Influence of Pretreatment Strategy on the Crushing of Spent Lithium-Ion Batteries
Abstract
:1. Introduction
2. Recycling of EOL LIBs
2.1. Design and Composition
2.2. Hazards and Waste Management
2.3. Thermal Treatment of LIB Components
2.3.1. Material Behavior
2.3.2. Crushing of LIB Compounds
2.4. Aim of This Contribution
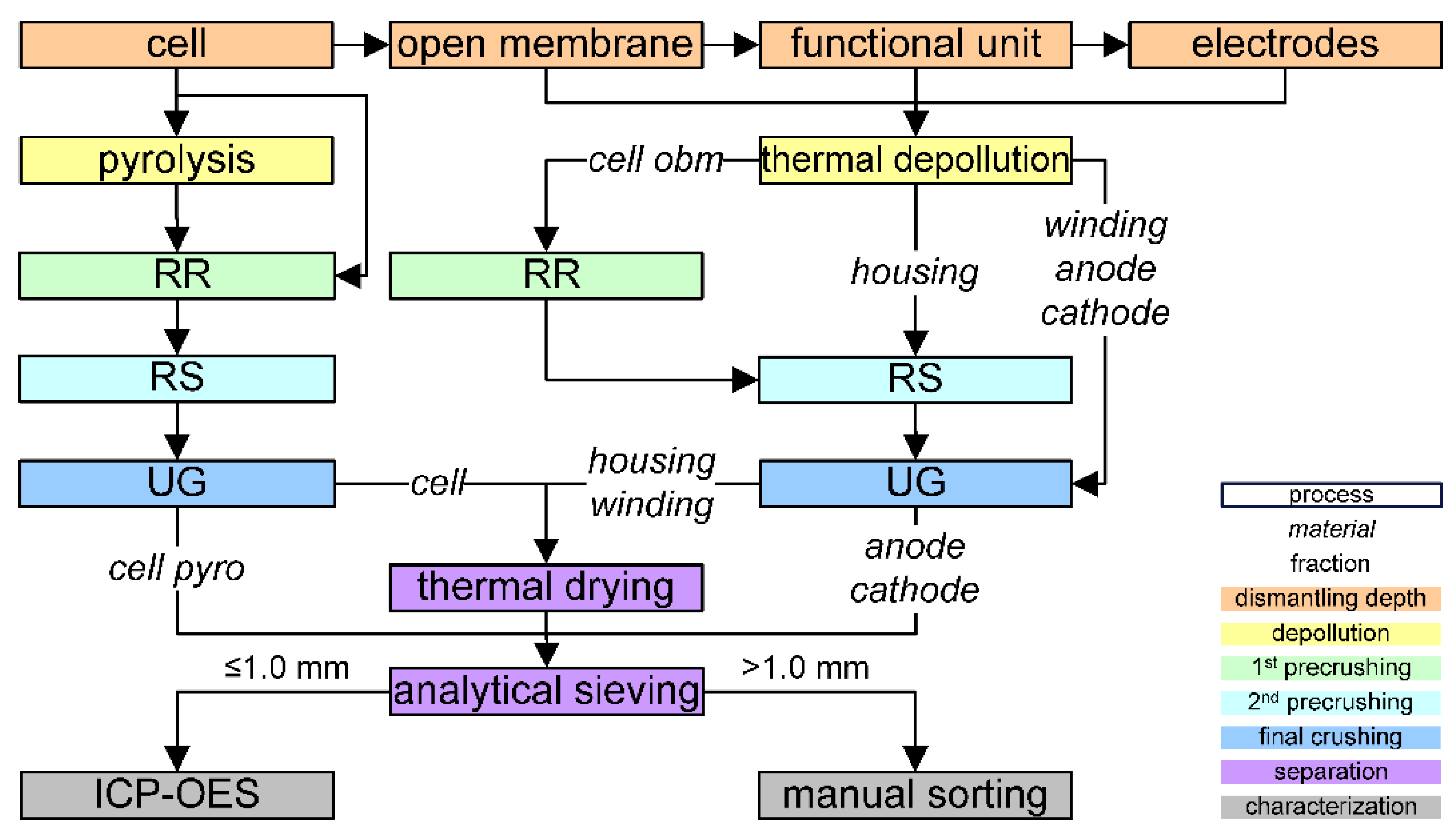
3. Materials and Methods
3.1. Materials
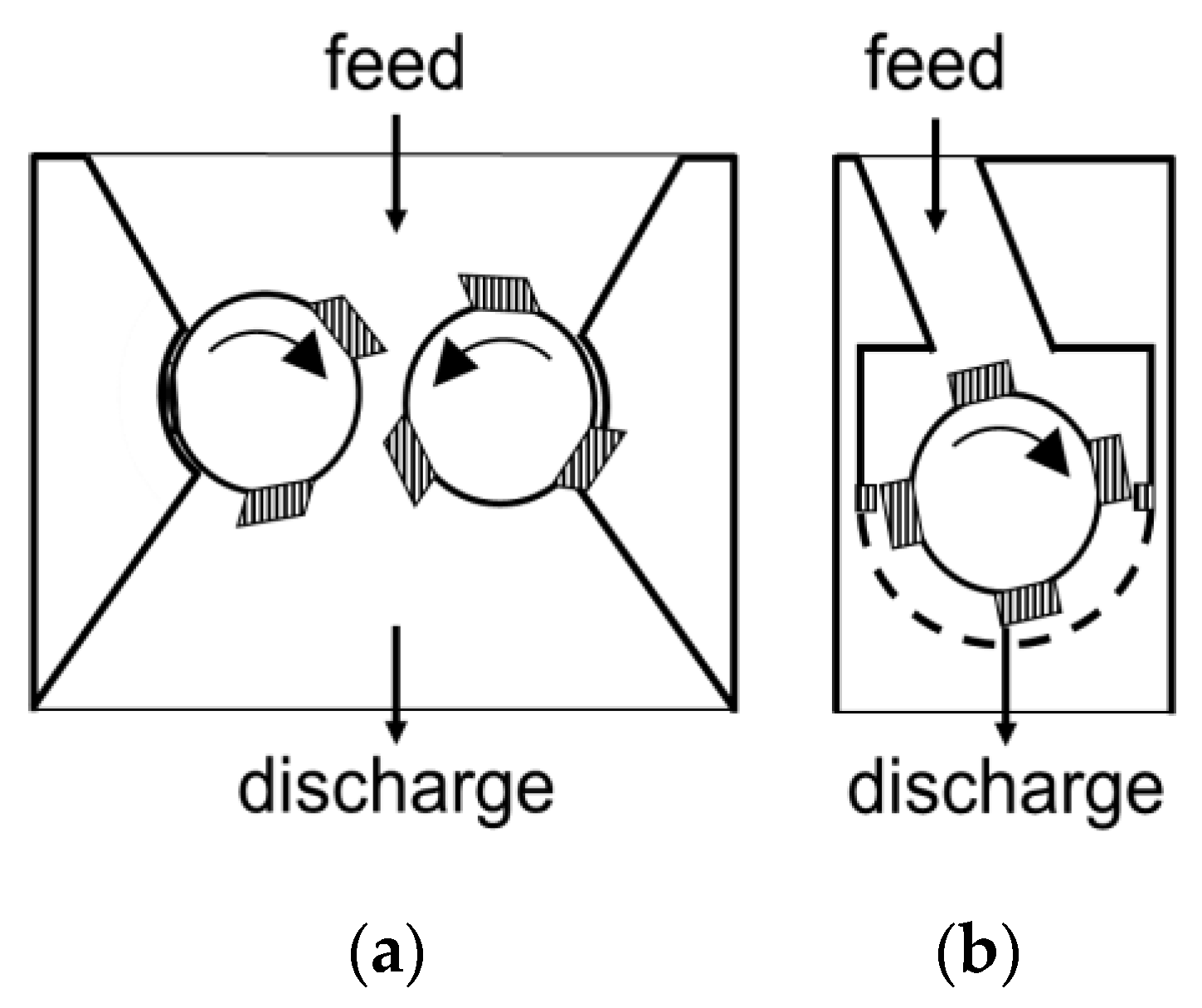
3.2. Equipmental Setup
3.3. Methods
4. Results and Discussion
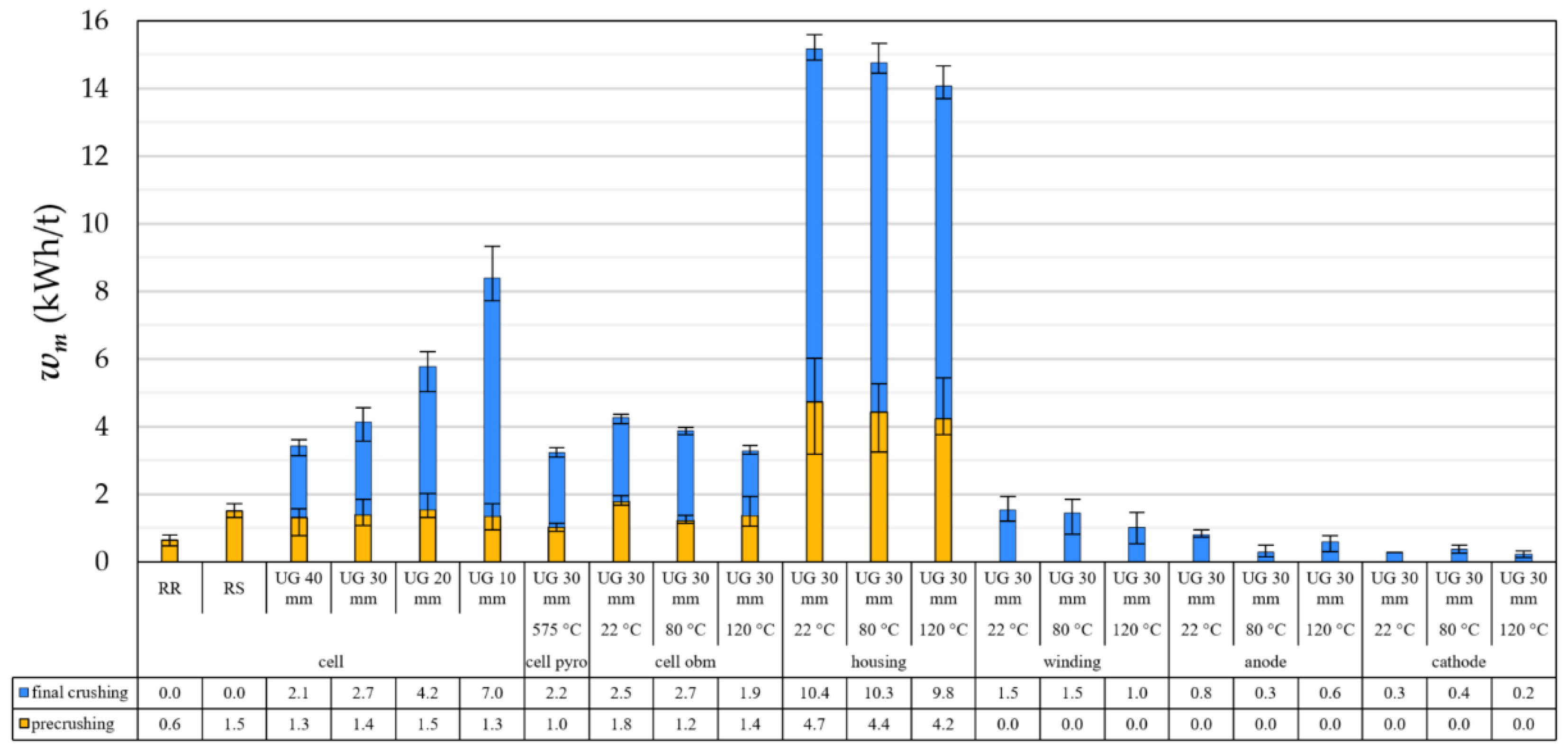
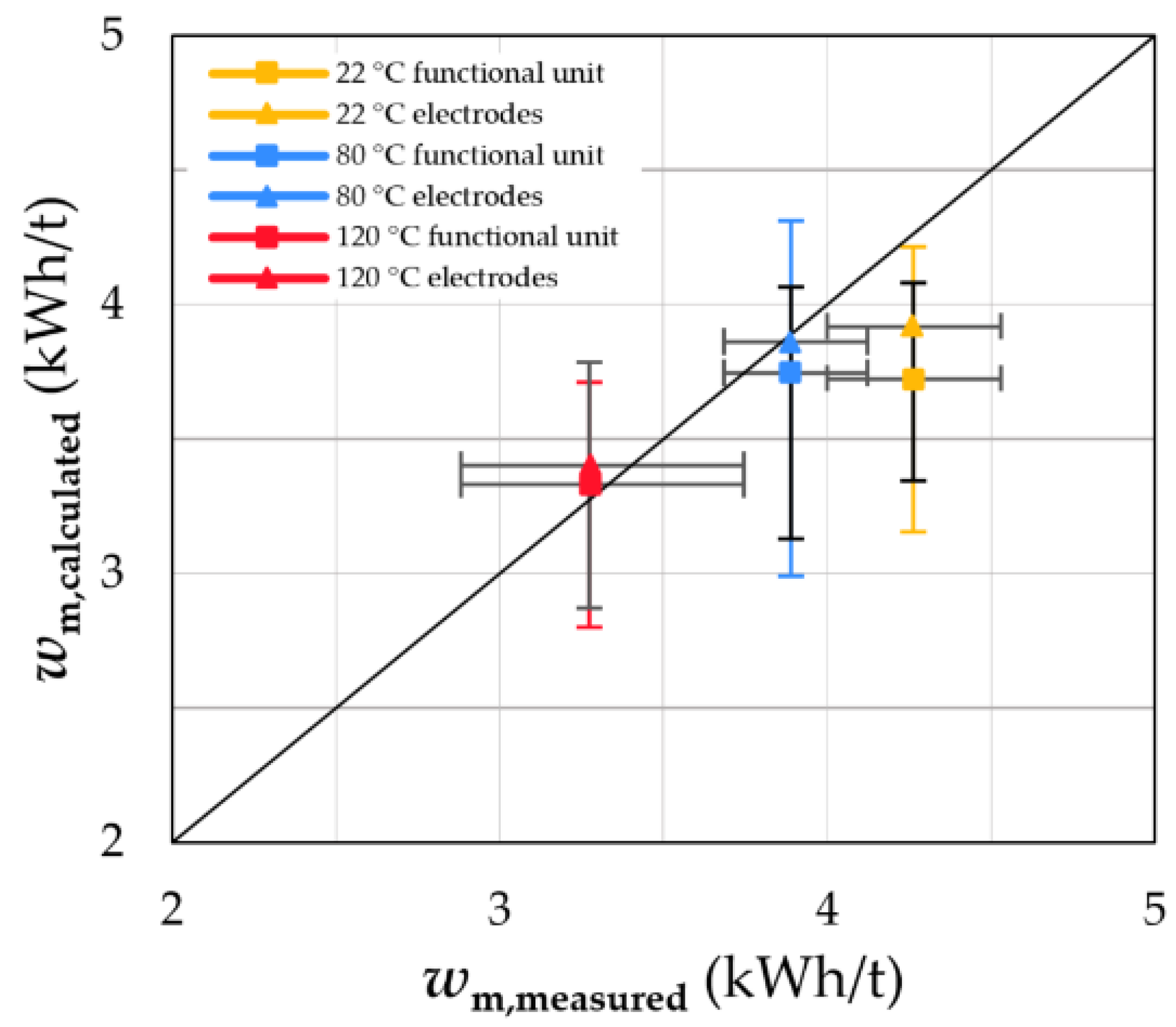
4.1. Energy Input for Crushing EOL LIBs
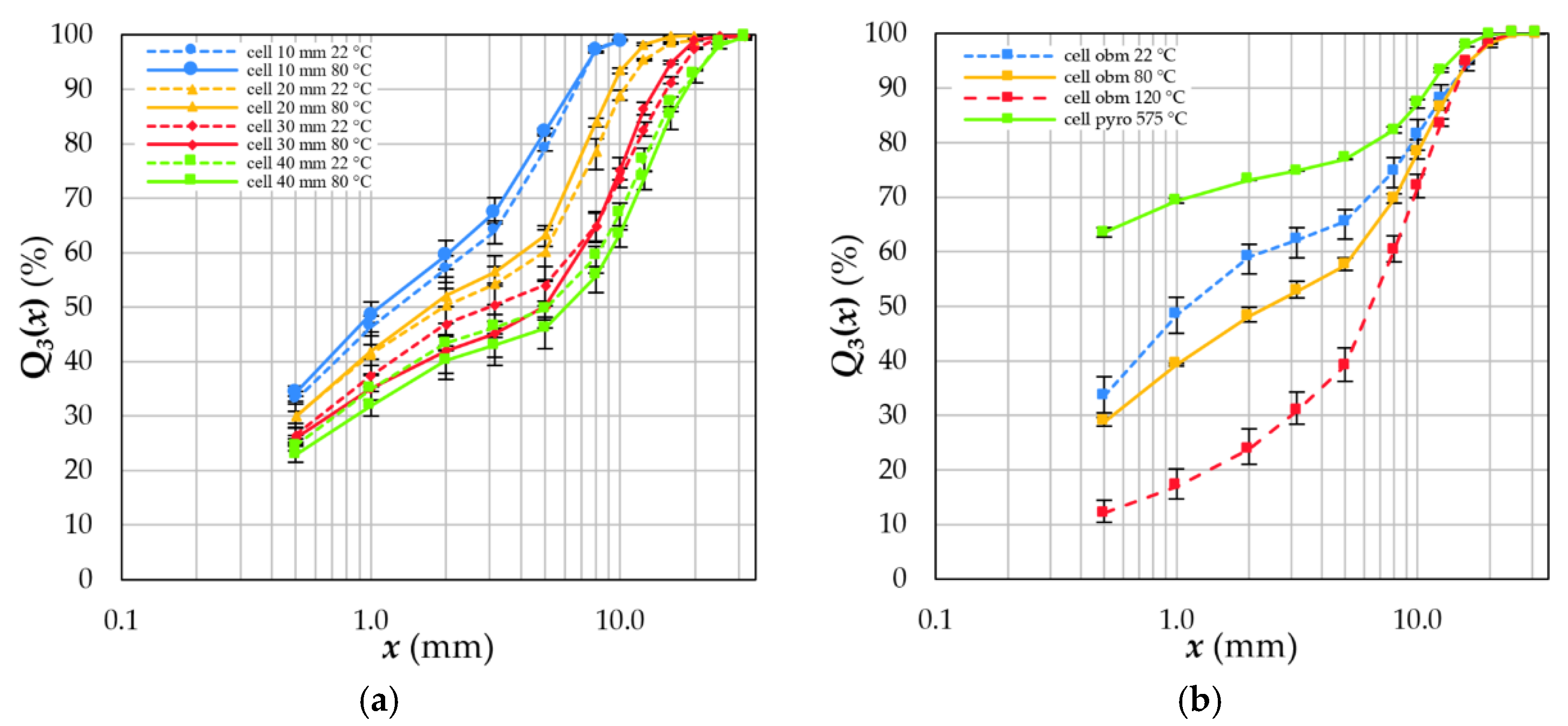
4.2. Fragment Size Distribution
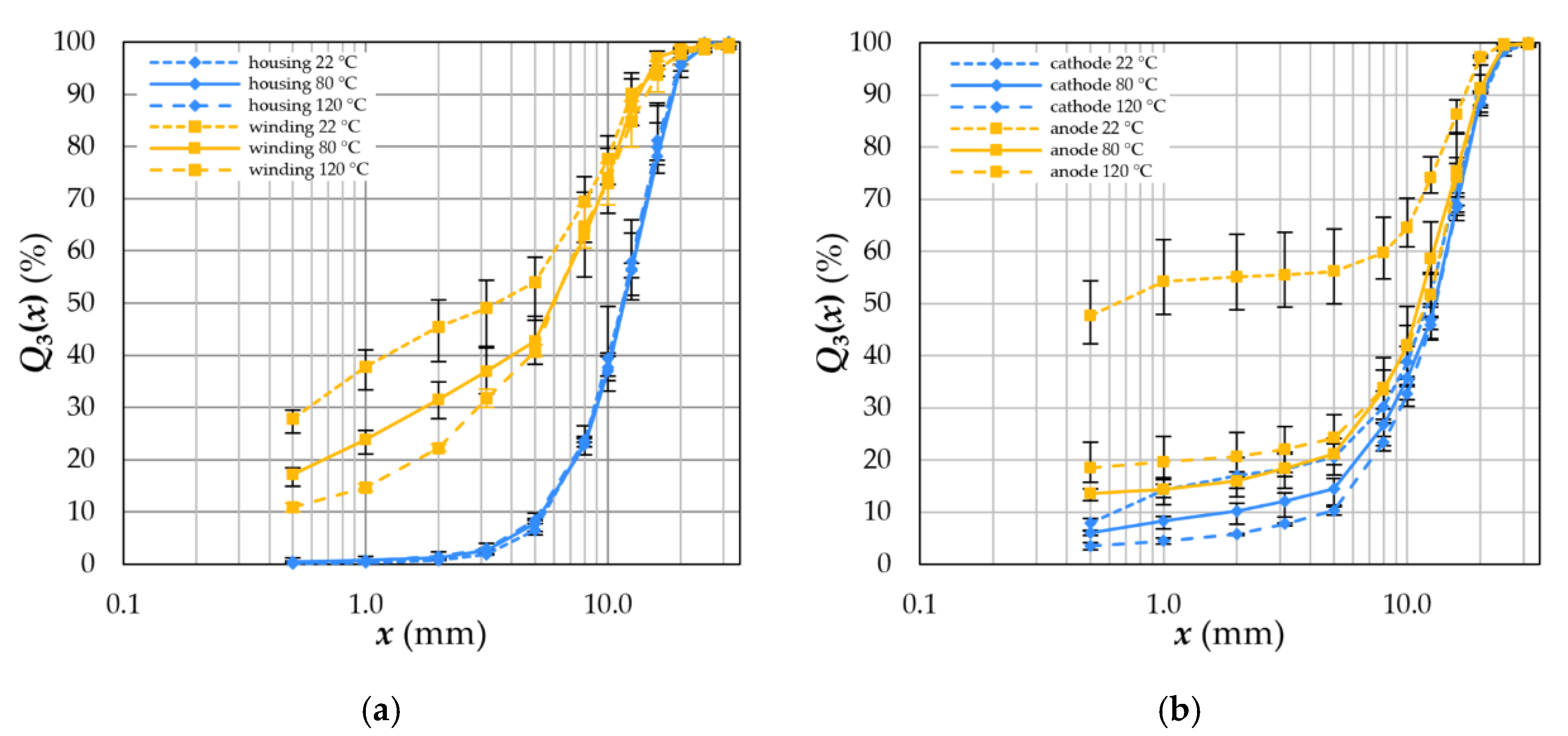
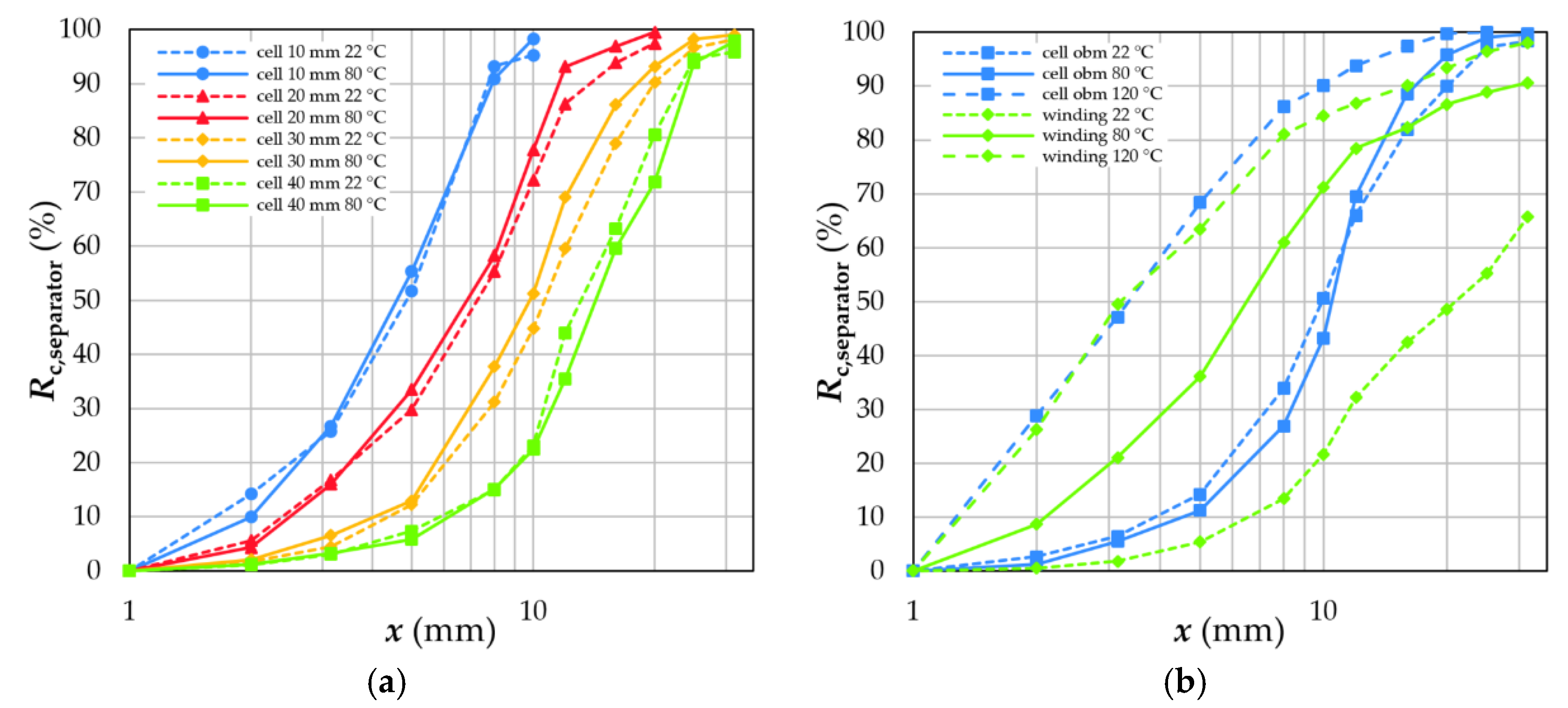
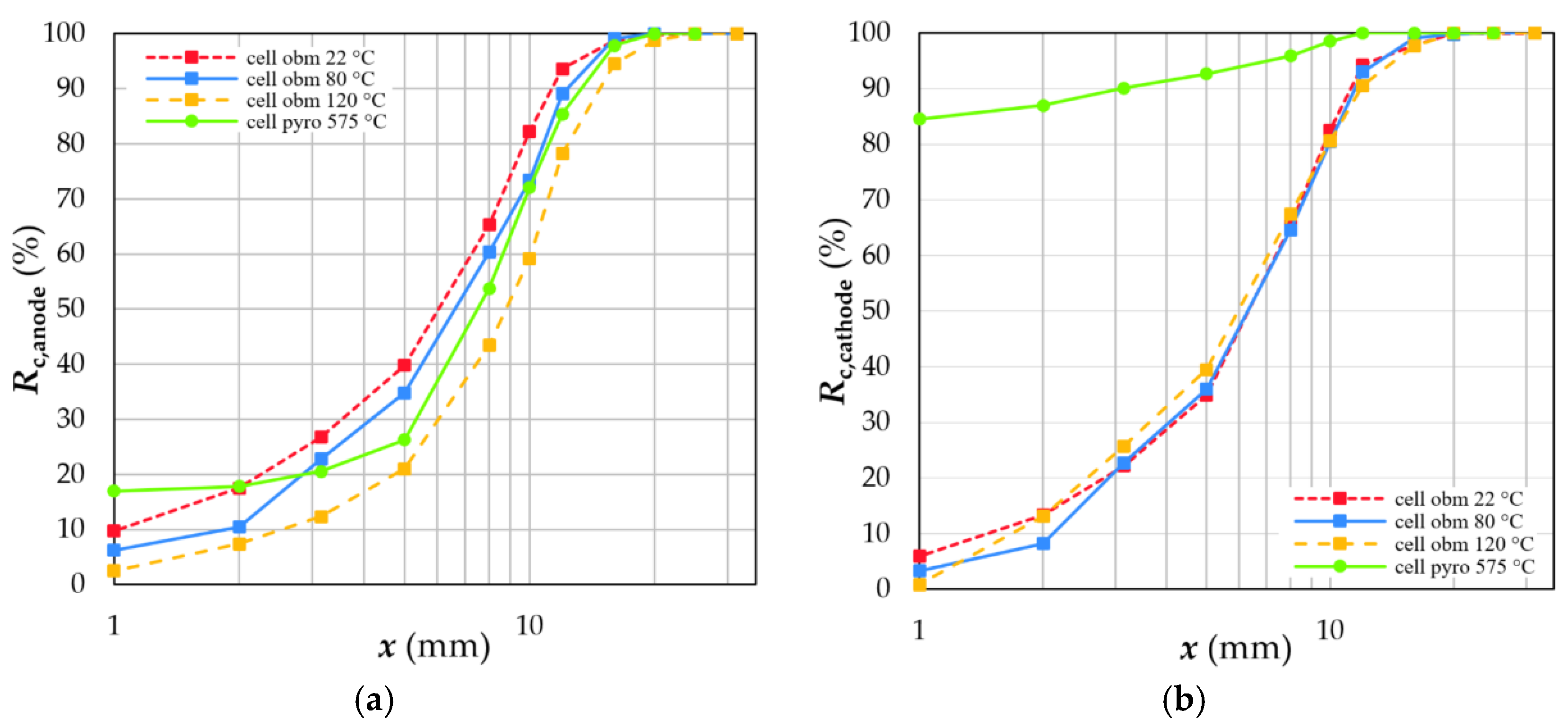
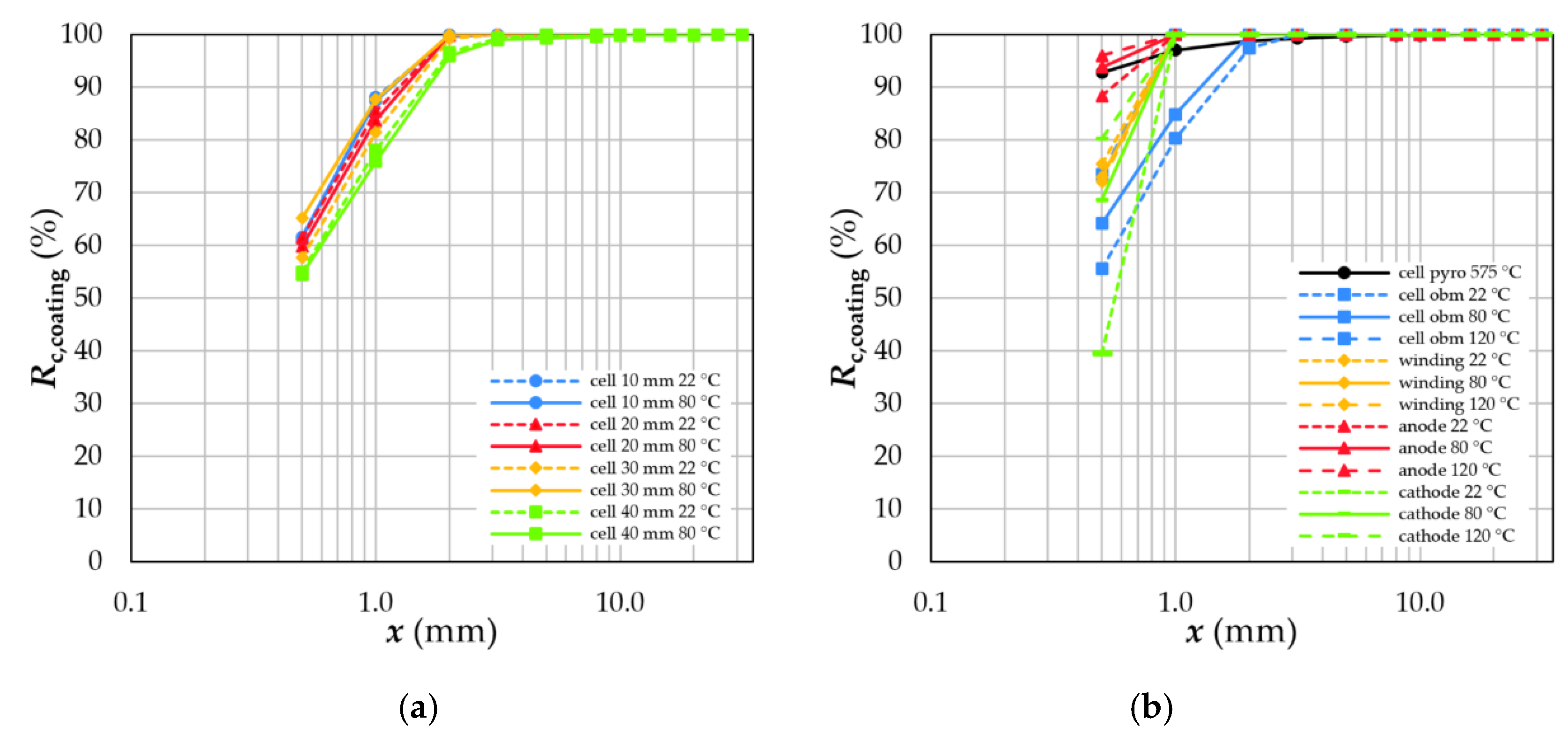
4.3. Degree of Liberation and Degree of Decoating
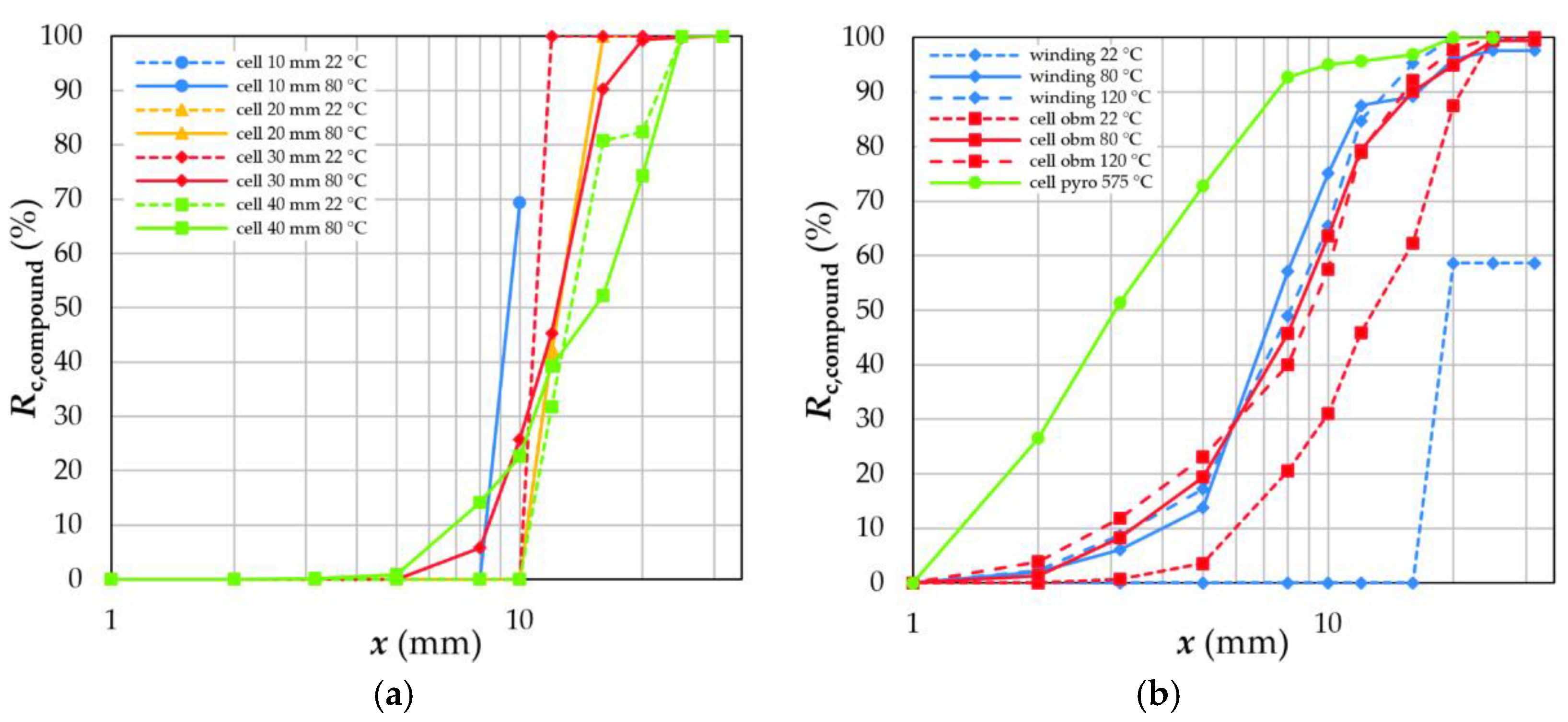
4.4. Exploitation of the Selective Breakage Behaviour
4.4.1. Separator Foil and Housing
4.4.2. Electrodes
4.4.3. Coating Material and Remaining Compounds
5. Conclusions
- After crushing in a cutting mill with grid sizes between 10 and 40 mm, fragments accumulate mainly in size fractions >1.0 mm. The finer size fractions <1.0 mm consists mainly of coating materials (see also [33,39,80]). Higher dismantling depths decrease the complexity of the feed compound, which has to be liberated by subsequent crushing steps.
- Elevated temperatures for depollution rearrange the crystal structure of the binder used for electrode coatings or even decompose them completely. Moreover, high temperatures weaken the tensile strength and stability of the plastics used as separator foils in LIBs. Consequently, compounds are formed, and electrode decoating is reduced to recoveries of less than 20%.
- The specific energy input necessary to crush a battery cell can be estimated by examining the crushing behaviour of its individual components. However, liberation and decoating cannot be predicted with this approach. In general, the energy input decreases with increasing grid size, increasing dismantling depth and depollution temperature.
- Thermal depollution between 80 °C and 120 °C partly removes organic solvents. Unfortunately, thermal depollution above the operating temperature of 60 °C is disadvantageous for subsequent separation steps due to the formation of compounds and a deteriorating decoating.
- Sieving can be used to separate a coating fraction since all other battery components are enriched in the coarser size classes. The mass recovery and quality of this fraction (black mass) is influenced by the thermal treatment and grid size in the crushing device.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AM | Active materials | Mn | Manganese |
| Al | Aluminium | NCA | Lithium Nickel Cobalt Aluminium Oxide |
| CMC | Sodium Carboxymethyl Cellulose | Ni | Nickel |
| Co | Cobalt | NMC | Lithium nickel manganese cobalt battery |
| Cu | Copper | NSD | Nail Penetration Safety Device |
| DEC | Diethyl carbonate | PC | Propylene carbonates |
| DMC | Dimethyl carbonate | PE | Polyethylene |
| EOL | End-of-life | PET | Polyethylene terephthalate |
| EC | Ethylene carbonate | PF5 | Phosphorus pentafluoride |
| EMC | Ethyl methyl carbonate | PP | Polypropylene |
| HF | Hydrofluoric acid | PVdF | Polyvinylidene fluoride |
| ICP-OES | Inductively coupled plasma optical emission spectrometry | RE | Recycling efficiency |
| LCO | Lithium cobalt oxide | RR | Slow-rotating, two-shaft rotary shear with axial gap |
| Li | Lithium | RR | Recovery rate |
| LIB(s) | Lithium-ion battery(ies) | RS | Slow-rotating, two-shaft rotary shear without axial gap |
| LFP | Lithium iron phosphate | SBR | Styrene butadiene rubber |
| LiF | Lithium fluoride | UG | One-shaft rotary shear with outlet grid final crushing |
| LiPF6 | Lithium hexafluorophosphate | WEEE | Waste electrical and electronic equipment |
| MRR | Material recovery rates |
References
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Elwert, T.; Römer, F.; Schneider, K.; Hua, Q.; Buchert, M. Recycling of Batteries from Electric Vehicles. In Behaviour of Lithium-Ion Batteries in Electric Vehicles—Battery Health, Performance, Safety, and Cost; Pistoia, G., Liaw, B., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar]
- Melin, H.E. The Lithium-Ion Battery End-of-Life Market—A Baseline Study; Circular Energy Storage: London, UK, 2019. [Google Scholar]
- Zhang, X.; Cao, H.; Xie, Y.; Ning, P.; An, H.; You, H.; Nawaz, F. A closed-loop process for recycling LiNi1/3Co1/3Mn1/3O2 from the cathode scraps of lithium-ion batteries: Process optimization and kinetics analysis. Sep. Purif. Technol. 2015, 150, 186–195. [Google Scholar] [CrossRef]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Perez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef] [PubMed]
- Vezzini, A. Manufacturers, Materials and Recycling Technologies. In Lithium-Ion Batteries Advances and Applications; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 529–551. [Google Scholar]
- Directive 2006/66/EC of the European Parliament and of the Council; European Union: Brussels, Belgium, 2006.
- Friedrich, B.; Träger, T.; Weyhe, R. Recyclingtechnologien am Beispiel Batterien. In Proceedings of the 25. Aachener Kolloquium Abfallwirtschaft, Forum M, Aachen, Germany, 29 November 2012. [Google Scholar]
- Regulation of the European Parliament and of the Council Concerning Batteries and Waste Batteries; European Commission: Brussels, Beglium, 2020; p. 130.
- Directive 2012/19/EU of the European Parliament and of the Council on Waste Electrical and Electronic Equipment (WEEE); European Commission: Brussels, Belgium, 2012; p. 34.
- Directive 2000/53/EC of the European Parliament and of the Council on End-Of Life Vehicles; L 269; European Commission: Brussels, Belgium, 2000; p. 8.
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- Dewulf, J.; Van der Vorst, G.; Denturck, K.; Van Langenhove, H.; Ghyoot, W.; Tytgat, J.; Vandeputte, K. Recycling rechargeable lithium ion batteries: Critical analysis of natural resource savings. Resour. Conserv. Recycl. 2010, 54, 229–234. [Google Scholar] [CrossRef]
- Zhao, S.; He, W.; Li, G. Recycling Technology and Principle of Spent Lithium-Ion Battery. In Recycling of Spent Lithium-Ion Batteries—Processing Methods and Environmental Impacts; An, L., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 1–26. [Google Scholar] [CrossRef]
- Zhao, G. Resource Utilization and Harmless Treatment of Power Batteries. In Reuse and Recycling of Lithium-Ion Power Batteries; Zhao, G., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 335–378. [Google Scholar]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Met.—Open Access Metall. J. 2020, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Hanisch, C.; Haselrieder, W.; Kwade, A. Recycling von Lithium-Ionen-Batterien—Das Projekt LithoRec. In Recycling und Rohstoffe; Thomé-Kozmienski, K.J., Goldmann, D., Eds.; TK Verlag: Neuruppin, Germany, 2012; Volume 5, pp. 691–698. [Google Scholar]
- Werner, D.M.; Mütze, T.; Kaas, A.; Peuker, U.A. Mechanical and physical processes of battery recycling. In Nano Technology for Battery Recycling, Remanufacturing, and Reusing; Siamak, F., Gupta, R.K., Yasin, T., Nguyen, T.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 455–486. [Google Scholar]
- Werner, D.; Mütze, T.; Peuker, U.A. Influence of cell opening methods on organic solvent removal during pretreatment in lithium-ion battery recycling. Waste Manag. Res. 2021, 40, 1015–1026. [Google Scholar] [CrossRef]
- Werner, D.; Mütze, T.; Peuker, U.A. Influence of cell opening methods organic solvent removal during processing in lithium-ion battery recycling. Metals 2022, 12, 663. [Google Scholar] [CrossRef]
- Stenzel, Y.P.; Börner, M.; Preibisch, Y.; Winter, M.; Nowak, S. Thermal profiling of lithium ion battery electrodes at different states of charge and aging conditions. J. Power Sources 2019, 433, 226709. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring Chemical, Mechanical, and Electrical Functionalities of Binders for Advanced Energy-Storage Devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef]
- Jeschull, F.; Brandell, D.; Wohlfahrt-Mehrens, M.; Memm, M. Water-Soluble Binders for Lithium-Ion Battery Graphite Electrodes: Slurry Rheology, Coating Adhesion, and Electrochemical Performance. Energy Technol. 2017, 5, 2108–2118. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W.; Bailey, C.; Ganter, M.J.; Landi, B.J. Economic and environmental characterization of an evolving Li-ion battery waste stream. J. Environ. Manag. 2014, 135, 126–134. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer Binders: Characterization and Development toward Aqueous Electrode Fabrication for Sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef]
- Vuorilehto, K. Materials and functions. In Lithium-Ion Batteries: Basics and Applications; Korthauer, R., Ed.; Springer: Berlin/Heidelberg Germany, 2019; Volume 2, pp. 21–28. [Google Scholar] [CrossRef]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Korthauer, R. (Ed.) Handbuch Lithium-Ionen-Batterien; Springer Vieweg: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kwade, A.; Diekmann, J. (Eds.) Recycling of Lithium-Ion Batteries—The LithoRec Way; Springer: New York, NY, USA, 2018. [Google Scholar]
- Arnberger, A.; Gresslehner, K.-H.; Pomberger, R.; Curtis, A. Recycling von Lithium Ionen Batterien aus EVs & HEVs. In Proceedings of the DepoTech 2012, Leoben, Austria, 6–9 November 2012. [Google Scholar]
- Gaines, L.; Sullivan, J.; Burnham, A. Life-Cycle Analysis for Lithium-Ion Battery Production and Recycling. In Proceedings of the 90th Annual Meeting of the Transportation Research Board, Washington, DC, USA, 23–27 January 2011. [Google Scholar]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutzc, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W. Targeting high value metals in lithium-ion battery recycling via shredding and size-based separation. Waste Manag. 2016, 51, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Arnberger, A.; Coskun, E.; Rutrecht, B. Recycling von Lithium-Ionen-Batterien. In Recycling und Rohstoffe; Thomé-Kozmiensky, K.J., Goldmann, D., Eds.; TK Verlag Karl Thomé-Kozmiensky: Neuruppin, Germany, 2018; Volume 11, pp. 583–599. [Google Scholar]
- Friedrich, B.; Schwich, L. E-Mobility Batteries Recycling—How to Reach the Demanded Recycling Efficiency? In Proceedings of the VDMA Webinar, online, 22 June 2021. [Google Scholar]
- Rahimzei, E. Begleit- und Wirkungsforschung Schaufenster Elektromobilität (BuW) Ergebnispapier Nr. 37—Sicherheit von Elektrofahrzeugen; Deutsches Dialog Institut GmbH: Frankfurt am Main, Germany, 2017. [Google Scholar]
- Gama, M. European Li-Ion Battery Advanced Manufacturing For Electric Vehicles—ELIBAMA. 2014. Available online: https://cordis.europa.eu/project/id/285385 (accessed on 28 June 2022).
- Hanisch, C.; Diekmann, J.; Stieger, A.; Haselrieder, W.; Kwade, A. Recycling of Lithium-Ion Batteries. In Handbook of Clean Energy Systems; Yan, J., Cabeza, L.F., Sioshansi, R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; Volume 5, pp. 2865–2888. [Google Scholar]
- Wuschke, L. Mechanische Aufbereitung von Lithium-Ionen-Batteriezellen. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 2018. [Google Scholar]
- Van Pels, W. Herausforderung hinsichtlich der Brandschutztechnologien in Recyclinganlagen insbesondere bei der Verarbeitung von Li-Ionen Traktionsbatterien. In Proceedings of the Recycling und Sekundärrohstoffe, Berlin, Germany, 2–3 March 2020; pp. 496–505. [Google Scholar]
- Wegener, K.; Andrew, S.; Raatz, A.; Dröder, K.; Herrmann, C. Disassembly of Electric Vehicle Batteries Using the Example of the Audi Q5 Hybrid System. In Proceedings of the Conference on Assembly Technologies and Systems, Dresden, Germany, 15–16 May 2014. [Google Scholar]
- Ekberg, C.; Petranikova, M. Lithium Batteries Recycling. In Lithium Process Chemistry—Resources, Extraction, Batteries and Recycling; Chagnes, A., Swiatowska, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–269. [Google Scholar] [CrossRef]
- Martens, H.; Goldmann, G. (Eds.) Recyclingtechnik; Springer Vieweg: Wiesbaden, Germany, 2016. [Google Scholar]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium-Ion Batteries, Part II: Laboratory-Scale Research Developments in Mechanical, Thermal, and Leaching Treatments. J. Sustain. Metall. 2020, 6, 142–160. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, X.; Han, J.; Qin, W.; Liu, T.; Zhao, C.; Chang, Z. Kinetic Study and Pyrolysis Behaviors of Spent LiFePO4 Batteries. ACS Sustain. Chem. Eng. 2019, 7, 1289–1299. [Google Scholar] [CrossRef]
- Träger, T.; Friedrich, B.; Weyhe, R. Recovery Concept of Value Metals from Automotive Lithium-Ion Batteries. Chem. Ing. Tech. 2015, 87, 1550–1557. [Google Scholar] [CrossRef]
- Lombardo, G.; Ebin, B.; Steenari, B.-M.; Alemrajabi, M.; Karlsson, I.; Petranikova, M. Comparison of the effects of incineration, vacuum pyrolysis and dynamic pyrolysis on the composition of NMC-lithium battery cathode-material production scraps and separation of the current collector. Resour. Conserv. Recycl. 2021, 164, 105142. [Google Scholar] [CrossRef]
- Rahimzei, E. Transportation of lithium batteries and lithium-ion batteries. In Lithium-Ion Batteries: Basics and Applications; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Park, Y.-S.; Oh, E.-S.; Lee, S.-M. Effect of polymeric binder type on the thermal stability and tolerance to roll-pressing of spherical natural graphite anodes for Li-ion batteries. J. Power Sources 2014, 248, 1191–1196. [Google Scholar] [CrossRef]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Xie, L.; Zhao, L.; Wan, J.-L.; Shao, Z.-Q.; Wang, F.-J.; Lv, S.-Y. The Electrochemical Performance of Carboxymethyl Cellulose Lithium as a Binding Material for Anthraquinone Cathodes in Lithium Batteries. J. Electrochem. Soc. 2012, 159, A499–A505. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Zhang, H.; Huang, B.; Ma, Y. Application of a novel binder for activated carbon-based electrical double layer capacitors with nonaqueous electrolytes. J. Solid State Electrochem. 2013, 17, 2035–2042. [Google Scholar] [CrossRef]
- Orendorff, C.J. The Role of Separators in Lithium-Ion Cell Safety. Electrochem. Soc. Interface 2012, 21, 61–65. [Google Scholar] [CrossRef]
- Weber, C.J.; Roth, M. Separators. In Lithium-Ion Batteries: Basics and Applications; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 75–88. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, W.; Han, J.; Jiao, F.; Qin, W.; Liu, T.; Zhao, C. Pyrolysis and physical separation for the recovery of spent LiFePO4 batteries. Waste Manag. 2019, 89, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, K.; Kaluzna-Czapliñska, J.; Józwiak, W.K. Thermal and thermo-catalytic degradation of polyolefins as a simple and efficient method of landfill clearing. Pol. J. Chem. Technol. 2010, 12, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Onu, P.; Vasile, C.; Ciocılteu, S.; Iojoiu, E.; Darie, H. Thermal and catalytic decomposition of polyethylene and polypropylene. J. Anal. Appl. Pyrolysis 1999, 49, 145–153. [Google Scholar] [CrossRef]
- Kim, J.; Gil Lee, J.; Kim, H.S.; Lee, T.J.; Park, H.; Ryu, J.H.; Oha, S.M. Thermal Degradation of Solid Electrolyte Interphase (SEI) Layers by Phosphorus Pentafluoride (PF5) Attack. J. Electrochem. Soc. 2017, 164, A2418–A2425. [Google Scholar] [CrossRef]
- Hartnig, C.; Schmidt, M. Elektrolytes and conducting salts. In Lithium-Ion Batteries: Basics and Applications; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2, pp. 59–74. [Google Scholar] [CrossRef]
- Haselrieder, W.; Westphal, B.; Bockholt, H.; Diener, A.; Höft, S.; Kwade, A. Measuring the coating adhesion strength of electrodes for lithium-ion batteries. Int. J. Adhes. Adhes. 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J. Thermal Degradation of Polymeric Materials; Rapra Technology Limited: Shawbury, Shrewsbury, Shropshire, UK, 2005. [Google Scholar]
- Wendler-Kalsch, E.; Gräfen, H. Korrosionsschadenkunde; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Kaesche, H. Die Korrosion der Metalle—Physikalisch-chemische Prinzipien und aktuelle Probleme, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Ostermann, F. Korrosion. In Anwendungstechnologie Aluminium; Ostermann, F., Ed.; Springer Vieweg: Berlin/Heidelberg, Germany, 2014; Volume 3, pp. 217–270. [Google Scholar]
- Sander, S.; Schubert, G.; Timmel, G. Characterisation of fragments produced by the comminution of metals especially considering the fragment shape. Powder Technol. 2002, 122, 177–187. [Google Scholar] [CrossRef]
- Woldt, D. Zerkleinerung nicht-spröder Stoffe in Rotorscheren und -reißern. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 2004. [Google Scholar]
- Timmel, G.; Sander, S.; Schubert, G. Comminution of scrap and metals in shredders with horizontally and vertically mounted rotor. Dev. Miner. Process. 2000, 13, C12a-11–C12a-18. [Google Scholar]
- Schubert, G. Comminution Equipment for Non-Brittle Waste and Scrap. Aufbereitungstechnik 2002, 43, 6–23. [Google Scholar]
- Despotopoulou, M.; Burchill, M.T. Coatings for electrochemical applications. Prog. Org. Coat. 2002, 45, 119–126. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.-Y.; Song, X.-F.; Yu, J.-G. Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning. Waste Manag. 2015, 46, 523–528. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- Wuschke, L.; Jäckel, H.-G.; Leißner, L.; Peuker, U.A. Crushing of large Li-ion battery cells. Waste Manag. 2019, 85, 317–326. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. End-of-Life Lithium-Ion Battery Component Mechanical Liberation and Separation. J. Miner. Met. Mater. Soc. 2019, 71, 4447–4456. [Google Scholar] [CrossRef]
- Schwarz, T.E.; Rübenbauer, W.; Rutrecht, B.; Pomberger, R. Forecasting Real Disassembly Time of Industrial Batteries based on Virtual MTM-UAS Data. In Proceedings of the 25th CIRP Life Cycle Engineering (LCE) Conference, Copenhagen, Denmark, 30 April–2 May 2018; pp. 927–931. [Google Scholar]
- Wuschke, L.; Jäckel, H.-G.; Gellner, M.; Peuker, U.A. Comminution of Li-Ion Traction Batteries. In Proceedings of the European Symposium on Comminution and Classification, Gothenborg, Sweden, 7–11 September 2015; p. 6. [Google Scholar]
- Woldt, D.; Schubert, G.; Jäckel, H.-G. Size reduction by means of low-speed rotary shears. Int. J. Miner. Process. 2004, 74, 405–415. [Google Scholar] [CrossRef]
- Schubert, G. Aufbereitung metallischer Sekundärrohstoffe—Aufkommen Charakterisierung Zerkleinerung; Springer: Wien, Austria, 2012. [Google Scholar]
- Hanisch, C.; Schünemann, J.-H.; Diekmann, J.; Westphal, B.; Loellhoeffel, T.; Prziwara, P.; Haselrieder, W.; Kwade, A. In-Production Recycling of Active Materials from Lithium-Ion Battery Scraps. ECS Trans. 2015, 64, 131–145. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Hayagan, N.; Bachmann, K.; Ferreira, A.; Werner, D.M.; Horn, D.; Peuker, U.A.; Serna-Guerrero, R.; Rudolph, M. Lithium-Ion Battery Recycling—Influence of Recycling Processes on Component Liberation and Flotation Separation Efficiency. ACS EST Eng. 2022. [Google Scholar] [CrossRef]
- Gellner, M. Mechanische Aufbereitung der Feinfraktion Zerkleinerter Lithium-Ionen-Batterien. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 2018. [Google Scholar]






| Component | Binder Type/ Material | Thermal Stability/ Melting Point T in °C | Decomposition/Degradation Temperature T in °C | Adhesive Strength Optimum/Shrinkage T in °C | |
|---|---|---|---|---|---|
| Pristine | Cycled | ||||
| anode | SBR | permanent: 70 | 350–450 2 | 400 1 | 300 3 |
| short-term: 120 1 | |||||
| CMC | stable 4,5 | 250–300 1 | 230 1, 247 4,5 | - | |
| Cu | 1085 | - | - | - | |
| cathode | PVdF | 185 5 | 450 2 | 410 1, 415 4 | 400 3 |
| Al | 660 | - | - | - | |
| separator | PE | 130–135 6,7 | 165 8,9 200 10 | - | 110 6 |
| PP | 160–165 6 | 120 6 | |||
| conducting salt | LiPF6 | 60 11 | - | 70 11,12 | - |
| Component | Function | Material | Mass Share w (%) |
|---|---|---|---|
| cathode | metal foil | Al | 3.0 |
| coating | NMC + PVdF + additives | 34.0 | |
| anode | metal foil | Cu | 7.2 |
| coating | graphite + SBR + CMC + additives | 17.9 | |
| housing | NSD contact | Cu | 0.9 |
| electrical contact | Cu | 0.7 | |
| electrical contact | Al | 0.3 | |
| case | Al | 11.8 | |
| retainer | PP | 0.7 | |
| sleeve | PET | 0.3 | |
| NSD foil | PP | 0.1 | |
| foils | PP | 0.2 | |
| glue | unknown | 0.2 | |
| others | not specified | 0.5 | |
| separator | foil | PP/PE/PP | 1.9 |
| electrolyte | organic solvents | DMC | 16.8 |
| EMC | |||
| DEC | |||
| EC | |||
| conductive salt | LiPF6 | 2.6 | |
| additives | unknown | 1.0 |
| Component | Mean Specific Energy Input | Dismantling Depth | Td in °C | ||
|---|---|---|---|---|---|
| Relative Mass | 22 | 80 | 120 | ||
| housing | wm,j in kWh/t | - | 15.2 | 14.8 | 14.1 |
| wj in % | functional unit | 16.1 | 17.2 | 17.7 | |
| electrodes | 17.5 | 18.1 | 18.3 | ||
| winding | wm,j in kWh/t | - | 1.5 | 1.5 | 1.0 |
| wj in % | functional unit | 83.9 | 82.8 | 82.3 | |
| anode | wm,j in kWh/t | - | 0.8 | 0.3 | 0.6 |
| wj in % | functional unit | 34.2 | 33.2 | 33.0 | |
| electrodes | 41.5 | 40.5 | 40.4 | ||
| cathode | wm,j in kWh/t | - | 0.3 | 0.4 | 0.2 |
| wj in % | functional unit | 44.7 | 45.6 | 45.7 | |
| electrodes | 54.1 | 55.6 | 55.9 | ||
| separator | wm,j in kWh/t | - | 23.7 | 29.2 | 17.6 |
| wj in % | functional unit | 3.6 | 3.2 | 3.0 | |
| electrodes | 4.4 | 3.9 | 3.7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, D.M.; Mütze, T.; Peuker, U.A. Influence of Pretreatment Strategy on the Crushing of Spent Lithium-Ion Batteries. Metals 2022, 12, 1839. https://doi.org/10.3390/met12111839
Werner DM, Mütze T, Peuker UA. Influence of Pretreatment Strategy on the Crushing of Spent Lithium-Ion Batteries. Metals. 2022; 12(11):1839. https://doi.org/10.3390/met12111839
Chicago/Turabian StyleWerner, Denis Manuel, Thomas Mütze, and Urs Alexander Peuker. 2022. "Influence of Pretreatment Strategy on the Crushing of Spent Lithium-Ion Batteries" Metals 12, no. 11: 1839. https://doi.org/10.3390/met12111839








