taniguchi, K.; Matsuda, H. Low superheat teeming with electromagnetic stirring. ISIJ Int. 1995, 35, 680–685. [Google Scholar] [CrossRef]
- An, H.; Bao, Y.; Wang, M.; Yang, Q.; Dang, Y. Numerical and experimental investigation of solidification structure evolution and reduction of centre segregation in continuously cast GCr15 bloom. Ironmak. Steelmak. 2020, 47, 1063–1077. [Google Scholar] [CrossRef]
- Cabrera-Marrewo, J.M.; Carreno-Galindo, V.; Morales, R.D.; Chávez-Alcalá, F. Macro-micro modeling of the dendritic microstructure of steel billets processed by continuous casting. ISIJ Int. 1998, 38, 812–821. [Google Scholar] [CrossRef]
- Weisgerber, B.; Hecht, M.; Harste, K. Investigations of the solidification structure of continuously cast slabs. Steel Res. 1999, 70, 403–411. [Google Scholar] [CrossRef]
- Gao, X.; Yang, S.; Li, J. Effects of micro-alloying elements and continuous casting parameters on reducing segregation in continuously cast slab. Mater. Des. 2016, 110, 284–295. [Google Scholar] [CrossRef]
- Wang, W.; Ji, C.; Luo, S.; Zhu, M. Modeling of dendritic evolution of continuously cast steel billet with cellular automaton. Metall. Mater. Trans. B 2017, 49, 200–212. [Google Scholar] [CrossRef]
- Jing, C.L.; Wang, X.H.; Jiang, M. Study on solidification structure of wheel steel round billet using FE-CA coupling model. Steel Res. Int. 2011, 82, 1173–1178. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, H.; Wang, F.; Li, B. Impact of electromagnetic stirring on grain structure of electroslag remelting ingot. JOM 2015, 67, 1821–1829. [Google Scholar] [CrossRef]
- Pierer, R.; Bernhard, C. On the influence of carbon on secondary dendrite arm spacing in steel. J. Mater. Sci. 2008, 43, 6938–6943. [Google Scholar] [CrossRef]
- Bai, L.; Liu, H.; Zhang, Y.; Miao, X.; Ruan, X. Numerical simulation of the microstructure of 22CrMoH billets and the effects of alloying elements. Chinese J. Eng. 2011, 33, 1091–1098. [Google Scholar] [CrossRef]
- Han, L.; Feng, Y.; Wu, H.; Chen, Z. Numerical simulation of effects of alloying elements on solidification structure of 20CrMnTi billets. Iron Steel 2016, 51, 36–42. [Google Scholar]
- Lally, B.; Biegler, L.; Henein, H. Finite difference heat transfer modeling for continuous casting. Metall. Mater. Trans. B 1990, 21, 761–770. [Google Scholar] [CrossRef]
- Rappaz, M.; Gandin, C.A. Probabilistic modelling of microstructure formation in solidification processes. Acta Metall. Mater. 1993, 41, 345–360. [Google Scholar] [CrossRef]
- Kurz, W.; Giovanola, B.; Trivedi, R. Theory of microstructural development during rapid solidification. Acta Metallurgica. 1986, 34, 823–830. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundaments of Solidification, 4th revised ed.; Trans Tech Publishers: Aedermannsdorf, Switzerland, 1998; p. 77. [Google Scholar]
- Bai, L.; Wang, B.; Zhong, H.; Ni, J.; Zhai, Q.; Zhang, J. Experimental and numerical simulations of the solidification process in continuous casting of slab. Metals 2016, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Li, W.C. Metallurgy and Physical Chemistry of Materials; Metallurgical Industry Press: Beijing, China, 2001; pp. 531–533. (In Chinese) [Google Scholar]
- Fang, Q.; Ni, H.W.; Zhang, H.; Wang, B.; Lv, Z.A. The effects of a submerged entry nozzle on flow and initial solidification in a continuous casting bloom mold with electromagnetic stirring. Metals 2017, 7, 146. [Google Scholar] [CrossRef] [Green Version]
- Pequet, C.; Rappaz, M.; Gremaud, M. Modeling of microporosity, macroporosity, and pipe-shrinkage formation during the solidification of alloys using a mushy-zone refinement method: Applications to aluminum alloys. Metall. Mater. Trans. A 2002, 33, 2095–2106. [Google Scholar] [CrossRef]
- Savage, J.; Pritchard, W.H. The problem of rupture of the billet in the continuous casting of steel. J. Iron Steel Inst. 1954, 178, 269–277. [Google Scholar]
- Hardin, R.A.; Liu, K.; Beckermann, C.; Kapoor, A. A transient simulation and dynamic spray cooling control model for continuous steel casting. Metall. Mater. Trans. B 2003, 34, 297–306. [Google Scholar] [CrossRef]
- Cai, K. Quality Control of Continuous Casting Billet; Metallurgical Industry Press: Beijing, China, 2010; pp. 279–282. (In Chinese) [Google Scholar]
- Hu, H.Q. Metal Solidification Principle; Machine Press: Beijing, China, 2000; pp. 131–133. (In Chinese) [Google Scholar]
- Yoo, H.; Viskanta, R. Effect of anisotropic permeability on the transport process during solidification of binary a mixture. Int. J. Heat Mass Transf. 1992, 35, 2335. [Google Scholar] [CrossRef]
- Piorier, D.R. Permeability for flow of dendritic liquid in columnar dendrite alloys. Metall. Trans. B 1987, 18, 245. [Google Scholar] [CrossRef]
- Cornelissen, M.C.M. Mathematical model for solidification of multicomponent alloys. Ironmak. Steelmak. 1986, 13, 204–212. [Google Scholar]
- Shen, H.F.; Beckermann, C. Experimental study on the mush deformation and solute redistribution. Acta Metall. Sin. 2002, 38, 352–358. [Google Scholar]







| Steel | C | Si | Mn | P | S | Cr | Al |
|---|---|---|---|---|---|---|---|
| S-1 | 0.1319 | 0.3639 | 1.4767 | 0.0182 | 0.0021 | \ | \ |
| S-2 | 0.1557 | 0.1326 | 0.5548 | 0.0168 | 0.0039 | \ | \ |
| S-3 | 0.1657 | 0.2802 | 1.4503 | 0.0184 | 0.0026 | \ | \ |
| S-4 | 0.3994 | 0.3071 | 1.4604 | 0.0198 | 0.0027 | 1.9 | 0.0153 |
| S-5 | 0.4878 | 0.2056 | 0.5308 | 0.0145 | 0.0026 | 0.0215 | 0.0189 |
| Parameters | ΔTS (K) | ΔTS,σ (K) | nS | ΔTV (K) | ΔTV,σ (K) | nV | α2 | α3 | Liquidus (°C) | Solidus (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| S-1 | 1 | 0.1 | 1 × 108 | 2.5 | 1 | 3.0 × 109 | 2.022 × 10−6 | 9.413 × 10−6 | 1513 | 1477 |
| S-2 | 1 | 0.1 | 1 × 108 | 1.9 | 1 | 3.0 × 109 | 5.227 × 10−6 | 1.630 × 10−5 | 1519 | 1483 |
| S-3 | 1 | 0.1 | 1 × 108 | 2.6 | 1 | 3.0 × 109 | 2.645 × 10−6 | 8.105 × 10−6 | 1511 | 1473 |
| S-4 | 1 | 0.1 | 1 × 108 | 4.0 | 1 | 3.0 × 109 | 1.551 × 10−6 | 1.412 × 10−6 | 1488 | 1431 |
| S-5 | 1 | 0.1 | 1 × 108 | 5.0 | 1 | 3.0 × 109 | 1.639 × 10−6 | 1.189 × 10−6 | 1489 | 1424 |
| Steel | Element | C | Si | Mn | P | S | Cr | Al | |
|---|---|---|---|---|---|---|---|---|---|
| Parameters | |||||||||
| S-1 | k | 0.16 | 0.58 | 0.72 | 0.33 | 0.045 | / | / | |
| m/K·(wt.%)−1 | −84.78 | −17.37 | −5.1 | −29.09 | −44.16 | / | / | ||
| S-2 | k | −79.8 | 0.16 | −5.2 | −27.2 | −38.0 | / | 4.0 | |
| m/K·(wt.%)−1 | 0.17 | 0.60 | 0.72 | 0.29 | 0.03 | / | 1.13 | ||
| S-3 | k | −80.7 | −16.7 | −5.2 | −28.4 | −38.4 | / | / | |
| m/K·(wt.%)−1 | 0.16 | 0.56 | 0.71 | 0.26 | 0.03 | / | / | ||
| S-4 | k | −87.2 | −16.7 | −5.3 | −32.3 | −44.9 | −1.7 | 5.0 | |
| m/K·(wt.%)−1 | 0.16 | 0.61 | 0.71 | 0.25 | 0.06 | 0.89 | 1.22 | ||
| S-5 | k | −88.1 | −17.2 | −5.2 | −30.8 | −48.4 | / | 3.9 | |
| m/K·(wt.%)−1 | 0.17 | 0.60 | 0.70 | 0.30 | 0.05 | / | 1.19 | ||
| All | D × 10−9/ m2·s−1 | 11 | 3.5 | 2.4 | 1.9 | 3.9 | 3.0 | 3.0 | |
| Γ/m·K | 3 × 10−7 | ||||||||
| Secondary Cooling Zone | Length, m | Water Flow Rate, L·min−1 | Computational Formula |
|---|---|---|---|
| Foot roller section (W) | 0.29 | 72.6 | hf = α·[581W0.541(1 − 0.0075TW) |
| Foot roller section (N) | 0.89 | 182.7 | |
| L2 | 3.45 | 174.2 | hk = γ·(130 + 350W) |
| L3 | 1.87 | 123.5 | |
| L4 | 2.14 | 66 | |
| L5 | 2.13 | 31 | |
| L6 | 9.2 | 66.2 | |
| L7 | 2.36 | 28.5 | |
| L8 | 22.68 | 68.75 | |
| Air cooling zone | / | qa = εσ(T4 − Ta4) | ε = 0.85/(1 + exp(42.68 − 0.02682Ts)0.0115) [26] |
| Steel | C | Si | Mn | P | S |
|---|---|---|---|---|---|
| C-0.20 | 0.2 | 0.2802 | 1.4503 | 0.0184 | 0.0026 |
| C-0.25 | 0.25 | 0.2802 | 1.4503 | 0.0184 | 0.0026 |
| C-0.30 | 0.3 | 0.2802 | 1.4503 | 0.0184 | 0.0026 |
| C-0.35 | 0.35 | 0.2802 | 1.4503 | 0.0184 | 0.0026 |
| Parameters | ΔTS (K) | ΔTS,σ (K) | nS | ΔTV (K) | ΔTV,σ (K) | nV | α2 | α3 |
|---|---|---|---|---|---|---|---|---|
| C-0.2 | 1 | 0.1 | 1 × 108 | 2.8 | 1 | 3.0 × 109 | 2.652 × 10−6 | 6.300 × 10−6 |
| C-0.25 | 1 | 0.1 | 1 × 108 | 3.0 | 1 | 3.0 × 109 | 2.572 × 10−6 | 4.535 × 10−5 |
| C-0.30 | 1 | 0.1 | 1 × 108 | 3.2 | 1 | 3.0 × 109 | 2.412 × 10−6 | 3.402 × 10−6 |
| C-0.35 | 1 | 0.1 | 1 × 108 | 3.4 | 1 | 3.0 × 109 | 2.230 × 10−6 | 2.634 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Wang, M.; Shi, P.; Xu, L. Effects of Alloying Elements on Solidification Structures and Macrosegregation in Slabs. Metals 2022, 12, 1826. https://doi.org/10.3390/met12111826
Zhang P, Wang M, Shi P, Xu L. Effects of Alloying Elements on Solidification Structures and Macrosegregation in Slabs. Metals. 2022; 12(11):1826. https://doi.org/10.3390/met12111826
Chicago/Turabian StyleZhang, Pan, Minglin Wang, Pengzhao Shi, and Lijun Xu. 2022. "Effects of Alloying Elements on Solidification Structures and Macrosegregation in Slabs" Metals 12, no. 11: 1826. https://doi.org/10.3390/met12111826
APA StyleZhang, P., Wang, M., Shi, P., & Xu, L. (2022). Effects of Alloying Elements on Solidification Structures and Macrosegregation in Slabs. Metals, 12(11), 1826. https://doi.org/10.3390/met12111826






