Influence of a Hydride-Forming Multi-Component Alloy on the Carbonization Behavior of Vulcanized Elastomer Composites
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winter, C.J. Hydrogen energy e abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrogen Energy 2009, 34, S1–S52. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroglu, T.N. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. Int. J. Hydrogen Energy 2005, 30, 795–802. [Google Scholar] [CrossRef]
- Peters, T.A.; Kaleta, T.; Stange, M.; Bredesen, R. Development of ternary Pd-Ag-TM alloy membranes with improved sulphur tolerance. J. Membr. Sci. 2013, 429, 448–458. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Salichev, G.A.; Tikhonovsky, M.A. Structure and mechanical properties of a light-weight AlNbTiV high entropy alloy. Mater. Lett. 2015, 142, 153–155. [Google Scholar] [CrossRef]
- Zou, Y.; Wheeler, J.M.; Ma, H.; Okle, P.; Spolenak, R. Nanocrystalline high entropy alloys: A new paradigm in high temperature strength and stability. Nano Lett. 2017, 17, 1569–1574. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Azenha, I.S.; Lin, Z.; Portugal, I.; Rodrigues, A.E.; Silva, C.M. Inorganic Membranes for Hydrogen Separation. Sep. Purif. Rev. 2018, 47, 229–266. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6, 36770. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, S.K.; Sheu, J.H.; Lin, J.T.; Lin, W.E.; Yeh, J.W.; Lin, S.J.; Liou, T.H.; Wang, C.W. Hydrogen storage properties of multiprincipal-component CoFeMnTixVyZrz alloys. Int. J. Hydrogen Energy 2010, 35, 9046–9059. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Microstructure and hydrogen storage properties of a TiZrNbMoV high entropy alloy synthesized using Laser Engineered Net Shaping (LENS). Int. J. Hydrogen Energy 2014, 39, 9904–9910. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Czujko, T. Microstructures and hydrogen storage properties of La-Ni-Fe-V-Mn alloys. Int. J. Hydrogen Energy 2017, 42, 27154–27164. [Google Scholar] [CrossRef]
- Zepon, G.; Leiva, D.R.; Strozi, R.B.; Bedoch, A.; Figueroa, S.J.A.; Ishikawa, T.T.; Botta, W.J. Hydrogen-induced phase transition of MgZrTiFe0.5Co0.5Ni0.5 high entropy alloy. Int. J. Hydrogen Energy 2018, 43, 1702–1708. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.; Tomilin, I.; Berdonosova, E.; Gammer, C.; Savvotin, I.; Shchetinin, I.; Zheleznyi, M.; Novikov, A.; Bazlov, A.; Serov, M.; et al. Composition design, synthesis and hydrogen storage ability of multi-principal-component alloy TiVZrNbTa. J. Alloys Compd. 2022, 901, 163638. [Google Scholar] [CrossRef]
- Sarac, B.; Zadorozhnyy, V.; Ivanov, Y.P.; Spieckermann, F.; Klyamkin, S.; Berdonosova, E.; Serov, M.; Kaloshkin, S.; Greer, A.L.; Sarac, A.S.; et al. Transition metal-based high entropy alloy microfiber electrodes: Corrosion behavior and hydrogen activity. Corros. Sci. 2021, 193, 109880. [Google Scholar] [CrossRef]
- Sarac, B.; Zadorozhnyy, V.; Berdonosova, E.; Ivanov, Y.; Klyamkin, S.; Gumrukcu, S.; Sarac, A.S.; Korol, A.; Semenov, D.; Zadorozhnyy, M.; et al. Hydrogen storage performance of the multi-principal-component CoFeMnTiVZr alloy in electrochemical and gas-solid reactions. RSC Adv. 2020, 10, 24613–24623. [Google Scholar] [CrossRef] [PubMed]
- Zadorozhnyy, V.; Sarac, B.; Berdonosova, E.; Karazehir, T.; Lassnig, A.; Gammer, C.; Zadorozhnyy, M.; Ketov, S.; Klyamkin, S.; Eckert, J. Evaluation of Hydrogen Storage Performance of ZrTiVNiCrFe in Electrochemical and Gas-Solid Reactions. Int. J. Hydrogen Energy 2020, 45, 5347–5355. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.; Soprunyuk, V.; Klyamkin, S.; Zadorozhnyy, M.; Berdonosova, E.; Savvotin, I.; Stepashkin, A.; Korol, A.; Kvaratskheliya, A.; Semenov, D.; et al. Mechanical spectroscopy of metal/polymer composite membranes for hydrogen separation. J. Alloy. Compd. 2021, 866, 159014. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Xin, X.; Johansson, R.; Wolff, M.; Horvarsson, B. Hydrogen in vanadium: Site occupancy and isotope effects. Phys. Rev. B 2016, 93, 134107. [Google Scholar] [CrossRef]
- Iwakura, Y.I.; Uno, K.; Takiguchi, T. Syntheses of aromatic polyketones and aromatic polyamide. J. Polym. Sci. Part A-1 Polym. Chem. 1968, 6, 3345–3355. [Google Scholar] [CrossRef]
- Marks, B.M. Boron Trifluoride-Hydrogen Fluoride Catalyzed Synthesis of Poly (Aromatic Ketone) and Poly (Aromatic Sulfone) Polymers. Patent No. US3441538A, 29 April 1969. [Google Scholar]
- Zhang, M.; Wang, Z.; Gao, L.; Ding, M. Polyimides from isomeric diphenylthioether dianhydrides. J. Polym. Sci. Part A-1 Polym. Chem. 2005, 44, 959–967. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.-Y.; Chen, M.-M.; Zhao, S.; Wang, J. Effects of carbonization temperature on microstructure and electrochemical performances of phenolic resin-based carbon spheres. J. Phys. Chem. Solids 2010, 71, 214–218. [Google Scholar] [CrossRef]
- Liu, X.-L.; Moriyama, K.; Gao, Y.-F.; Jin, R.-H. Polycondensation and carbonization of phenolic resin on structured nano/chiral silicas: Reactions, morphologies and properties. J. Mater. Chem. B 2016, 4, 626–634. [Google Scholar] [CrossRef]
- Kawahara, Y.; Otoyama, S.; Yamamoto, K.; Wakizaka, H.; Shinahara, Y.; Hoshiro, H.; Ishibashi, N.; Iwashita, N. Direct Carbonization of High-performance Aromatic Polymers and the Production of Activated Carbon Fibers. J. Text. Sci. Eng. 2015, 5, 1000219. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Woszidlo, S.; Koehler, R.; Kopinke, F.-D. Hydrothermal carbonization of poly(vinyl chloride). Chemosphere 2015, 119, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Stepashkin, A.; Chukov, D.; Kaloshkin, S.; Pyatov, I.; Deniev, M. Carbonized elastomer based composites filled with carbon fillers and silicon carbide. Mater. Lett. 2018, 215, 288–291. [Google Scholar] [CrossRef]
- Chukov, D.I.; Stepashkin, A.A.; Salimon, A.I.; Kaloshkin, S.D. Highly filled elastomeric matrix composites: Structure and property evolution at low temperature carbonization. Mater. Des. 2018, 156, 22–31, 5552. [Google Scholar] [CrossRef]
- Ruban, E.; Stepashkin, A.; Gvozdik, N.; Konev, D.; Kartashova, N.; Antipov, A.; Lyange, M.; Usenko, A. Carbonized elastomer composite filled with hybrid carbon fillers for vanadium redox flow battery bipolar plates. Mater. Today Commun. 2021, 26, 101961. [Google Scholar] [CrossRef]
- Antsiferov, V.N.; Serov, M.M.; Lezhnin, V.P.; Smetkin, A.A. About fabrication, properties and application of rapidly cooled fibers, Izv. Vysh. Uchebn. Zaved. Poroshk. Metall. Funkts. Pokryt 2013, 1, 55–58. (In Russian) [Google Scholar] [CrossRef]
- Serov, M.M. Microcrystalline and amorphous alloys obtained by the method of rapid quenching of melt, Tekhnol. Legk. Splav 2008, 4, 34–41. [Google Scholar]
- Zadorozhnyy, V.Y.; Klyamkin, S.N.; Kaloshkin, S.D.; Zadorozhnyy, M.Y.; Bermesheva, O.V. Mechanochemical Synthesis and Hydrogen Sorption Properties of Nanocrystalline TiFe. Inorg. Mater. 2011, 47, 1081–1086. [Google Scholar] [CrossRef]
- Zadorozhnyi, V.Y.; Skakov, Y.A.; Milovzorov, G.S. Appearance of Metastable States in Fe–Ti and Ni–Ti Systems in the process of Mechanochemical Synthesis. Met. Sci. Heat Treat. 2008, 50, 404–410. [Google Scholar] [CrossRef]
- Gong, C.; Chen, Y.; Li, T.; Liu, Z.; Zhuang, Z.; Guo, B.; Wang, H.; Dai, L. Free volume based nonlinear viscoelastic model for polyurea over a wide range of strain rates and temperatures. Mech. Mater. 2021, 52, 103650. [Google Scholar] [CrossRef]
- Qiao, J.; Amirkhizi, A.V.; Schaaf, K.; Nemat-Nasser, S.; Wu, G. Dynamic mechanical and ultrasonic properties of polyurea. Mech. Mater. 2011, 43, 598. [Google Scholar] [CrossRef]
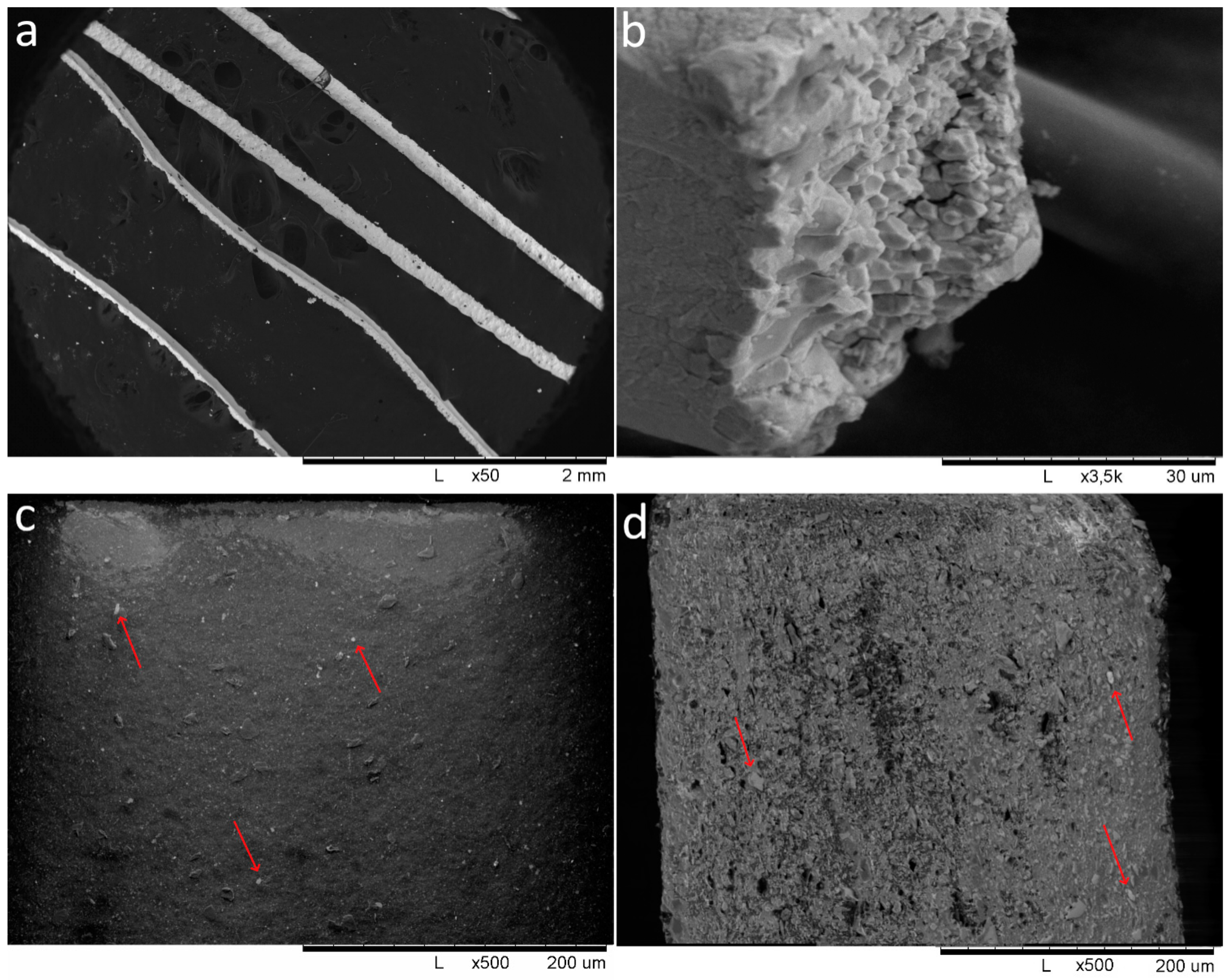
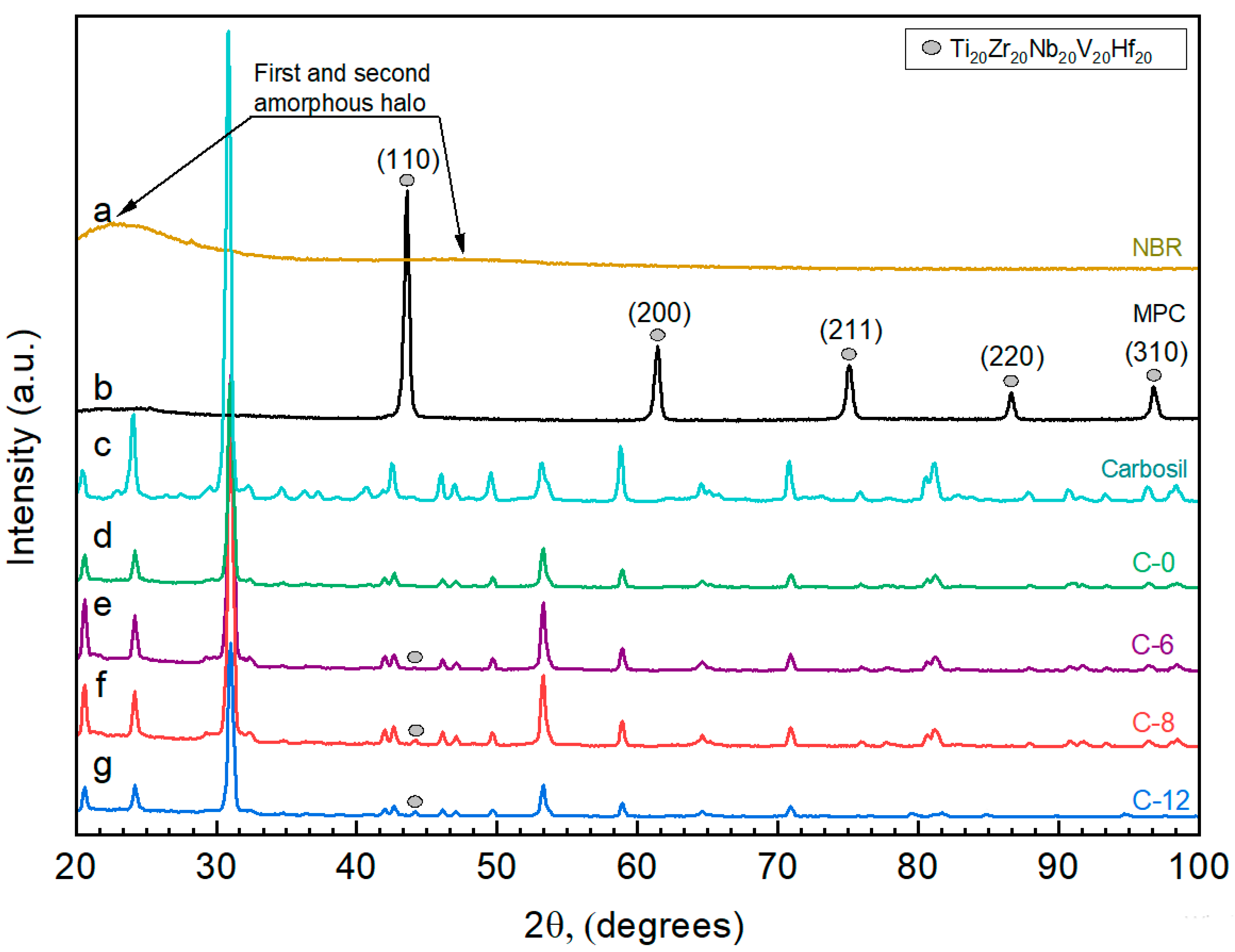
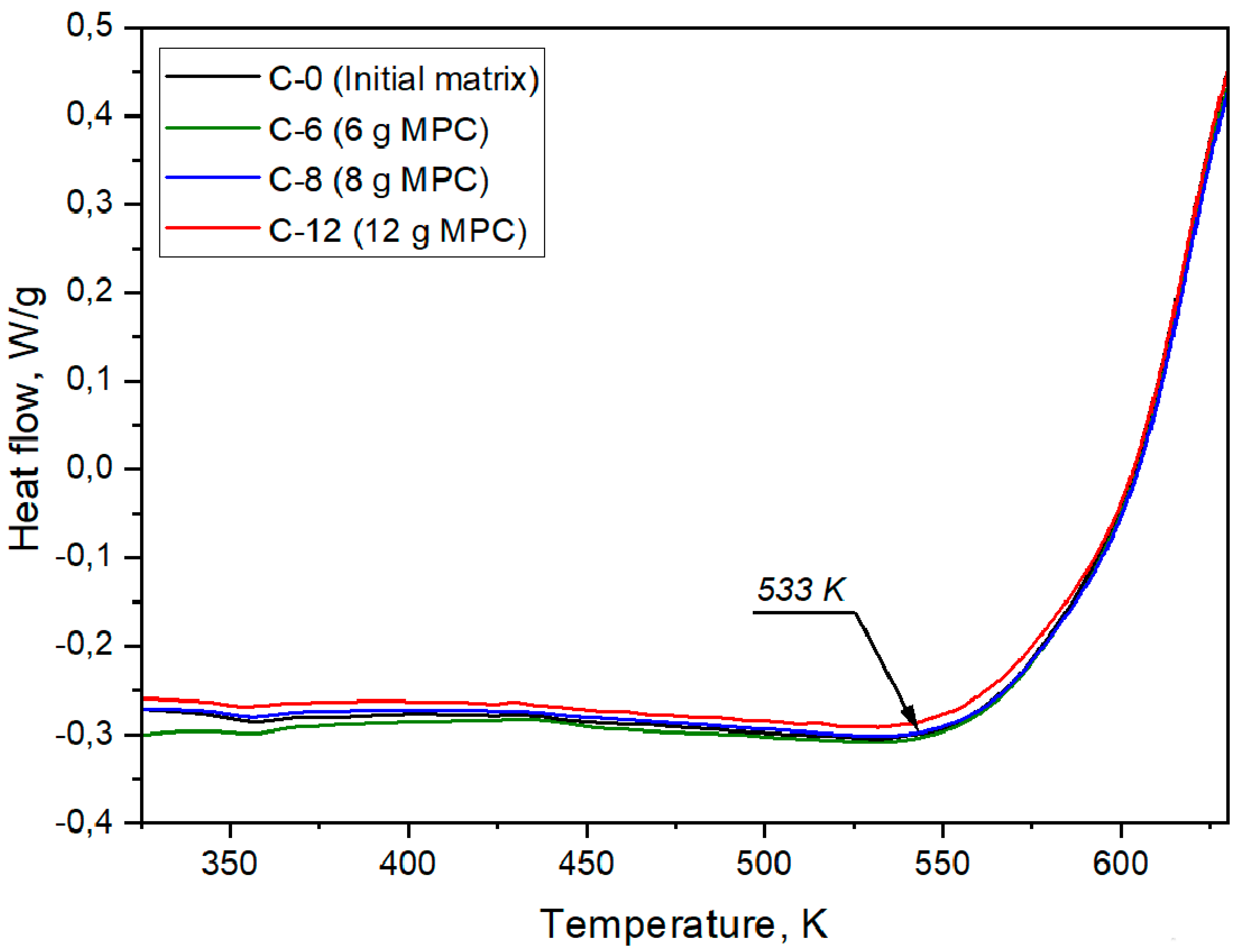
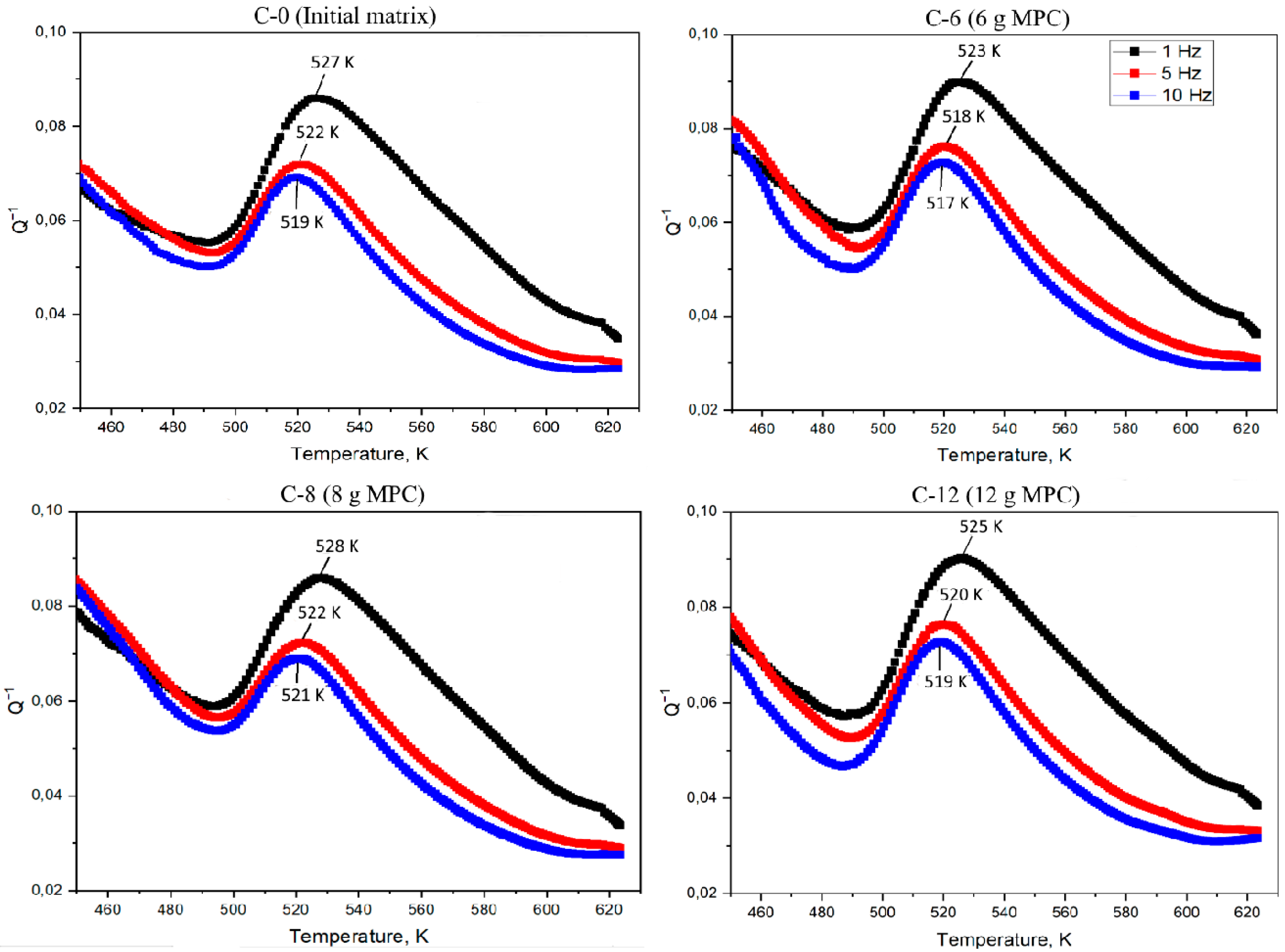

| Designation | Content of the Mixture Components (g) | |||
|---|---|---|---|---|
| NBR | Carbosil | Dicumyl Peroxide | MPC Ti20Zr20Nb20V20Hf20 | |
| C-0 | 100 | 100 | 1 | - |
| C-6 | 100 | 100 | 1 | 6 |
| C-8 | 100 | 100 | 1 | 8 |
| C-12 | 100 | 100 | 1 | 12 |
| Sample Name | ѓ, Hz | T, K | Q−1 | Ea, kJ/mol |
|---|---|---|---|---|
| C-0 | 1 | 527 ± 1 | 0.086 | 274 ± 2 |
| 5 | 522 ± 1 | 0.072 | ||
| 10 | 519 ± 1 | 0.069 | ||
| C-6 | 1 | 523 ± 1 | 0.089 | 241 ± 2 |
| 5 | 518 ± 1 | 0.076 | ||
| 10 | 517 ± 1 | 0.072 | ||
| C-8 | 1 | 528 ± 1 | 0.085 | 224 ± 2 |
| 5 | 522 ± 1 | 0.072 | ||
| 10 | 521 ± 1 | 0.068 | ||
| C-12 | 1 | 525 ± 1 | 0.090 | 174 ± 2 |
| 5 | 520 ± 1 | 0.076 | ||
| 10 | 519 ± 1 | 0.072 |
| Sample Name | Permeability Coefficient, Barrer * | |||||
|---|---|---|---|---|---|---|
| N2 | CO2 | He | CH4 | Ar | H2 | |
| C-0 | 0.7 | 11.1 | 4.5 | 1.3 | 1.4 | 5.6 |
| C-6 | 0.5 | 9.7 | 3.5 | 1.0 | 1.1 | 5.0 |
| C-8 | 0.8 | 12.6 | 5.3 | 2.2 | 1.6 | 7.0 |
| C-12 | - | 9.5 | 4.5 | - | 1.4 | 5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zadorozhnyy, M.; Savvotin, I.; Berdonosova, E.; Klyamkin, S.; Stepashkin, A.; Korol, A.; Zadorozhnyy, V. Influence of a Hydride-Forming Multi-Component Alloy on the Carbonization Behavior of Vulcanized Elastomer Composites. Metals 2022, 12, 1847. https://doi.org/10.3390/met12111847
Zadorozhnyy M, Savvotin I, Berdonosova E, Klyamkin S, Stepashkin A, Korol A, Zadorozhnyy V. Influence of a Hydride-Forming Multi-Component Alloy on the Carbonization Behavior of Vulcanized Elastomer Composites. Metals. 2022; 12(11):1847. https://doi.org/10.3390/met12111847
Chicago/Turabian StyleZadorozhnyy, Mikhail, Ivan Savvotin, Elena Berdonosova, Semen Klyamkin, Andrey Stepashkin, Artem Korol, and Vladislav Zadorozhnyy. 2022. "Influence of a Hydride-Forming Multi-Component Alloy on the Carbonization Behavior of Vulcanized Elastomer Composites" Metals 12, no. 11: 1847. https://doi.org/10.3390/met12111847
APA StyleZadorozhnyy, M., Savvotin, I., Berdonosova, E., Klyamkin, S., Stepashkin, A., Korol, A., & Zadorozhnyy, V. (2022). Influence of a Hydride-Forming Multi-Component Alloy on the Carbonization Behavior of Vulcanized Elastomer Composites. Metals, 12(11), 1847. https://doi.org/10.3390/met12111847








